Abstract
This article uses a demographic approach and data from the Health and Retirement Survey, a nationally representative sample of the U.S. population, to investigate sex differences in the length of life lived with heart disease and after a heart attack for persons in the United States age 50 and older. On average, women live longer than men with heart disease. At age 50 women can expect to live 7.9 years and men 6.7 years with heart disease. The average woman experiences heart disease onset three years older and heart attacks 4.4 years older than men.
Keywords: Heart disease, multistate life table, sex differences
INTRODUCTION
Heart disease is the most prevalent fatal chronic disease afflicting older Americans. It accounts for approximately 32 percent of all deaths and is the leading cause of death for both men and women aged 65 years and older (Anderson & Smith, 2005). It is also a major cause of illness; 37 percent of men and 27 percent of women over age 65 reported having a heart condition in 2001/2002 (Federal Interagency Forum on Aging-Related Statistics, 2004). Men have higher heart disease mortality rates than women at all ages with the gap declining as age increases (Khaw & Barrett-Connor, 1992; Markides, 1992), although in recent years the declines have been less for women than for men (American Heart Association, 2004). For this reason, many Americans do not perceive heart disease as a woman’s health issue although estimates indicate that from 40 to 50 percent of postmenopausal women will develop heart disease (Col et al., 1997; Lloyd-Jones et al., 2006). There is also evidence that women have heart attacks 5 to 10 years older than men, that women who have a heart attack have more confounding health problems, and that women who are hospitalized after a heart attack are less likely to receive certain types of drugs and diagnostic and treatment procedures (Clarke, Gray, Keating & Hampton, 1994; Eastwood & Doering, 2005; Fuchs, 1999). Controversy continues about whether sex differences occur in initial hospital survival and long-term survival after a heart attack (Coronado, Griffith, Beshansky & Selker, 1997; Maynard, Every, Martin, Kudenchuk & Weaver, 1997; Vaccarino, Krumholz, Yarzebski, Gore & Goldberg, 2001; Vaccarino, Parsons, Every, Barron & Krumholz, 1999).
Despite glimpses into the heart disease experiences of men and women, basic information on gender differences in heart disease morbidity, incidence and duration for the national population of the U.S. is elusive. Unclear, for example, is how much of men’s higher prevalence of heart disease signifies a higher incidence of heart disease and longer periods of life spent with heart disease; or whether men’s higher death rates result in fewer years lived with heart disease. It is also unclear how the different age structure of the female and male populations affects some of the generalizations about men and women. Incorporating the age patterning of heart disease onset and death into a multistate life table model allows us to calculate the length of life “with and without heart disease,” which identifies the average period of heart disease development or progression and the period of treatment or monitoring in the population. Clarification of sex differences in this disease process will inform our understanding of the likely effects of potential intervention.
Demographic models of chronic disease onset and outcome have been used previously to examine life expectancies with and without disease or impairment (Jagger & Matthews, 2002; Robine, Jagger & Cambois, 2002), and are well-suited to clarifying the effects of sex differences in morbidity and mortality rates for the length of life with and without disease, expected ages at major health events, and population disease composition (Laditka & Hayward, 2003; Robine, Jagger, Mathers, Crimmins & Suzman, 2003). We estimate the age-specific likelihood of a series of health events using hazard models. We use the parameters of these models to calculate multistate life table models of heart disease–free life expectancy for Americans over 50 by gender.
MATERIALS AND METHODS
Data
Incidence measures of heart disease experience are calculated using data from the first two waves of the Health and Retirement Survey (HRS) including the older sample which began as the Assets and Health Dynamics Among the Oldest Old (AHEAD) Survey. The HRS sample was nationally representative of community-dwelling persons aged 51–61 in 1992 and their spouses. The AHEAD was representative of persons 70 years of age and older in 1993 and their spouses. The pooled sample covers the entire age range over 50 at the initial interview because spouses fill in the ages 62 to 69. Wave 2 surveys for both the HRS and AHEAD occurred approximately two years after the baseline survey, that is, in 1994 and 1995, respectively.
We use the sample from age 50 through 97 years of age (N = 18,160) who had information on heart disease status or death at waves 1 and 2 (unweighted N = 18,034). Persons with missing information were more likely to be older and female. The sample has more women (N = 10,110) than men (N = 8,050). The sample is racially and ethnically representative of the U.S. national community-dwelling population when sample weights are used as in this analysis.
