Abstract
OKT3 monoclonal antibody therapy was added to preexisting baseline immunosuppressive treatment with ciclosporin and steroids to treat rejection in 52 recipients of cadaveric livers and 10 recipients of cadaveric kidneys. Rejection was controlled in 75% of patients treated, often after high-dose steroid therapy had failed. Rejection recurred during the 17-month follow-up period, after completion of OKT3, in only 25% of the patients who had responded. The safety and effectiveness of this monoclonal therapy, added to ciclosporin and steroids, has been established in this study.
Keywords: Transplantation, Monoclonal antibody, Allograft rejection
Introduction
Numerous clinical studies in renal, hepatic and cardiac transplantation have shown the value of antilymphocyte globulin (ALG) given prophylactically with azathioprine and steroids [1-4] or for the specific purpose of reversing an established rejection [5-7]. However, several inherent properties of conventional ALG prevented its full exploitation [8, 9]. ALG could not be fully standardized from batch to batch; it frequently contained irrelevant antibodies directed against formed blood elements other than lymphocytes, and the amount of heterologous protein administered was excessive. Avoidance of these disadvantages became possible with the development of monoclonal antibody preparations with defined specificities using the hybridoma technology developed by Kohler and Milstein [10].
Much of what has been learned about lymphocyte functions in the past few years has led to the hope that subsets of the lymphocyte population could be specifically manipulated by monoclonal antibodies for therapeutic purposes. Antigen recognition and induction of activation of mature T lymphocytes appear to reside within the T3/antigen receptor complex on the T cell surface [11, 12]. Modulation of the T3/antigen receptor complex has been shown to occur following antigen stimulation [13] and with murine monoclonal antibodies, such as Orthoclone OKT 3, directed against one component of the T3 antigen [14]. In vitro studies have demonstrated an effect of OKT3 on both the cytotoxic and the proliferative capacities of T cells [15, 16]. In vivo, OKT3 has been shown to have a profound effect on both the primate [17] and the human [18] immune response.
In 1981, Cosimi et al. [18, 19] reported the first clinical trials with OKT3 for the treatment of acute renal allograft rejection. In those studies, in other similar pilot investigations [20] and in a multicenter clinical trial [21] OKT3 was reported to reverse acute rejection of kidney transplants significantly better than treatment with high-dose steroids. The major drawback was a high incidence of recurrent rejections when the patients were returned to maintenance therapy with azathioprine and prednisone.
We report here results of our pilot trials of OKT3 therapy in 62 recipients of cadaveric kidneys or livers whose baseline immunosuppression was with ciclosporin and steroids. A ten- to 14-day course of OKT3 was generally started because rejection had developed in spite of ciclosporin-steroid therapy, but in several liver recipients, OKT3 was begun because optimal therapy with ciclosporin could not be given in the face of poor postoperative renal function.
Materials and Methods
Case Material
The patients were treated between August 1984 and May 1985 with follow-up through January 1986.
Cadaveric Renal Transplantation
The average age of the ten renal recipients was 36.0 ± (SD) 16.0 years (range, 24–55 years). Four of the ten had diabetes mellitus with diabetic nephropathy as the cause of renal failure. Nine were primary graft recipients, and the 10th had undergone retransplantation after the primary graft had been rejected 36 months earlier. Except for avoidance of AB0 blood group incompatibilities and positive lymphocytotoxic antibody crossmatches, donor-recipient matching was random. At the HLA A, B, and DR loci, an average of 1.1 antigens out of a maximum of 6 were matched.
Orthotopic Liver Transplantation
Twenty-two of the 52 liver recipients were in the pediatric age range of 6 months to 17 years and averaged 6.9±(SD) 4.5 years. The age of the 30 adults averaged 39.1 ± 8.6 years (range, 19–54 years). Forty-three (83%) of the 52 patients were bearing their first grafts, and the other 9 had undergone retransplantation.
All of the grafts used for hepatic recipients were selected without knowledge of the HLA types. At the HLA A, B, and DR loci, the antigens matched averaged 1.2 out of a possible 6. Positive cytotoxic crossmatches were present in 3 (9%) of the 34 cases in whom the test was performed. In 6 patients, there was a breach of the conventional guidelines of AB0 blood group compatibility, which were originally defined in kidney transplantation [22]. The resistance of the liver to hyperacute rejection from preformed antibody states has been noted before [23, 24].
In 3 patients whose first liver grafts failed in spite of OKT3 therapy, the murine immunoglobulin was used again after retransplantation. The results of treatment in these 3 patients, who were given a second course of OKT3 1, 2, and 3 months after a first course, were analyzed separately from the results obtained with the 52 first courses.
Primary Immunosuppression
Kidney Transplantation
For kidney recipients, an oral dose of 17.5 mg ciclosporin/kg body weight was given 5–6 h preoperatively. Postoperatively, the same daily amount was provided in divided doses every 12 h. Four patients who did not have time for oral preoperative medication, or who could not eat postoperatively, were given intravenous ciclosporin for a few days in doses of 4 to 6 mg/kg/day. Postoperatively, ciclosporin therapy was monitored by frequent determinations, with radioimmunoassay of the whole-blood drug level in samples obtained at trough, just before administration of the next dose [25]. The whole-blood concentrations were kept in the range of 750–1,000 ng/ml if renal function remained or became satisfactory. If good function was not obtained, potential nephrotoxicity was suspected and the dose of ciclosporin was adjusted downward after 10 days, so that the whole-blood trough concentration would fall to the 400–600 ng/ml range or slightly lower (fig. 1).
Fig. 1.
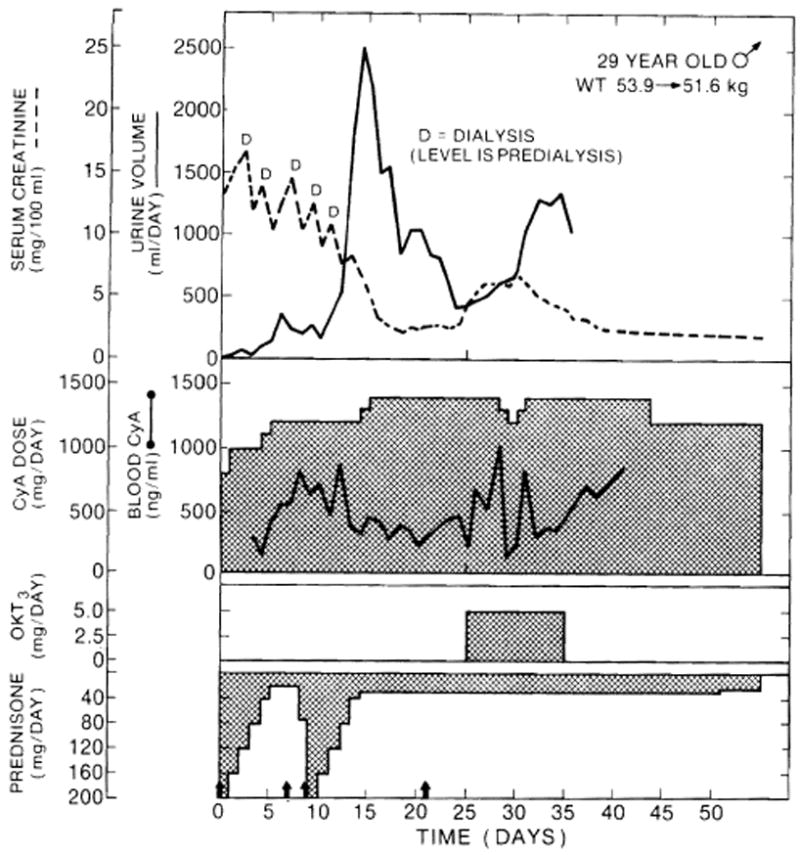
Clinical course of a cadaveric kidney recipient whose graft underwent an initial period of acute tubular necrosis, with a beginning subsequent diuresis that was interrupted by rejection. Arrows: 1 g methylprednisolone. CyA = Ciclosporin.
Steroids were also given systemically [26]. With revascularization of the kidney allografts, 1 g methylprednisolone was administered intravenously in the operating room, and subsequently, a 5-day burst of methylprednisolone was started intravenously or orally, beginning with 200 mg on the 1st postoperative day. The prednisone doses were reduced by 40 mg/day until the 6th postoperative day, when a maintenance level of 20 mg was reached. If rejection was thought to have developed in spite of this therapy, the patient was submitted to a second 5-day steroid course (fig. 1). Those who were refractory to this therapy or who responded only temporarily became candidates for OKT3 therapy. Their rejections were therefore considered ‘steroid-resistant’.