Measures
Information on the presence of heart disease at the two interviews is provided by self-report and is used to classify individuals according to initial heart disease state at each wave. Each person can be allocated to one of three states: “No heart disease,” never having been told by a doctor that they have coronary heart disease, angina, congestive heart failure, or other heart problems; “Heart disease without heart attack” means that a respondent has been told that he/she has one of the conditions listed above but has never had a “heart attack or myocardial infarction”; and having had a “heart attack.” Heart attack is a state that can be entered after heart disease but one cannot return to the state of only heart disease after having had a heart attack; nor can one return to never having heart disease.
Self-reports of conditions in the HRS produce similar levels of disease prevalence as in other national surveys, for example, the National Health Interview Survey (Fisher et al., 2005). Several studies have compared self-reported history of heart disease and medical record report and generally found good agreement (Bush, Miller, Golden & Hale, 1989; Lampe, Walker, Lennon, Whincup & Ebrahim, 1999; Psaty et al., 1995; Voalklander et al., 2006). The question wording in the AHEAD survey allows us to identify only those who have had a heart attack in the last five years so there will be some underestimate at the start of the population in this state at the older ages; however, it is the rates of onset rather than prevalence that are the building blocks of the multistate life tables.
Multistate life tables allow us to estimate state-specific mortality rates from the three heart disease states. Reports of death supplied by informants at the time of the second interview are supplemented with information from the National Death Index (NDI) to determine the vital status and to determine cause of death. To incorporate information on heart disease development among those who died within the interval and to put them at risk in an appropriate manner for our demographic model, decedents with heart disease designated as a cause of death are classified as getting heart disease mid-interval if it was not reported at the initial interview. Decedents from heart attacks are classified as having a heart attack mid-interval if they have not reported an earlier heart attack. Some decedents did not give permission to be linked to the NDI and thus death certificates indicating cause of death are not obtained. We preserve the population’s level of mortality from heart disease and other causes by randomly assigning a cause (i.e., death due to heart disease, heart attack, or other causes) for decedents with missing information based on age-sex specific distributions of cause of death in the U.S. population. About one-third of all decedents are classified as dying from heart disease (N = 335 out of 1,045) and about one-third of these (N = 110) die from a heart attack.
Methods
The multistate life table method has been described and compared to others in a number of methodological papers on estimating healthy life expectancy (Laditka & Hayward, 2003). It involves two stages, the estimate of a series of incidence-based health state transition schedules which are then used as the input to the multistate life table which is used to derive the lifetime events for a synthetic cohort experiencing all of the estimated age-specific transition rates. The health transition schedules indicate the annual likelihood of getting heart disease or of having a heart attack. Mortality schedules represent the mortality rates experienced by those with heart disease, those who have had a heart attack, and those with no disease. Incidence rates of morbidity track whether disease events occurred over the 2-year observation interval among the appropriate “at risk” populations. All events, including censoring, are assumed to occur at the end of the interval unless a person dies of previously unreported heart disease–which is assumed to occur mid-interval. For this reason, we calculate the rates by separating all intervals into two one-year periods.
We estimate the incidence rates for the multistate life table using a discrete-time hazard model (Crimmins, Hayward & Saito, 1994, 1996; Land et al., 1994). In this approach, the probability, pijx, is the probability of moving from state i to state j at age x where x refers to the age at last birthday. We model the probabilities separately for men and women using a log-linear model, that is,
The model’s parameter estimates are used to calculate predicted transition probabilities, pij(x) which serve as the inputs for the multistate life table. The multistate life table develops the lifetime implications for the life table cohort of experiencing the health transitions and mortality as observed in the population. These are represented by traditional life table functions such as survivors at a given age but in a given state, deaths at a given age, and life expectancy and years lived after a specified age.
Two types of multistate life tables are constructed: population-based and status-based. The population-based life table provides the experience for the average person in the population. It allocates a life table cohort to each disease state at age 50, the initial age of the life table, according to observed disease prevalence at that age. Status-based life tables provide information on the average outcome for persons with a given disease status at a given age. The status-based table begins with an entire cohort in a given state at a set age, for example, no heart disease at age 60. The projected disease lifecycle of that hypothetical cohort is then determined according to the transition probabilities.
RESULTS
At baseline, 14 percent (1,131 of 8,050) of male respondents 50 years of age and over report having heart disease; an additional 9 percent have had a heart attack. Women’s prevalence rates are 15 and 5 percent, respectively. About 200 men and 300 women reporting no disease at baseline were diagnosed by Wave 2, and about 100 of each sex had a heart attack. Approximately 50 persons of each sex who had heart disease at baseline reported a heart attack by Wave 2. About 500 of each sex died over the observation period. These transitions are the basis for calculating our life tables.
The parameters of the hazard models of the disease transitions indicate that all of the transitions are significantly more likely to occur among older persons, except for heart attacks among men who have heart disease (Table 1). The age effects for the two models predicting heart attack are about twice as large for women indicating that heart attack risk increases more steeply with age among women. The other age effects are similar for both sexes. By pooling the data for the two sexes, models were estimated to test the significance of the differences between the two groups (results not shown but described below).
TABLE 1.
Summary of Equations for Heart Disease Hazard Models
| Event | Men | Women | ||
|---|---|---|---|---|
| Intercept | Age | Intercept | Age | |
| No Heart Disease to Heart Disease (not Attack) | −7.120** | 0.049** | −7.077** | 0.047** |
| No Heart Disease to Heart Attack | −6.615** | 0.032** | −9.504** | 0.066** |
| Heart Disease (not Attack) to Heart Attack | −4.737** | 0.017 | −6.115** | 0.031* |
| No Heart Disease to Death | −10.854** | 0.098** | −10.345** | 0.086** |
| Heart Disease (not Attack) to Death | −7.874** | 0.070** | −7.663** | 0.064** |
| Heart Attack to Death | −7.125** | 0.065** | −6.938** | 0.063** |
p < 0.01,
p < 0.001
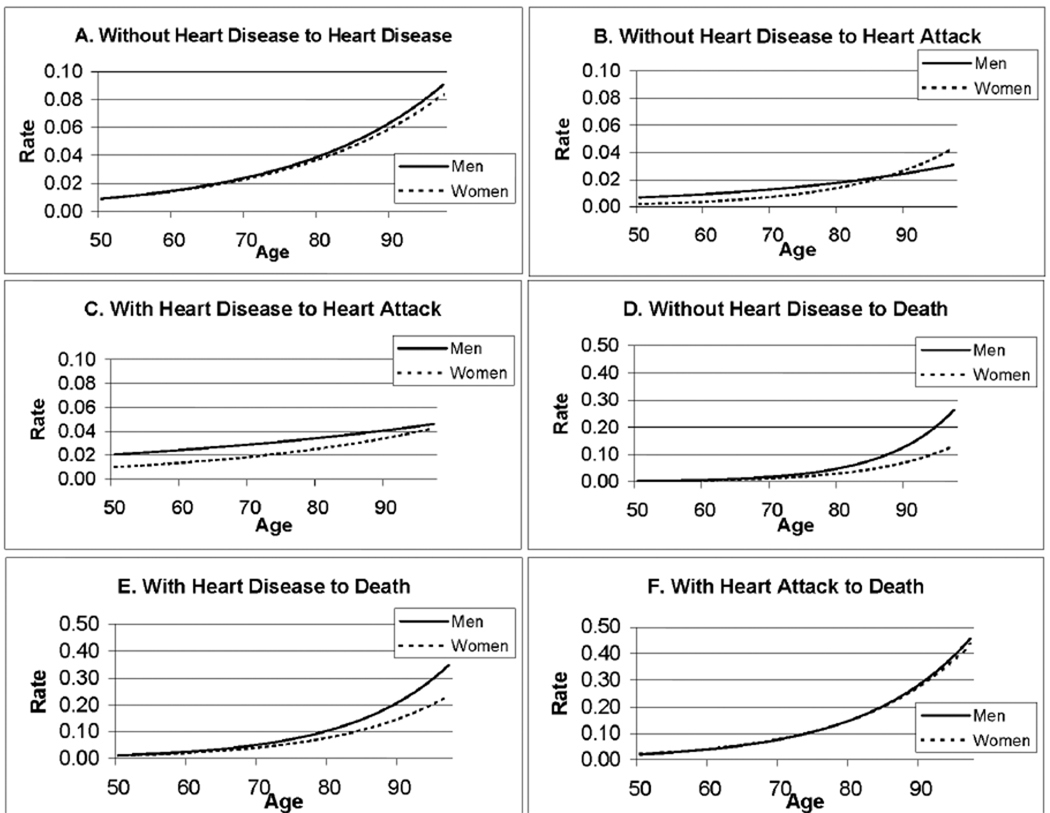
Based on the sex-specific models’ parameter estimates shown in Table 1, the age-specific rates of heart disease and heart attack onset and death from each state are calculated and shown in Figure 1. These figures clarify the age and sex differences in the rates of heart disease onset and progression. Most of the events indicating progression to worse cardiovascular health or death, for example, are more frequent among men; however, there is no sex difference in the onset of heart disease among those without heart disease initially (Figure 1A). On the other hand, men who begin the interval without reported heart disease, or who have heart disease but have not had a heart attack are more likely to have a heart attack up until the older ages (Figures 1B, 1C). An examination of death rates from the various states shows that men who do not have heart disease or who have heart disease but have not had a heart attack have higher rates of death than women (Figures 1D, 1E). Men and women who have had a heart attack do not differ in their death rates (Figure 1F).