Liver Transplantation
The principles of the ciclosporin-steroid therapy for liver transplant cases were the same as for renal recipients, but the details were vastly more complicated. Most liver recipients required intravenous ciclosporin and intravenous steroids for several days postoperatively. With resumption of dietary intake, the absorption of oral ciclosporin was generally unreliable and unpredictable, necessitating the overlapping of intravenous therapy (fig. 2), sometimes for periods of weeks before adequate blood levels could be sustained with the oral route alone [27, 28]. The achievement of therapeutic blood levels of ciclosporin was made difficult in many patients by the concurrent incipient renal failure, which is a common precondition in hepatic transplant recipients [29, 30] and which can be aggravated by ciclosporin. As a consequence, the steroid component of therapy was individualized more than with the renal recipients in an effort to compensate for irregular ciclosporin schedules.
Fig. 2.
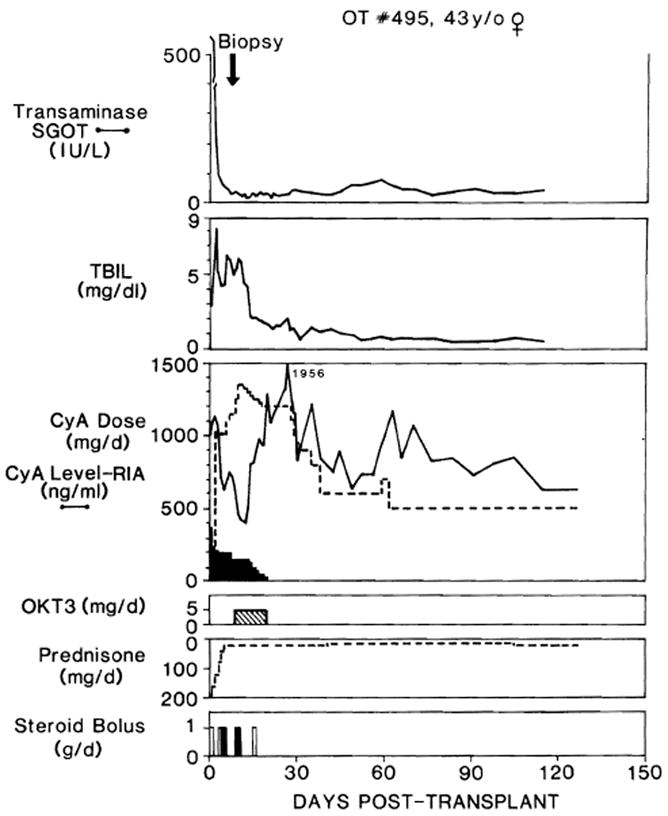
Clinical course of liver recipient OT 495, who developed biopsy-proved rejection after 10 days while undergoing ciclosporin(CyA)-steroid therapy. Note that ciclosporin was given at first by both intravenous (dark shading) and oral (no shading) routes. TBIL = Total bilirubin; SGOT = serum glutamic-oxaloacetic transaminase; steroid bolus: solid bars = methylprednisolone, intravenously; open bars = hydrocortisone intravenously.
Indications and Timing of OKT3 Therapy
Renal Transplantation
Rejection was the reason for OKT3 treatment in all 10 patients. Therapy was begun a median interval of 25 days after transplantation (range 10–167 days). Rejection was diagnosed from the usual multiple lines of evidence, not all of which were present in every case. These included the physical signs of wound tenderness and graft swelling; secondary deterioration of grafts that had functioned well immediately postoperatively; failure of five grafts with poor initial function to recover from their presumed acute tubular necrosis, and secondary deterioration of allografts that were recovering from acute tubular necrosis (fig. 1). Serial radionuclide scans showed the progressive reductions in renal blood flow and function that have been associated with rejection [31, 32]. Needle biopsies of the grafts were obtained in 3 patients prior to starting OKT3 therapy, 10, 48, and 167 days postoperatively. Histologically, these biopsies contained the mononuclear cell infiltrations and edema of acute rejection, as well as evidence of chronic rejection in a graft that had been in place for 167 days. Follow-up biopsies after OKT3 therapy were not obtained.
Liver Transplantation
The 52 hepatic allograft recipients were treated with OKT3 from 2 to 460 days after transplantation. Of the 41 patients in whom liver biopsies were obtained, 36 were done just before or shortly after starting monoclonal therapy. In the other 5 patients, liver biopsies were done 2–4 weeks prior to initiation of OKT3 therapy. In analyzing the results, the liver-transplant recipients were stratified into three groups:
– Group 1: OKT3 treatment started less than 10 days postoperatively;
– Group 2: OKT3 treatment started 10 days to 3 months postoperatively;
– Group 3: OKT3 treatment started more than 3 months postoperatively.
Group I
Therapy in this group was started a median of 6 days following transplantation. Eight of the eighteen patients treated during the first 9 days after transplantation had poor renal function (table I), which precluded aggressive treatment with ciclosporin (fig. 3). When present, renal failure was always associated with hepatic dysfunction. Contributing factors leading to liver dysfunction were thought to be ischemic injury of the graft and/or rejection, but an absolute differential diagnosis between these potentially coexisting factors was difficult to make. In several patients, liver biopsies were either deferred until shortly after initiation of OKT3 therapy or not done because of their unstable clinical condition or because their coagulation studies were severely deranged. The associations of hepatic and renal complications, as these could be classified from clinical events, hepatic biopsies, and autopsy tissues, are summarized in table II.
Table I.
Indications for OKT3 therapy in hepatic allograft recipients 1
| Number of patients | Renal failure | Poor initial graft function | Rejection | |
|---|---|---|---|---|
| Group 1 | 18 | 8 | 9 | 152 |
| Group 2 | 22 | 0 | 3 | 21 |
| Group 3 | 12 | 0 | 0 | 12 |
| Total | 52 | 8 | 12 | 48 |
As documented by clinical and laboratory criteria (see text).
See table II for factors associated with rejection in group 1.
Fig. 3.
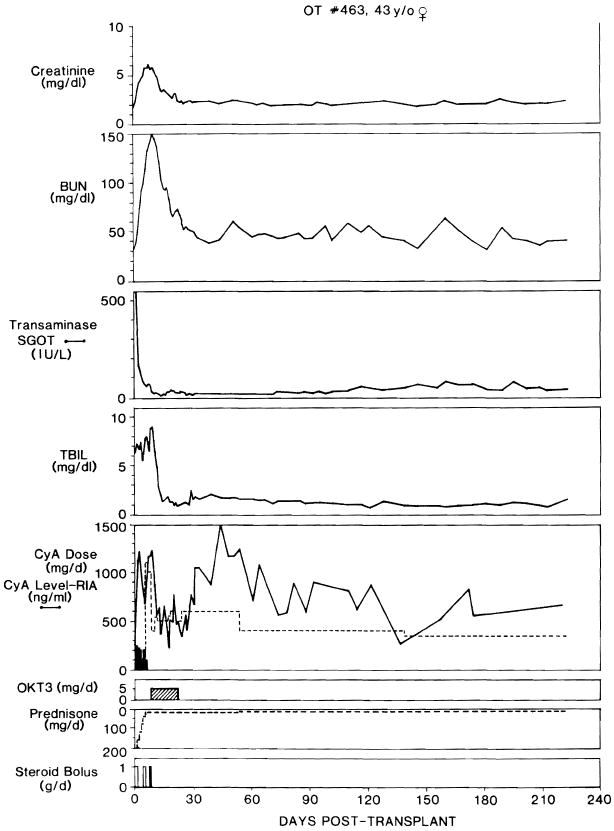
Clinical course of liver recipient OT 463, whose OKT3 course was started after 9 days in part because her renal failure precluded giving therapeutic doses of ciclosporin to treat her presumed rejection. Note resolution of azotemia during OKT3 therapy. Abbreviations and symbols, same as in figure 2.
Table II.
Complex nature of coexisting indications for OKT3 therapy in group 1 patients
| Indication | Number of patients |
|---|---|
| Renal failure alone | 0 |
| Renal failure plus primary graft dysfunction | 2 |
| Renal failure plus rejection | 3 |
| Primary graft dysfunction alone | 1 |
| Primary graft dysfunction plus rejection | 3 |
| Rejection alone | 6 |
| Renal failure, primary graft dysfunction plus rejection | 3 |
| Total | 18 |
Six of the ten patients in group 1 who had hepatic dysfunction without renal failure had clinical and histologic evidence of liver rejection. Three others had a combination of ischemic injury and rejection, which explained persistent liver function abnormalities. Only 1 patient had primary hepatic graft dysfunction alone.
Group 2
The decision to use OKT3 therapy between 10 days and 3 months was based invariably upon a secondary deterioration of hepatic function tests after fair to good function had been achieved during the first 9 days. Among the 22 patients in this group, OKT3 was started a median of 21 days posttransplantation. Before starting OKT3, hepatitis secondary to B virus infection was ruled out with a hepatitis serologic screen. Ultrasound and/or angiographic studies were used to prove hepatic arterial and portal vein patency. Biliary obstruction was ruled out with ultrasonography, computerized axial tomography, radionuclide imaging techniques, or cholangiography. Liver biopsies, verifying acute cellular rejection (fig. 4), were obtained in 21 of the 22 patients in this group.