FIGURE 1.
Estimated Annual Transition Rates Across Disease States
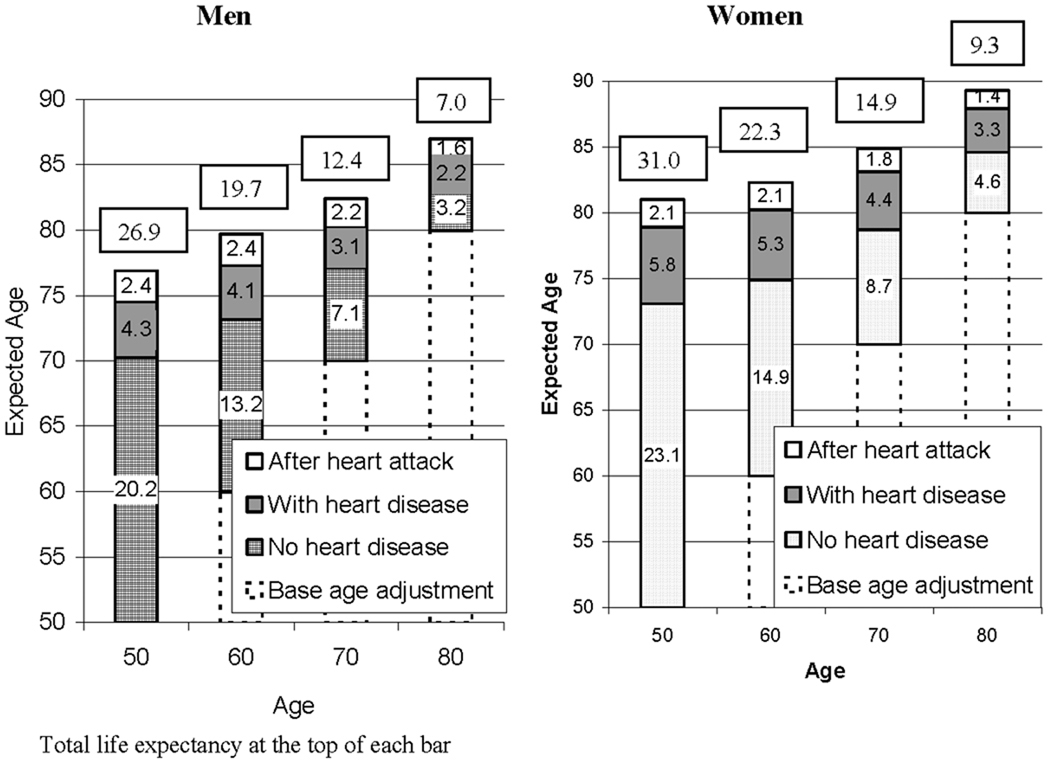
The multistate life expectancies based on this combination of transition rates identify how long men and women can expect to live with heart disease and after a heart attack. These are shown for the population at ages from 50 to 80 (Figure 2). The average length of life men at age 50 can expect to live is 26.9 years in total. Approximately 20 years of life are free of heart disease and 6.7 are spent with heart disease with 2.4 of those after having had a heart attack and 4.3 with heart disease but not having had a heart attack. Women at age 50 average 31 years in total with about 23 heart-disease free years. They live more years than men disease-free, more years with heart disease but no heart attack (5.8 vs. 4.3), but not more years after having had a heart attack (2.1 vs. 2.4).
FIGURE 2.
Total Life Expectency and Life Expectency by Heart Disease Status and Age and Expected Age at Heart Disease Onset, Heart Attack, and Death: Population-Based Life Table
On average, women experience heart disease at older ages than men. The average age at onset of heart disease in the population aged 50 and over is 70 for men and 73 for women. On average, men will have a heart attack at 74.5 and women at 78.9. At age 50, both men and women can expect to have heart disease for approximately 1/4 of their remaining lives. As individuals survive, the relative proportion of life that is free of heart disease declines. By age 70, for example, 57 percent of men’s remaining life is free of heart disease; the figure for women is almost identical (58 percent). This is because the average years free of disease are smaller at the later ages and the average years with disease are not reduced as much.
In terms of absolute years spent with heart disease, Figure 2 shows that at every age the average woman can expect to live with heart disease more years than the average man. At age 50, the average woman can expect to live 7.9 years with heart disease, while the figure for the average man is 6.7 years. At each age, women are more likely to spend time diagnosed with disease and without a heart attack. Men at every age can expect to spend more years after a heart attack than women.
Examination of the full life table (data not shown) can provide information on the health status of the life table population. Given these morbidity and mortality rates, out of 100,000 men in a cohort at age 50, one-third (33,368) are expected to have a heart attack at some time in their lives; for women, about one-quarter (26,599) will have a heart attack. Three-fourths (72 percent) of both men and women will develop heart disease over their lifetimes.
The results for the population-based life tables show life expectancy for the average person alive at a given age irrespective of his or her disease status. Status-based tables show the consequences of being in a disease state at a given age for subsequent health. Men who have no heart disease at age 50, for example, can expect to live free of heart disease until age 71; for women, the figure is 74. Surviving in good health has significant payoffs in terms of one’s future health; for both men and women, we observe a compression of life spent with heart disease as persons survive to later ages without heart disease. Among men, surviving to 70 without heart disease, the average man is free of heart disease until 80. Women free of heart disease at 70 are likely to survive disease-free to 82.
Women who have heart disease but who have not had a heart attack at a given age live longer with the disease than men. Men who have heart disease by age 50, can expect to live two years less than women who have heart disease, 21.3 years versus 23.3 years. Among people who have had a heart attack at a given age, life expectancy is strikingly similar for men and women. At age 50, both men and women who have had a heart attack can expect to live 18 more years. By age 70, both sexes can expect to live 8 more years–a pattern produced by the similar age-specific male/female death rates after heart attack noted earlier. These figures indicate the potential years of treatment for persons who get heart disease at a young age.
Disease prevalence implied by the life table is a useful means to gauge what current rates portend for the future prevalence of heart disease in the population as they indicate the health of the population after the observed incidence rates have been in effect for some years. In general, if incidence and mortality rates reported in this survey continue, the life tables point to small decreases in heart disease prevalence at younger ages and increases at older ages. Half of surviving men will have heart disease by age 77 and half of women by age 87–currently prevalence peaks at about 40 percent. This increase reflects current higher levels of survival with heart disease than experienced historically. The percentage of the surviving population who will have had a heart attack is considerably higher at each age for men than for women. At age 75, one-quarter of men in the life table population have had a heart attack; for women, this is halved.
DISCUSSION
Using a life table approach which controls for difference in population age composition, we have documented some similarities and some differences in the heart disease experience of American men and women. The average woman lives more years with heart disease at every age after 50 because of the lower survival among men both with and without heart disease and because men progress to heart attacks faster than women. Persons who have had a heart attack have higher mortality than others; once men and women have a heart attack, however, their survival is similar. Men and women over age 50 do not differ in the likelihood of onset of heart disease without heart attack, but men are more likely to have a heart attack.
Our estimate is that women are 4.4 years older on average when they have a heart attack which is near the lower end of previous estimates. The fact that women are older at heart attack could be a factor in the different treatment that men and women receive upon hospital admission (Clarke et al., 1994; Eastwood & Doering, 2005; Fuchs, 1999).
Men are more likely to end up having had a heart attack than women, and a heart attack appears to be more likely as the first sign of heart disease for men than for women from age 50 through sometime in their early 80s. This could result from sex differences in the disease process but reports could also be affected by gender differentials in the use of medical care during which one becomes aware of having heart disease. Because younger men are less likely to visit doctors, men with heart disease may be less likely to be diagnosed with heart disease before heart attack. This could partly explain the fact that observed heart disease onset rates among those without disease are similar for men and women.
It is interesting that men and women who know that they have had a heart attack do not survive differently. One can assume that most of these people are under a physician’s care. We must also consider the possibility that heart attacks may be less recognized in women. “Silent myocardial infarctions (MIs)” are thought to be more common among women (de Bruvne et al., 1997).