Fig. 4.

Histologic findings of cellular rejection typical in group 2 liver patients consist of portal mononuclear cell infiltration with damage to bile duct epithelium and endothelium of vessels (arrow). The patient, OT 407, had a repeat biopsy after therapy (fig. 8). a × 125. b × 315.
Group 3
Twelve recipients have given a course of OKT3 from 3 to 16 months after transplantation (median, 230 days posttransplantation) because of a delayed deterioration of liver function (fig. 5). The workup, including a percutaneous liver biopsy in all group 3 patients, was the same as in group 2. If histopathologic findings of acute rejection were found, a steroid bolus was given and eventually a course of OKT3 was started, even if there were morphologic stigmata of chronic rejection, including loss of lobular bile ductules, arteriolar thickening, and portal fibrosis (fig. 6).
Fig. 5.
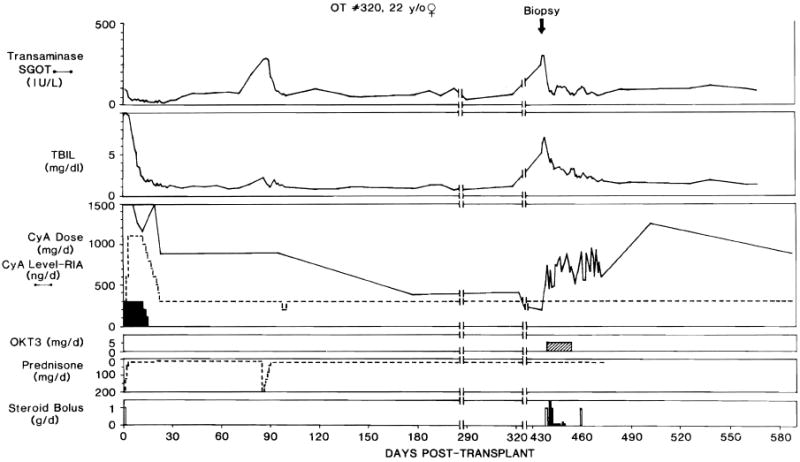
Clinical course ofliver recipient OT 320 treated with OKT3 for acute rejection 14 months after transplantation. Abbreviations and symbols, same as in figure 2.
Fig. 6.
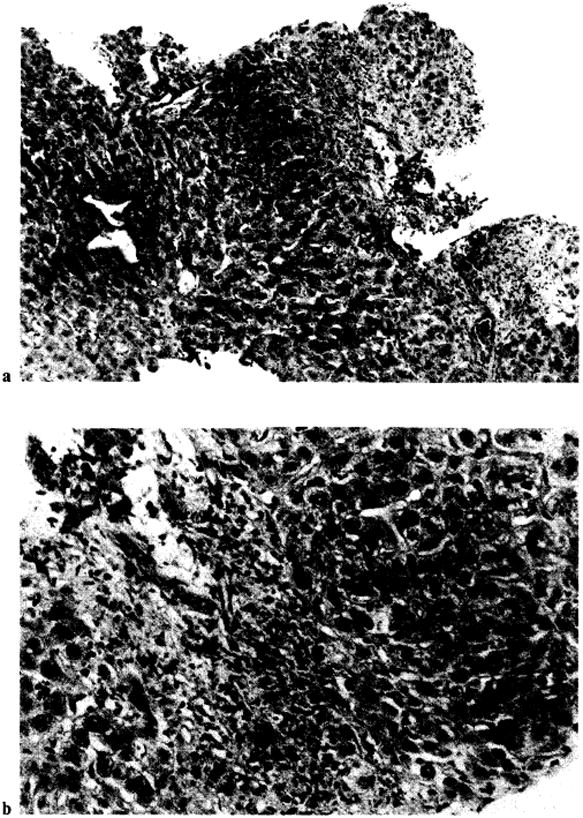
Patient OT 377. Histologic findings of chronic rejection. Note paucity of bile ducts and fibrosis in portal tracts. a × 125. b × 315.
OKT3 Administration and Precautions
The intravenous dose of OKT3 was 5 mg in adults and 2.5 mg in children, delivered by syringe push over 2–5 min. Premedication with 50 mg i.v. diphenhydramine and 500–1,000 mg i.v. hydrocortisone was carried out approximately 1 h prior to administration of the first two doses. The patients were watched continuously by a physician for the first 2 h. Wheezing, dyspnea, cardiovascular instability, and fever were considered potential precursors of more serious reactions.
Because severe pulmonary edema has been reported in patients with preexisting fluid overload [21], this factor was excluded or treated prior to treatment with OKT3. Furthermore, monoclonal antibody therapy was never undertaken without the capability for emergency endotracheal intubation and ventilatory support. The steps for the treatment of anaphylactoid reactions, which included bronchospastic reactions, severe cardiovascular instability, and pulmonary edema, were throughly impressed upon all physicians in attendance. The sequence began with 1 g hydrocortisone intravenously, progressed to an epinephrine infusion if necessary, and concluded with intubation and ventilatory support. This chain of events never evolved in any of the kidney patients, but 1 of the 52 liver recipients required intubation and an epinephrine drip for 24 h.
Adjustments in prednisone therapy from the maintenance level were not usually made during the 10- to 14-day period of OKT3 therapy. In contrast, efforts were made to adjust the ciclosporin doses to those that would be kept permanently, using daily measurements of ciclosporin blood levels for guidance. The objective was to have all factors of the maintenance immunosuppression stable at the end of therapy (fig. 3). Since ciclosporin was often suspected as the cause of renal dysfunction, downward adjustments of ciclosporin dose were common at the beginning of therapy, with restoration to higher levels toward the end when renal function parameters stabilized.
Immunologic Monitoring of Peripheral Blood
The following immunologic parameters were also studied in the majority of adult recipients: serum OKT3 levels, presence of antimurine antibodies, and T cell subpopulations. OKT3 and antimurine antibody titers were determined by enzyme-linked immunosorbent assay (ELISA) using kits furnished by Ortho Pharmaceutical Corporation, Immunobiology Division, according to previously reported methods [33].
T-cell subpopulation determinations were done by flow cytometry on a FACS IV [Becton Dickinson, Mountainview, Calif.], using slight modifications of methods described previously [34]. The following fluorescein-labeled anti-T-cell surface marker monoclonal antibodies were used: T3 (OKT3, Leu 4), T4 (OKT4, Leu 3 a+b), and T8 (OKT8, Leu 2a). The OKT series was purchased from Ortho Diagnostics; the Leu series was purchased from Becton Dickinson. At least 5 × 104 cells were analyzed for each monoclonal antibody determination.
Histopathologic Studies in Liver Recipients
Biopsy specimens were fixed in neutral buffered formalin and routinely stained with hematoxylin and eosin, trichrome, and reticulin stains. The biopsy slides were reviewed by three pathologists (AJD, RJ, KAP) in a blind fashion, independent of clinical correlation. A consensus pathologic diagnosis was given for each specimen. There were minor differences of opinion among the pathologists in only 2 cases.
The following histologic criteria were used for the pathologic diagnosis of acute hepatic rejection (fig. 4):
– A predominantly mononuclear portal tract infiltrate in which the inflammatory infiltrate consisted of 50–60% mononuclear cells intermixed with polymorphonuclear cells and eosinophils;
– Characteristic localization of the inflammatory cells around and beneath the swollen endothelium of portal capillaries and small veins, with infiltration and damage of the epithelium of small bile ductules (the number of ‘damaged’ bile ductules and the degree of damage generally increased with time. The most severe changes, with ductular loss, were seen in ‘chronic rejection’).
– Absence of the following histologic findings: significant panlobular inflammation; piecemeal necrosis; cholangiolar proliferation; disarray, with ballooning and spotty individual hepatocyte necrosis; and prominent lymphohistiocytic infiltration of the hepatic lobule, with inflammatory cell destruction of hepatocytes. (The foregoing are not prominent features of rejection under immunosuppression and suggest alternative diagnoses, such as viral hepatitis or large-duct obstruction.)
A diagnosis of chronic hepatic rejection (fig. 6) was made if there was evidence of
– The obliterative arteriolar lesions that have been found in liver as well as other solid organs [35, 36];
– Loss of intrahepatic bile ducts, often with only a mild periductal and intraductal chronic inflammatory infiltrate;
– Portal fibrosis, especially if linkage had occurred between portal tracts or central veins;
– The above must be accompanied by an absence of lobular changes as outlined earlier for acute rejection.
In 24 cases in which the pretreatment biopsies showed cell-mediated rejection, follow-up biopsies were obtained from 2 days before completion to 5 weeks after completion of OKT3 therapy. Nineteen of these 24 follow-up biopsies were done within 2 weeks after stopping the monoclonal therapy.