Interestingly, our estimates of total life expectancy and life expectancy without heart disease for a national sample representative of all race-ethnic groups in the American population are similar to results derived from the more local Framingham study, which has a low participation rate and an ethnically unrepresentative sample of the U.S. In addition to geographic coverage, our estimates differ from those developed from Framingham in that they are derived from self-reports of conditions in the 1990s, while the Framingham estimates are derived from examination and laboratory results for over 40 years for persons free of disease at age 40 in approximately 1950. We estimate that women aged 50 would live 31 more years with 23 free of heart disease; the estimates from the Framingham data were 32 years and 26 free of heart disease (Peeters, Mamun, Willekens & Bonneux, 2002). For men, the estimate for the Framingham cohort was 26 years of life with 20 free of disease; while ours was 27 years of life with 20 free of disease. Our models indicate that more persons will experience heart disease over their lifecycles than estimates arising from the Framingham data. Our data indicate that almost 3 out of 4 men and women will experience heart disease before death, while the estimates from the Framingham data are 52 percent for men and 39 percent for women (Lloyd-Jones et al., 2006). Our more recent data may reflect the recently observed increases in heart disease prevalence in the U.S. population (Crimmins & Saito, 2000; Cutler & Richardson, 1997).
This article provides the first estimates of life expectancy with and without heart disease for the U.S. national population based on multistate life table methods. Earlier estimates of life expectancy with heart disease developed using cross-sectional prevalence data appear to be lower than those obtained here. Using age-specific prevalence data from the National Health Interview Survey with heart disease presence defined using a simple question about the presence of heart disease, both men and women have about 4.5 years of heart disease at age 65 (Crimmins, Kim & Hagedorn, 2002). The estimate for both sexes developed here would be 6.8 years. In addition to having a different definition of heart disease, the estimates based on cross-sectional data did not incorporate different mortality rates by disease state. This is probably the reason that the estimated length of life with heart disease in the previous study is lower than that estimated with the multistate method employed in this analysis which has clearly documented a difference in mortality by heart disease state.
CONCLUSIONS
The multistate approach to estimating time spent with and without disease clarifies that heart disease is a major health problem for both sexes. The importance of behavioral changes and medical advances that would delay the onset of fatal conditions such as heart disease is clear (Franco, de Laet, Peeters, Jonker, Mackenbach & Nusselder, 2005a; Franco, Peeters, Bonneux & de Laet, 2005b; Laditka & Laditka, 2000). If no one got heart disease before age 70 and all other rates stayed the same as observed, the number of years spent with heart disease would decrease by about a third.
The number of years lived with heart disease indicates the tremendous burden of heart disease for both sexes and for the medical care system. The multistate approach and the models developed in this article provide an ideal mechanism for understanding the implications of projected medical and technological innovation or changes in behavior or population characteristics on future population health. For instance, the basic model parameters can be changed using assumptions about the future benefit of new technology on the prevention of disease and death in order to derive implied changes in the burden of disease represented by years spent with heart disease. Further research using multistate models of disease which incorporates the potential of health innovations and behaviors to change model parameters which can be derived from epidemiological or clinical data is the next step in extending the use of the multistate life table approach and examining the potential difference in the effect of new health innovations, policy changes, and behavioral modification for men and women (Laditka & Laditka, 2000).
Acknowledgments
Support for this project was provided by Grants No. P30 AG17265, R01 AG11235 and R01 AG11758 from the National Institute on Aging.
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
Contributor Information
Eileen M. Crimmins, Andrus Gerontology Center, University of Southern California, Los Angeles, CA..
Mark D. Hayward, Population Research Center, The University of Texas, Austin, TX..
Hiroshi Ueda, World Health Organization Kobe Centre, Centre for Health Development, Japan..
Yasuhiko Saito, Center for Information Networking, Nihon University, Japan..
Jung Ki Kim, Andrus Gerontology Center, University of Southern California, Los Angeles, CA..
REFERENCES
- American Heart Association. Women and cardiovascular diseases–Statistics (Revised) [Retrieved March 15, 2006];2004 from http://www.americanheart.org/downloadable/heart/1136818052118Females06.pdf.