Definition of Therapeutic Response
Kidney Recipients
A full response to OKT3 therapy was defined as a return of serum creatinine to less than 2 mg/dl (SI units: 176.8 μmol/l) during or within 2 weeks after completion of therapy. In addition, reversal of graft tenderness and swelling, improvement in the radionuclide flow scans, and diuresis were generally seen. A less marked but definite improvement was termed a partial response.
Liver recipients
A complete response to therapy in the liver recipients during OKT3 therapy or within the first 2 weeks following its completion was defined as return of abnormalities in liver function to normal or near normal, with objective improvement in the histologic findings in follow-up biopsy studies, when these were obtained. Partial response was defined as improvement of biochemical parameters, with or without objective improvement in histology. Patients were assigned to the no-response category if there was no improvement or if there was worsening of biochemical and histologic parameters.
Results
Kidney Transplantation
Period of Follow-Up
The mean follow-up of the 10 renal-transplant recipients after treatment with OKT3 is 13.6 ± (SD)2.0 months (range, 10–17 months).
Morbidity
There were no life-threatening side effects, such as pulmonary edema, attributable to administration of OKT3 in renal patients. Fever was the most common side effect, and was seen in 80% of the patients during OKT3 therapy. No major infections occurred, but 40% experienced minor infections, of which the most common was cutaneous herpes simplex (table III). One patient had a cecal perforation during the course of OKT3 therapy, necessitating right ileocolectomy. However, the patient recovered and did not lose allograft function. The cecal perforation was not considered a complication of OKT3 therapy.
Table III.
Morbidity and mortality in 10 kidney-transplant recipients receiving OKT3 therapy
| Percentages | ||
|---|---|---|
| Morbidity | Fever | 80 |
| Hemodynamic instability | 60 | |
| GI symptoms | 70 | |
| Dyspnea | 20 | |
| Chills | 70 | |
| Anaphylactoid reaction | 0 | |
| Infections: major | 0 | |
| minor | 40 | |
| Mortality | Overall | 0 |
Mortality
All of the renal patients treated with OKT3 were alive at the end of the follow-up period.
Response to OKT3 Therapy
Resolution of the physical findings of rejection, an increase in urine output, and a progressive decline in serum blood urea nitrogen (BUN) and creatinine were seen in 8 of the 10 patients. Four (40%) had a full response and four (40%) had a partial response. Rebiopsy was not performed in any of these patients. In one failure, the transplanted kidney was found at graft nephrectomy to have undergone humoral mediated rejection, as documented by pathologic findings. The other failure was from continued, uncontrolled, cell-mediated rejection. Reversal of rejection, when this occurred, usually was evident within 2–3 days, but continued improvement was seen up to 2 weeks following completion of OKT3.
Recurrence of Rejection
Following completion of monoclonal therapy, the patients were maintained on a chronic ciclosporin-steroid regimen in the same manner as other renal recipients. Two patients slowly rejected their grafts 2.5 and 6 months after a partial response to OKT3. With a mean follow-up of 15.5 months, recurrent rejection has not been noted in the other 6 patients who had responded to OKT3 therapy. Thus, 60% of these patients with steroid-resistant rejection have achieved long-term graft function. Five of the six patients have a mean creatinine of 1.7 ± (SD) 0.5 mg/dl (SI units: 150.3 ± 44.2 μmol/l); the 6th has a stable creatinine of 2.4 mg/dl (SI units: 212.2 μmol/l), with a BUN of 111 mg/dl (SI units: 39.6 mmol/l).
Liver Transplantation
Period of follow-up
The mean follow-up is 12.2±(SD) 1.7 months after treatment with OKT3. The mean follow-up has been 12.1 ± 1.5, 11.4 ± 2.0 and 13.3 ± 1.4 months for groups 1, 2, and 3, respectively.
Morbidity
The majority of the side effects attributable to OKT3 were not life threatening (table IV). While 32% of the patients had subjective symptoms of shortness of breath, only 1 patient had severe bronchospasms requiring intubation and ventilatory support. One patient had polyarthralgias.
Table IV.
Morbidity and mortality in 52 liver transplant patients who were treated with OKT3
| Percentages | ||
|---|---|---|
| Morbidity | Fever | 75 |
| Hemodynamic instability | 62 | |
| GI symptoms | 55 | |
| Dyspnea | 32 | |
| Chills | 28 | |
| Rash | 20 | |
| Anaphylactoid reaction | 2 | |
| Infections: major | 15 | |
| minor | 56 | |
| Mortality | Overall1 | 23 |
| Group 1 | 33 (6/18) | |
| Group 2 | 18 (4/22) | |
| Group 3 | 17 (2/18) |
No deaths attributable to OKT3.
Major infections occurred in 15% of the patients during OKT3 therapy, including Pneumocystis carinii pneumonia in 1 patient and cytomegalovirus infections in two patients. Other major infections included bacterial systemic sepsis, first diagnosed by the presence of bacteremia, which in all but 1 case was of graft origin. Urinary tract infections, cutaneous herpes simplex, and trivial wound infections were seen in 56% of the recipients (table II). Almost one third of the patients had cutaneous herpes simplex at some point during or shortly after OKT3 therapy.
Mortality
The mortality in 52 liver transplant recipients was 23% during the mean follow-up of 12.2 months. In the pediatric recipients, there was 25% mortality compared with 22% in the adults. Patients in group 1 had an overall mortality of 33%, reflecting the gravity of their condition when OKT3 treatment was started. Patients in group 2 had a mortality of 18%, while those in group 3 had a 17% mortality.
The deaths bore no obvious relation to OKT3 therapy. All 6 of the patients in group 1 who died had primary hepatic graft failure from the time of transplantation. In retrospect, the poor graft function in 2 cases was caused by hepatic artery thrombosis, while poor initial function in the other 4 recipients in group 1 may have been partly due to ischemia during procurement. Two of these six patients were considered too ill to have biopsies prior to starting OKT3 therapy.
Two of the four patients who died in group 2 probably received damaged grafts. One of these 2 patients suffered a cerebral vascular accident, the other had a massive wound infection, and both developed serious infections of their liver grafts. A 3rd patient from group 2 died of metastases from his original hepatoma 7 months after therapy, without evidence of rejection. The 4th patient died shortly after retransplantation for uncontrollable recurrent rejection, 6 months after an initial reversal with OKT3.
One death in group 3 occurred at retransplantation for chronic rejection that had not been reversed with OKT3. The other death was from systemic herpes zoster, 9 months after OKT3 therapy.
Response to OKT3 Therapy
The response rate was 78%, with 53% of patients showing full reversal of rejection, while 25% had a partial response. The highest response rate was in group 2, with a 91% incidence of objective improvement. This group also had the best ‘full’ response rate, with 73% of patients having resolution of all biochemical parameters of rejection. Evidence for reversal of rejection often was not obvious for 3 or 4 days, and continued improvement thereafter was the general rule (fig. 7).
Fig. 7.
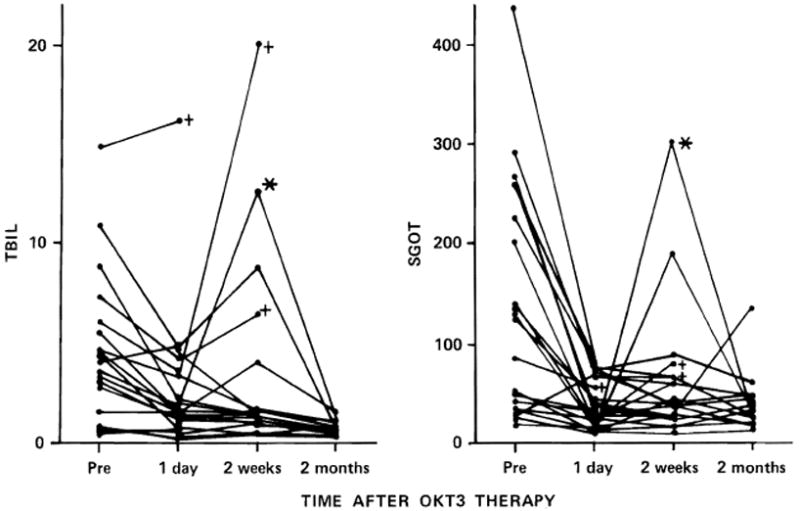
Response of serum bilirubin (TBIL) and serum glutamic oxaloacetic transaminase (SGOT) in the patients of group 2, whose rejection was diagnosed between 10 days and 3 months after liver replacement. The crosses indicate the loss of the graft, and the asterisk is on the curve of a patient who developed herpes virus hepatitis after OKT3 therapy.
The individual serum bilirubin and transaminase levels of group 2 patients before, during, and after therapy are shown in figure 7. Among the patients who responded to OKT3, there was a 52% decrease in total bilirubin from the onset to the termination of OKT3 therapy, with a further 27% reduction, to 21% of pretreatment values, by the end of 2 months. Meanwhile, there were corresponding 72% and 79% decreases in the serum glutamic oxaloacetic transaminase (fig. 7).
In contrast, the recipients of group 1 treated in the first 9 postoperative days had only a 72% response rate, of which the response was more often partial than complete (table V). The failure rate was 28%.
Table V.
Response of liver recipients to OKT3 for reversal of acute rejection
| No response % | Partial response % | Full response % | |
|---|---|---|---|
| Overall | 22 | 25 | 53 |
| Group 1 | 28 (5/18) | 39 (7/18) | 33(6/18) |
| Group 2 | 9 (2/22) | 18 (4/22) | 73 (16/22) |
| Group 3 | 33 (4/12) | 25 (3/12) | 42 (5/12) |
The total and partial response rates and the failure rate in the patients of group 3, who were treated after 3 months posttransplant, were 42, 25, and 33% respectively (table V).
Histopathologic Studies
Forty-one of the 52 patients had a needle biopsy of the liver prior to or shortly after initiation of OKT3 therapy. Cell-mediated rejection was documented in 95% (31/33) of group 2 and group 3 patients (table VI). In addition, biopsies from group 3 patients also had findings of chronic rejection in 75% of the specimens. In contrast, the 8 biopsies that were obtained in group 1 patients had three examples of ischemic injury, with only slight – and in one case no – evidence of rejection.
Table VI.
Predominant histologic findings in liver biopsies taken before or within 48 h of beginning OKT3 therapy
| Group | n | Cell-mediated rejection | Chronic rejection | Ischemic injury | Ischemia plus rejection | Hepatitis | Other |
|---|---|---|---|---|---|---|---|
| 1 | 8 | 5 | 0 | 3 | 0 | 0 | 0 |
| 2 | 22 | 19 | 1 | 0 | 1 | 0 | 11 |
| 3 | 12 | 11 | 9 | 0 | 0 | 1 | 0 |
This patient had evidence of bile duct obstruction, a diagnosis that was later confirmed. Secondary bile duct reconstruction was carried out successfully.
Follow-up biopsies at the end of the monoclonal therapy were obtained in 24 of the liver recipients with pretreatment evidence of cell-mediated rejection (table VII). 79% had improvement in the histologic picture following OKT3 therapy; 8% had no change, and in 8% of the biopsies, there was an increase in infiltrates or evidence of continued immune-mediated destruction. In addition, one patient developed evidence of herpes virus hepatitis following therapy.
Table VII.
Histologic findings in 24 post-OKT3 liver biopsies in which pretreatment biopsies showed cellular rejection
| Group | n | Histologic findings |
|||
|---|---|---|---|---|---|
| cellular rejection |
hepatitis | ||||
| improved | no change | worse | |||
| 1 | 5 | 4 | 0 | 1 | 0 |
| 2 | 11 | 9 | 0 | 1 | 1 |
| 3 | 81 | 6 | 2 | 0 | 02 |
| Total | 24 | 19 | 2 | 2 | 1 |
Evidence of chronic changes in pretreatment samples (6/8) did not change in posttreatment samples.
The pre-OKT3 biopsy findings with cellular rejection: the findings resolved post-treatment, but changes consistent with herpes hepatitis appeared.
In patients who were rebiopsied, histopathologic evidence of amelioration of acute rejection (fig. 4, 8) was commonly found, regardless of whether OKT3 therapy was started early, at an intermediate period, or late following transplantation (table VII). In one patient in group 2, there was improvement of rejection, but a histologic picture developed that was consistent with viral hepatitis (OT 428). This patient subsequently developed abnormal liver function, which responded to intravenous acyclovir. The findings due to chronic rejection in pretreatment specimens did not change following OKT3 therapy (fig. 6, 9). These persistent stigmata included loss of bile ductules; arteriolar thickening; and portal, central vein, and sinusoidal fibrosis.
Fig. 8.
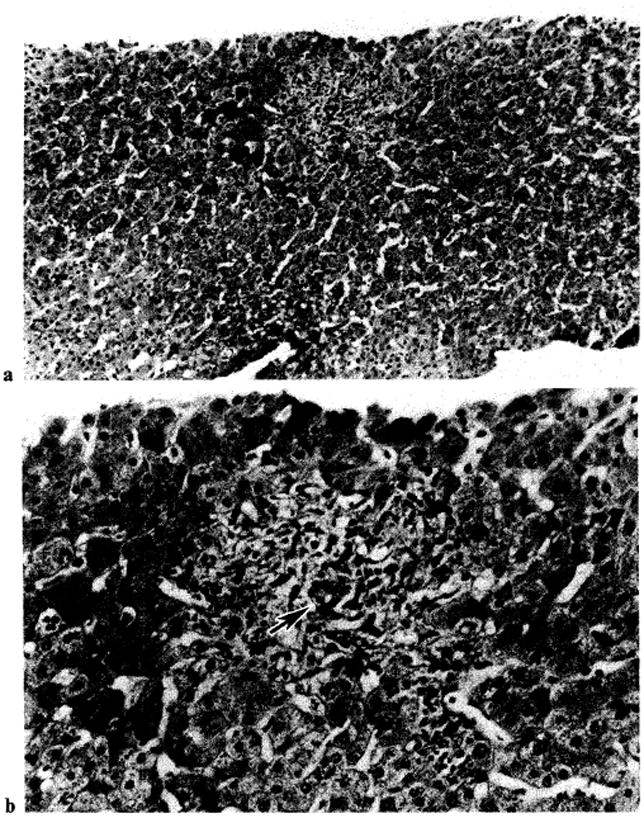
Improvement of histologic findings of liver patient OT 407, whose biopsy before OKT3 therapy is shown in figure 4. Note marked decrease of inflammatory cells after OKT3 therapy and intact bile ductule (arrow). a × 125. b × 315.
Fig. 9.
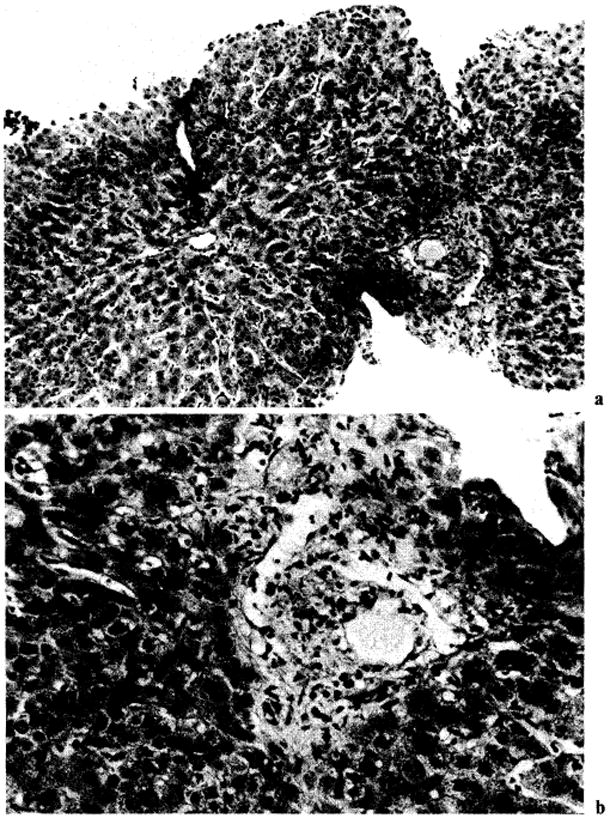
Improvement of histologic findings of liver patient OT 377 after OKT3 therapy. The pretreatment biopsy is shown in figure 6. Note reduction of mononuclear cells. However, no bile ductules were seen in this biopsy, and there was an increase in centrilobular canalicular cholestasis. a × 125. b × 315.
In patients who underwent rebiopsy, the histopathologic grading was prognostic of the clinical response to OKT3. Clinical improvement, despite a lack of histologic improvement, occurred in only 43% of cases. In contrast, there was a 94% correlation between improvement of the histologic picture and a favorable clinical response. By the χ2 test, a statistically significant correlation between the change in the histologic pattern and the clinical outcome was found (p < 0.01).
Recurrence of Rejection
During the average follow-up of more than 12 months, only 10 (25%) of the 40 patients who responded to OKT3 experienced further rejection episodes. The recurrent rejection episodes occurred a mean of 6.75 ± (SD) 3.25 months following completion of OKT3 (range, 0.5–12 months). Five of the ten recurrent rejections were successfully treated with high-dose steroids. The other 5 patients subsequently lost their grafts and required retransplantation.
After an average follow-up of 12.2 months, 35 (67%) of the 52 patients who received OKT3 therapy now have satisfactory hepatic function of their originally treated allograft. Group 1 patients have a 61% graft function rate, as compared with 77% in group 2 and only 33% in group 3.