- Anderson R, Smith B. Death: Leading causes for 2002. National vital statistics reports. 2005;53:14. [PubMed] [Google Scholar]
- Bush T, Miller S, Golden A, Hale W. Self-report and medical record report agreement of selected medical conditions in the elderly. American Journal of Public Health. 1989;79:1554–1556. doi: 10.2105/ajph.79.11.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke K, Gray D, Keating N, Hampton J. Do women with acute myocardial infarction receive the same treatment as men? British Medical Journal. 1994;309:563–566. doi: 10.1136/bmj.309.6954.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Col NF, Eckman MH, Karas RH, Pauker SG, Goldberg RJ, Ross EM, et al. Patient-specific decisions about hormone replacement therapy in postmenopausal women. Journal of the American Medical Association. 1997;277:1140–1147. doi:10.1001/jama.277.14.1140. [PubMed] [Google Scholar]
- Coronado BE, Griffith JL, Beshansky JR, Selker HP. Hospital mortality in women and men with acute cardiac ischemia: A prospective multicenter study. Journal of the American College of Cardiology. 1997;29:1490–1496. doi: 10.1016/s0735-1097(97)00077-6. doi:10.1016/S0735-1097(97)00077-6. [DOI] [PubMed] [Google Scholar]
- Crimmins EM, Hayward MD, Saito Y. Changing mortality and morbidity rates and the health status and life expectancy of the older population. Demography. 1994;31:159–175. doi:10.2307/2061913. [PubMed] [Google Scholar]
- Crimmins EM, Hayward MD, Saito Y. Differentials in active life expectancy in the older population of the United States. Journal of Gerontology: Social Sciences. 1996;51B:S111–S120. doi: 10.1093/geronb/51b.3.s111. [DOI] [PubMed] [Google Scholar]
- Crimmins EM, Kim JK, Hagedorn A. Life with and without disease: Women experience more of both. Journal of Women and Aging. 2002;14(1/2):47–59. doi: 10.1300/J074v14n01_04. doi: 10.1300/J074v14n01_04. [DOI] [PubMed] [Google Scholar]
- Crimmins EM, Saito Y. Change in the prevalence of disease among older Americans: 1984–1994. [Retrieved August 13, 2006];Demographic Research. 2000 November 1;3 Article 9. from http://www.demographic-research.org/Volumes/Vol3/9/3-9.pdf.
- Cutler DM, Richardson E. Measuring the health of the U.S. population. In: Baily M, Reiss P, Winston C, editors. Brookings papers on economic activity: Microeconomics. Washington DC: The Brookings Institution; 1997. [Google Scholar]
- de Bruvne MC, Mosterd A, Hoes AW, Kors JA, Kruijssen DA, van Bemmel JH, et al. Prevalence, determinants, and misclassification of myocardial infarction in the elderly. Epidemiology. 1997;8:495–500. doi: 10.1097/00001648-199709000-00004. [DOI] [PubMed] [Google Scholar]
- Eastwood JA, Doering LV. Gender differences in coronary artery disease. Journal of Cardiovascular Nursing. 2005;20:340–351. doi: 10.1097/00005082-200509000-00008. [DOI] [PubMed] [Google Scholar]
- Federal Interagency Forum on Aging-Related Statistics. Washington, D.C.: U.S. Government Printing Office; Older Americans 2004: Key indicators of well-being. 2004;88
- Fisher G, Faul J, Weir D, Wallace RB. Documentation of chronic disease measures in the Health and Retirement Study. HRS Documentation Report DR-009. 2005 [Google Scholar]
- Franco OH, de Laet C, Peeters A, Jonker J, Mackenbach J, Nusselder W. Effects of physical activity on life expectancy with cardiovascular disease. Archives of Internal Medicine. 2005a;165:2355–2360. doi: 10.1001/archinte.165.20.2355. doi:10.1001/archinte.165.20.2355. [DOI] [PubMed] [Google Scholar]
- Franco OH, Peeters A, Bonneux L, de Laet C. Blood pressure in adulthood and life expectancy with cardiovascular disease in men and women. Hypertension. 2005b;46:280–286. doi: 10.1161/01.HYP.0000173433.67426.9b. doi:10.1161/01.HYP.0000173433.67426.9b. [DOI] [PubMed] [Google Scholar]
- Fuchs VR. Health care for the elderly: How much? Who will pay for it? Health Affairs. 1999;18:11–21. doi: 10.1377/hlthaff.18.1.11. doi:10.1377/hlthaff.18.1.11. [DOI] [PubMed] [Google Scholar]