Serologic Studies during OKT3 Therapy
An excess of OKT3 antibody was detected in all 20 patients who were tested during therapy. The validity of this observation was confirmed by addition of test serum samples to normal lymphocytes, with subsequent binding of free OKT3 to these cells, as detected by complement-mediated lymphocytotoxicity.
Development of antimurine antibodies was also monitored during and after 33 courses of OKT3. Prior to administration of OKT3, no antimurine antibody could be detected in any patient. Afterwards 20 (61%) of the 33 patients developed detectable antimurine antibodies, usually by day 12 after start of treatment.
T-Cell Subpopulations during OKT3 Therapy
Depletion of T3-bearing T cells occurred during the first few days of OKT3 administration (fig. 10a). A corresponding decrease in the number of total peripheral circulating lymphocytes could be demonstrated, to an average of 76% of the pretreatment lymphocyte count on day 1 of therapy. Initially, there was concurrent depletion of T4- and T8-bearing cells (fig. 10b), but antigenic modulation of the T3 receptor occurred, allowing expression of significant percentages of T4-positive and T8-positive (but T3-negative) lymphocytes by 8 days after onset of OKT3 therapy. Following cessation of monoclonal antibody administration, a prompt reappearance of the T3 marker occurred (fig. 10a).
Fig. 10.
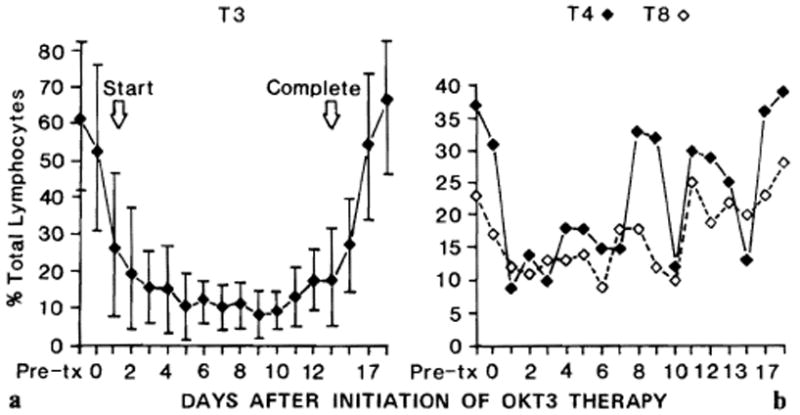
Influence of OKT3 therapy on T-cell subpopulations in liver recipients during rejection. a Percent of total circulating lymphocytes that bear the T3 antigen as determined by direct immunofluorescence (with standard deviations). b Percent of total circulating lymphocytes bearing T4 and T8 by direct immunofluorescence in the same patients as in a. Average number of observations per data point was 10.
The large standard deviation of the percent of T3-bearing T cells seen in OKT3-treated liver patients was largely accountable by T-cell profiles of several patients, who had marked abnormalities in T-cell clearance, which resulted in a large percentage of residual circulating lymphocytes continuing to bear the T3 marker. An example of one such patient is shown in figure 11. A statistical correlation between the ability or inability to ‘clear’ residual T3-bearing cells and clinical outcome could not be established, possibly because of the small sample size. Direct enumeration of lymphocytes, as determined by incubating peripheral lymphocytes with fluorescein-conjugated antimouse IgG antibodies, confirmed the presence of a large number of OKT3-coated lymphocytes in the circulation of liver recipients throughout OKT3 therapy.
Fig. 11.
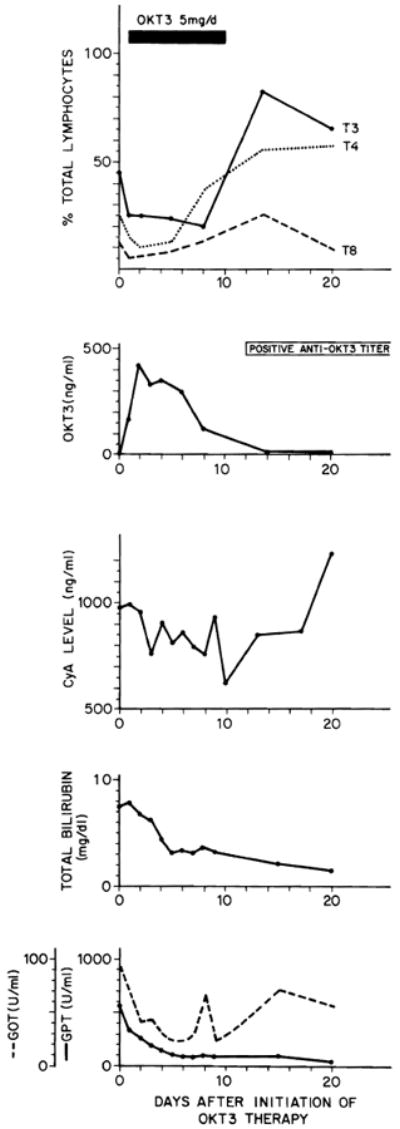
Events in patient OT 469, whose biopsy-proved rejection was reversed with OKT3 therapy. Treatment was started on the 6th postoperative day. Note the persistence of T3-positive lymphocytes in the circulation and T3 modulation during OKT3 therapy as well as the lymphocyte rebound after discontinuance of therapy. CyA = Ciclosporin. GOT = glutamic oxaloacetic transaminase; GPT = glutamic pyruvate transaminase.
Retreatment with OKT3
Three liver recipients were given a second full course of OKT3, 1–3 months after a first course. The second course was used to treat rejection of second grafts, which had been placed after the first grafts had failed in spite of OKT3 therapy. Complete and long-term remission from rejection was accomplished in 2 of the 3 patients without further episodes of recurrent rejection 3 and 6 months after completion of their second course of OKT3. In the unsuccessful case, the patient developed a high titer of antimurine antibodies during the second OKT3 course. This patient continued to reject the second liver but was successfully retransplanted with a third allograft 2 months later.
The acute reactions to the mouse immunoglobulins were no more severe than in the average patient undergoing primary OKT3 therapy, but in the patient with antimurine antibodies, fever with simultaneous demonstration of rising titers of antimurine antibodies, and serologic evidence of immune complexes supported the diagnosis of a delayed serum-sickness reaction. Renal function did not deteriorate, and this patient recovered promptly following discontinuance of OKT3.
Discussion
Heterologous antilymphoid serum (ALS) or its globulin derivative, ALG, has been used clinically since 1966 for the prevention or reversal of graft rejection [1]. The ability of ALG to ‘erase’ immunologic memory [37-39] was a particularly striking quality, which prompted Smith et al. [40] and Lance and Medawar [41] to show how solid organs or skin grafts could be rescued from established rejection in animal models.
With monoclonal antibody preparations, the potent therapeutic qualities of ALG can be retained while the dangers of irrelevant foreign protein administration are reduced. Kung et al. [42] were able to produce mouse monoclonal antibodies directed against varying T-cell surface antigens [42]. They found that OKT3 reacted with more than 95% of peripheral, postthymic T cells without affecting immature thymocytes. Thus, the selective depletion of peripheral T lymphocytes in our study and in other studies [19, 20] was not surprising.
Despite the fact that the amount of heterologous immunoglobulin administered as monoclonal antibody is 1/50 to 1/600 of that used with conventional polyclonal ALG, the side effects are not different from those reported with the crude preparations more than 15 years ago [8, 9]. Fever, rashes, and gastrointestinal disturbances are probably not life-threatening, but reactions that were referred to as ‘anaphylactoid’ in the old literature [8, 9] have been a factor in at least 2 reported deaths during monoclonal treatment [21]. Typically, such reactions can occur up to several hours after ALG or OKT3 injection. Cardiovascular instability and symptoms or findings suggestive of an acute asthmatic attack are ominous warnings that emergency therapy with ventilator support, massive doses of steroids, and, more specifically, intravenous epinephrine, may be required. It has been speculated that release of lymphokines from the disrupted lymphocytes generates secondary amplification effects in widely distributed and biologically important target cells.
The swift changes in lymphocyte traffic and dynamics help explain not only the toxicity but also the effectiveness of OKT3 in the rapid reversal of renal allograft rejection, as described by Cosimi et al [19] and others [20, 21]. In these earlier reports, in which baseline immunosuppression was with azathioprine and prednisone, a disquieting feature was a high rate of recurrent rejection, ranging from 50 to 80% shortly after completion of OKT3 therapy.
A factor that may have contributed to these recurrences was the systematic downward adjustment of the doses of azathioprine and prednisone during monoclonal therapy that was carried out primarily because of a fear of uncontrolled infection [19]. Instead of this practice, we have used the interval of OKT3 therapy as a breathing spell during which baseline immunosuppression therapy has been adjusted to an acceptable level that would be used chronically.