- Jagger C, Matthews F. Gender differences in life expectancy free of impairment at older ages. Journal of Women and Aging. 2002;14:85–97. doi: 10.1300/J074v14n01_06. doi:10.1300/J074v14n01_06. [DOI] [PubMed] [Google Scholar]
- Khaw KT, Barrett-Connor E. Sex differences, hormones, and coronary heart disease. In: Marmot M, Elliott P, editors. Coronary heart disease epidemiology from etiology to public health. New York, NY: Oxford University Press; 1992. pp. 274–286. [Google Scholar]
- Laditka J, Laditka S. The morbidity compression debate: risks, opportunities, and policy options for women. Journal of Women and Aging. 2000;12:23–38. doi: 10.1300/J074v12n01_03. doi:10.1300/J074v12n01_03. [DOI] [PubMed] [Google Scholar]
- Laditka S, Hayward MD. The evolution of demographic methods to calculate health expectancies. In: Robine J-M, Jagger C, Mathers C, Crimmins E, Suzman R, editors. Determining health expectancies. London: John Wiley & Sons, Limited; 2003. pp. 221–234. [Google Scholar]
- Lampe F, Walker M, Lennon L, Whincup P, Ebrahim S. Validity of a self-reported history of doctor-diagnosed angina. Journal of Clinical Epidemiology. 1999;52:73–81. doi: 10.1016/s0895-4356(98)00146-2. doi:10.1016/S0895-4356(98)00146-2. [DOI] [PubMed] [Google Scholar]
- Land KC, Guralnik JM, Blazer DG. Estimating increment-decrement life tables with multiple covariates from panel data. The case of active life expectancy. Demography. 1994;31:297–319. doi:10.2307/2061887. [PubMed] [Google Scholar]
- Lloyd-Jones DM, Leip EP, Larson MG, D’Agostino RB, Beiser A, Wilson PW, et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;113:791–798. doi: 10.1161/CIRCULATIONAHA.105.548206. doi:10.1161/CIRCULATIONAHA.105.548206. [DOI] [PubMed] [Google Scholar]
- Markides KS. Risk factors, gender and health. In: Glasse L, Hendricks J, editors. Gender and aging. Amityville, NY: Baywood; 1992. pp. 25–32. [Google Scholar]
- Maynard C, Every NR, Martin JS, Kudenchuk PJ, Weaver WD. Association of gender and survival in patients with acute myocardial infarction. Archives of Internal Medicine. 1997;157:1379–1384. doi:10.1001/archinte.157.12.1379. [PubMed] [Google Scholar]
- Peeters A, Mamun AA, Willekens F, Bonneux L. A cardiovascular life history: A life course analysis of the original Framingham Heart Study cohort. European Heart Journal. 2002;23:458–466. doi: 10.1053/euhj.2001.2838. doi:10.1053/euhj.2001.2838. [DOI] [PubMed] [Google Scholar]
- Psaty B, Kuller L, Bild D, Burke G, Kittner S, Mittelmark M, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Annals of Epidemiology. 1995;5:270–277. doi: 10.1016/1047-2797(94)00092-8. doi:10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- Robine J-M, Jagger C, Cambois E. European perspectives on healthy aging in women. Journal of Women and Aging. 2002;14:119–133. doi: 10.1300/J074v14n01_08. doi:10.1300/J074v14n01_08. [DOI] [PubMed] [Google Scholar]
- Robine J-M, Jagger C, Mathers C, Crimmins E, Suzman R, editors. Determining health expectancies. London: John Wiley & Sons, Limited; 2003. [Google Scholar]
- Vaccarino V, Krumholz HM, Yarzebski J, Gore JM, Goldberg RJ. Sex differences in 2-year mortality after hospital discharge for myocardial infarction. Annals of Internal Medicine. 2001;134:173–181. doi: 10.7326/0003-4819-134-3-200102060-00007. [DOI] [PubMed] [Google Scholar]
- Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex-based differences in early mortality after myocardial infarction. National Registry of Myocardial Infarction 2 participants. New England Journal of Medicine. 1999;341:217–225. doi: 10.1056/NEJM199907223410401. [DOI] [PubMed] [Google Scholar]
- Voaklander D, Thommasen H, Michalos A. The Relationship between Health Survey and Medical Chart Review Results in a Rural Population. Social Indicators Research. 2006;77(2):287–305. doi:10.1007/s11205-005-2551-1. [Google Scholar]