Even more fundamentally, it may be suggested that the full value of OKT3 therapy could not be exploited using azathioprine and prednisone. With the more potent combination of ciclosporin and steroids, which was used clinically with OKT3 for the first time in this study, the prospect of reversing rejection with long subsequent periods of an enhanced survival under continuing ciclosporin and steroids seemed brighter, providing there were not pharmacologic incompatibilities between the individual agents. The mechanism of ciclosporin is to block interleukin-2 synthesis by activated T cells with a consequent reduction of T-cell proliferation [43]. Suthanthiran et al. [44] have reported that methylprednisolone can enhance the immunosuppressive capability of cyclosporine in vitro. While the in vivo effects of OKT3 are primarily immunosuppressive in nature, OKT3 has the converse effect of being mitogenic to an appropriate subpopulation of T cells in vitro [45] and, in addition, it can induce interleukin-2 secretion [46, 47]. Thus, it is conceivable that OKT3 could cancel the effects of being ciclosporin-steroid therapy. Fortunately, such fears of pharmacologic chaos have proved groundless, at least as judged by clinical observations of our patients, whose immunosuppression was demonstrably intensified by OKT3.
The use of OKT3 with ciclosporin-steroids was not accompanied by an alarming increase of serious infections as was feared when OKT3 was first added to azathioprine-steroid therapy [19, 48]. Although there was an increase in the incidence of viral infections during or shortly after completion of OKT3 therapy, these were limited for the most part to annoying localized herpes. No deaths have occurred among our renal transplant recipients, and the 23% mortality among the liver recipients from infections or other complications have not been, in any instance, directly attributable to specific toxicity from OKT3.
In view of the relative safety of ciclosporin-steroid-OKT3 therapy, the ability to control and reverse difficult rejections has been encouraging. In the renal allograft recipients who had been highly selected for their resistance to steroid therapy, the salvage of organs was striking. Eight of ten renal grafts that were apparently lost to rejection recovered function – four completely and four partially. Furthermore, the benefit seemed long-lasting; the four patients with complete resolution have not had recurrent rejection during the follow-up period ranging from 10 to 12 months, although two of the four grafts with only a partial response slowly failed over the next few months.
Analysis of the efficacy of OKT3 in reversal of acute rejection was more complex in the liver recipients. However, the conclusions were no less definite. In the 22 patients in group 2, who developed rejection 10 days to 3 months after transplantation, the ability of OKT3 to reverse rejection was marked. The rejections were controlled in 91% of the 22 recipients. While 18% of these patients only had a partial response, 73% had complete resolution during or shortly after completion of OKT3 therapy. Confidence in the clinical assessment was heightened by the good correlation between the clinicians’ evaluation and the histopathologic studies of needle biopsies. Almost all of the biopsies, which were taken before OKT3 therapy, showed rejection, and improvement in rejection criteria was the most common change seen in subsequent biopsies. The best clinical responses were in patients whose livers showed unequivocal histologic changes of acute rejection as opposed to those whose liver showed significant chronic rejection or ischemic injury.
The effectiveness of OKT3 therapy was also evident, although not as universal, in patients treated before 10 days (group 1) and after 3 months (group 3). In group 1, not all of the patients suspected of having rejection actually had this diagnosis, but the needle biopsies necessary to clarify the situation were often considered too dangerous to be justifiable as a precondition of treatment. The most complex conditions for the use of OKT3 therapy were in the subgroup of group 1, in which patients developed severe hepatorenal complications during the first few postoperative days. Some such recipients probably did not yet have rejection, and it can be argued further that the testing of a new drug under such circumstances created an unfair disadvantage for its evaluation. Yet the introduction of OKT3 into the therapeutic scheme at this time has already been life-saving in a number of instances. By starting OKT3, it has been possible to markedly decrease the intravenous ciclosporin component of therapy. This has allowed renal function to return without the handicap of drug nephrotoxicity while ensuring that lethal rejection would not supervene. Even when a biopsy could not be obtained because of excessive risks, the OKT3 therapy could serve as a ‘diagnostic trial’, in which abrupt improvement of liver function was evidence that a component of early hepatic dysfunction was due to rejection. In several patients who improved clinically enough to allow a biopsy 1–3 days following initiation of OKT3, residual lymphoid infiltration and other findings of rejection were still present.
The patients in group 3, whose liver allografts underwent rejection after 3 postoperative months, were given OKT3 with some hesitancy, since the biopsies of 75% of these patients showed evidence of chronic rejection in addition to acute changes, thereby limiting the function that could be restored. In spite of the artifact created by treating patients for rejection either very early or very late after transplantation, 67% of the recipients in the combined groups 1 and 3 had either a partial or a complete restoration of graft function during OKT3 therapy, and only 2 patients had subsequent episodes of recurrent rejection.
When our clinical trial with OKT3 liver recipients was first started, randomization for the treatment of acute rejection was planned between monoclonal therapy versus high-dose steroids, both in conjunction with ciclosporin and prednisone. The inappropriateness of randomization was soon realized. Some of the patients consigned by chance to the OKT3 limb had rejections that might have responded easily to temporarily increased steroid therapy. Others with very severe rejections were committed by chance to an obviously futile and potentially dangerous trial with high-dose steroid therapy, from which rescue with OKT3 therapy was delayed. The necessity for such frequent use of the OKT3 ‘safety net’ in the steroid limb (table VIII) caused the randomized trial to be terminated. Our present policy is to treat all rejections with one or two 5-day bursts of high-dose steroid therapy. If this adjustment, plus careful adjustment of the ciclosporin doses, does not bring a rejection under control, OKT3 is started.
Table VIII.
Summary of OKT3 versus high-dose steroid randomization trials in reversal of acute liver rejection
| n | Reversal | Rescue needed | Rescue successful | |
|---|---|---|---|---|
| OKT3 | 7 | 6 | 0 | – |
| Prednisone | 11 | 4 | 71 | 6/7 |
Patients who did not respond to high-dose steroids were given a full course of OKT3 as rescue therapy.
Observations in 3 of our patients have suggested the possibility of reusing OKT3. The possibility of retreatment with OKT3 has been raised before [49] but, to our knowledge, has not been clinically tried. Two patients had complete reversal of rejection of second transplants. The third effort failed in a patient whose serum contained antimurine antibodies after the first course of OKT3. Thistlethwaite et al. [50] have described the use of cyclophosphamide during OKT3 therapy to decrease the sensitization rate, but have considered this effort at reduction of antibody formation to be dangerous.
The first clinical trials of ciclosporin use by Calne et al. [51] have opened a new era in transplantation. As more experience has been acquired with the use of ciclosporin, there has been a drift toward polypharmaceutical therapy, which initially began by combining ciclosporin with steroids [52] and which will undoubtedly encompass increasing use of monoclonal antibodies as these become commercially available. As was the case with the original double-drug and triple-drug regimens of azathioprine-prednisone [53] and azathioprine-prednisone-ALG [38], it can be easier to work with smaller dose ranges of multiple agents than to work within the limitations imposed by the toxicity of any single agent. This will be particularly important with ciclosporin, a drug whose margin is fairly sharply defined by nephrotoxicity.
Acknowledgments
The authors would like to express their appreciation to the Ortho Pharmaceutical Corporation for their support. In addition, we also acknowledge the help of Amy Roundtree and Joeta Kowalski for their help in collecting data.
Footnotes
Supported by research grants from the Veterans Administration, by NIH grant AM-29961, and a grant from the Competitive Medical Research Fund-Richard King Mellon Foundation.
NB: This analysis was attempted only for the first 62 liver and kidney recipients for whom minimal follow-ups of at least 9 months are available. An additional 26 renal recipients and 84 hepatic recipients have been treated from 1 week to 9 months prior to completion of this report. The early results in the more recent 110 cases have been similar to the findings reported here. The total experience in the treatment of kidney rejection with OKT3 is given by Gordon et al. elsewhere in this symposium collection.
References
- 1.Starzl TE, Marchioro TL, Porter KA, et al. The use of heterologous antilymphoid agents in canine renal and liver homotransplantation and in human renal homotransplantation. Surgery Gynec Obstet. 1967;124:301–318. [PMC free article] [PubMed] [Google Scholar]
- 2.Najarian J, Simmons R, Condie R, et al. Seven years’ experience with antilymphoblast globulin for renal transplants from cadaver donors. Ann Surg. 1976;184:352–368. doi: 10.1097/00000658-197609000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Starzl TE, Groth CG, Brettschneider L, et al. Orthotopic transplantation of the human liver. Ann Surg. 1968;168:392–415. doi: 10.1097/00000658-196809000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griepp R, Stinson E, Dong E, et al. The use of antithymocyte globulin in human heart transplantation. Circulation. 1972;45(suppl 1):147–153. [PubMed] [Google Scholar]
- 5.Glass N, Miller D, Sollinger H, et al. A comparative study of steroid and heterologous antiserum in the treatment of acute renal allograft rejection. Transplant Proc. 1983;15:617–621. [Google Scholar]
- 6.Shield C, Cosimi A, Rubin R, et al. Use of antithymocyte globulin for reversal of acute allograft rejection. Transplantation. 1979;28:461–464. [PubMed] [Google Scholar]
- 7.Nelson P, Cosimi A, Delmonico F, et al. Antithymocyte globulin as the primary treatment for renal allograft rejection. Transplantation. 1984;36:587–589. [PubMed] [Google Scholar]
- 8.Starzl TE. Experience in hepatic transplantation. Saunders; Philadelphia: 1969. pp. 193–241. [Google Scholar]
- 9.Starzl TE. Experience in hepatic transplantation. Saunders; Philadelphia: 1969. pp. 242–276. [Google Scholar]
- 10.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature, Lond. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 11.Weiss A, Stobo J. Requirement for the coexpression of T3 and the T cell antigen receptor on a malignant human T cell line. J exp Med. 1984;160:1284–1299. doi: 10.1084/jem.160.5.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reinherz E, Meuer S, Fitzgerald K, et al. Antigen recognition by human T lymphocytes is linked to surface expression of the T3 molecular complex. Cell. 1982;30:735–743. doi: 10.1016/0092-8674(82)90278-1. [DOI] [PubMed] [Google Scholar]
- 13.Zanders E, Lamb J, Feldman M, et al. Tolerance of T cell clones is associated with membrane antigen changes. Nature, Lond. 1983;303:625–627. doi: 10.1038/303625a0. [DOI] [PubMed] [Google Scholar]
- 14.Weiss A, Imboden J, Schoback D, et al. Role of T3 surface molecules in the activation of human T cells: a two stimulus requirement for IL-2 production reflects events occurring at a pre-translational level. J Immun. 1984;133:123–128. [PubMed] [Google Scholar]
- 15.Chang T, Kung P, Gingras S, et al. Does OKT3 monoclonal antibody react with an antigen-recognition structure on human T cells? Proc natn Acad Sci USA. 1981;78:1805–1808. doi: 10.1073/pnas.78.3.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang T, Gingras S. OKT3 monoclonal antibody inhibits cytotoxic T lymphocyte mediated cell lysis. Int J Immunopharmacol. 1981;3:183–186. doi: 10.1016/0192-0561(81)90011-4. [DOI] [PubMed] [Google Scholar]
- 17.Cosimi A, Burton R, Kung P, et al. Evaluation in primate renal allograft recipients of monoclonal antibody to human T-cell subclasses. Transplant Proc. 1981;13:499–503. [PubMed] [Google Scholar]
- 18.Cosimi A, Colvin R, Burton R, et al. Use of monoclonal antibodies to T cell subsets for immunologic monitoring and treatment in recipients of renal allografts. New Engl J Med. 1981;305:308–314. doi: 10.1056/NEJM198108063050603. [DOI] [PubMed] [Google Scholar]
- 19.Cosimi A, Burton R, Colvin R, et al. Treatment of acute allograft rejection with OKT3 monoclonal antibody. Transplantation. 1981;32:535–539. doi: 10.1097/00007890-198112000-00018. [DOI] [PubMed] [Google Scholar]
- 20.Norman D, Barry J, Henell K, et al. Reversal of acute allograft rejection with monoclonal antibody. Transplant Proc. 1985;17:39–41. [Google Scholar]
- 21.Ortho Multicenter Transplant Study Group. A randomized clinical trial of OKT3 monoclonal antibody for acute rejection of cadaveric renal transplants. New Engl J Med. 1985;313:337–342. doi: 10.1056/NEJM198508083130601. [DOI] [PubMed] [Google Scholar]
- 22.Starzl TE. Experiences in renal transplantation. Saunders; Philadelphia: 1964. pp. 37–47. [Google Scholar]
- 23.Starzl TE, Koep LJ, Halgrimson CG, et al. Fifteen years’ experiences of clinical liver transplantation. Gastroenterology. 1979;77:375–388. [PMC free article] [PubMed] [Google Scholar]
- 24.Iwatsuki S, Iwaki Y, Kano T, et al. Successful liver transplantation from crossmatch positive donors. Transplant Proc. 1981;13:286–288. [PMC free article] [PubMed] [Google Scholar]
- 25.Burckart G, Venkataramanan R, Ptachcinski R. Cyclosporine monitoring. Am Ass clin Chern. 1985;6:1–11. [Google Scholar]
- 26.Starzl TE, Halaka TR, Rosenthal JT, et al. Variable convalescence and therapy after cadaveric renal transplantation under cyclosporine A and steroids. Surgery Gynec Obstet. 1982;154:1–7. [PMC free article] [PubMed] [Google Scholar]
- 27.Starzl TE, Iwatsuki S, Shaw BW, et al. Orthotopic liver transplantation in 1984. Transplant Proc. 1985;17:250–258. [PMC free article] [PubMed] [Google Scholar]
- 28.Venkataramanan R, Burckart G, Ptachcinski R. Pharmacokinetics and monitoring of cyclosporine following orthotopic liver transplantation. Semin Liver Dis. 1985;5:357–368. doi: 10.1055/s-2008-1040633. [DOI] [PubMed] [Google Scholar]
- 29.Shear L, Kleinerman J, Gabuzda G. Renal failure in patients with cirrhosis of the liver. Am J Med. 1965;39:184–209. doi: 10.1016/0002-9343(65)90041-0. [DOI] [PubMed] [Google Scholar]
- 30.Ellis D, Avner ED, Starzl TE. Renal failure in children with hepatic failure undergoing evaluation for orthotopic liver transplantation. J Pedial. 1986;3:393–398. doi: 10.1016/s0022-3476(86)80879-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stables D, Klingensmith C, III, Johnson M. Renal transplantation. In: Rosenfield, Glickman, Hodson, editors. Diagnostic imaging in renal disease. Appleton-Century-Crofts; New York: 1979. pp. 167–213. [Google Scholar]
- 32.Hor G, Pabst H, Pfiefer K, et al. Radionuclides in renal transplantation. J nucl Med. 1972;13:795–800. [PubMed] [Google Scholar]
- 33.Jaffers G, Colvin R, Cosimi A, et al. The human immune response to murine OKT3 monoclonal antibody. Transplant Proc. 1983;15:646–648. [Google Scholar]
- 34.Becton Dickinson: Monoclonal antibody source book. Becton Dickinson Monoclonal Center; Mountain View: [Google Scholar]
- 35.Porter KA. Pathological changes in transplanted kidneys. In: Starzl, editor. Experiences in hepatic transplantation. Saunders; Philadelphia: 1964. pp. 299–359. [Google Scholar]
- 36.Porter KA. Pathology of the orthotopic homograft and heterograft. In: Starzl, editor. Experiences in hepatic transplantation. Saunders; Philadelphia: 1969. pp. 422–471. [Google Scholar]
- 37.Monaco A, Wood M, van der Werf B, et al. Effects of antilymphocyte serum in mice, dogs and man. In: Wolstenholme, O’Connor, editors. CIBA Foundation Study Group No. 29. Antilymphocytic serum. Little, Brown; Boston: 1967. pp. 111–138. [Google Scholar]
- 38.Starzl TE, Porter KA, Iwasaki Y, et al. The use of heterologous antilymphocyte globulin in human renal homotransplantation. In: Wolstenholme, O’Connor, editors. CIBA Foundation Study Group No. 29. Antilymphocytic serum. Little, Brown; Boston: 1967. pp. 4–40. [Google Scholar]
- 39.Levey R, Medawar P. Some experiments on the action of antilymphoid antisera. Ann NY Acad Sci. 1966;129:164–177. [Google Scholar]
- 40.Smith GV, Kolff J, Kashiwagi N, et al. Modification of established rejection of canine kidney and liver homografts with antilymphocyte gamma G globulin. Surgery. 1969;66:546–549. [PMC free article] [PubMed] [Google Scholar]
- 41.Lance E, Medawar P. Survival of skin heterografts under treatment with antilymphocytic serum. Lancet. 1968;i:1174–1176. doi: 10.1016/s0140-6736(68)91868-0. [DOI] [PubMed] [Google Scholar]
- 42.Kung P, Goldstein G, Reinherz E, et al. Monoclonal antibodies defining distinctive human T cell surface antigens. Science. 1979;206:347–349. doi: 10.1126/science.314668. [DOI] [PubMed] [Google Scholar]
- 43.Palacios R. Mechanisms of T-cell activation; role and functional relationship of HLA DR antigens and interleukins. Immuno Rev. 1982;63:73–110. doi: 10.1111/j.1600-065x.1982.tb00412.x. [DOI] [PubMed] [Google Scholar]
- 44.Suthanthiran M, Evelyn M, Rubin A, et al. A reappraisal of the effects of monoclonal antibodies directed at T cell differentiation antigens. Transplantation. 1984;38:720–726. doi: 10.1097/00007890-198412000-00034. [DOI] [PubMed] [Google Scholar]
- 45.Van Wauwe J, De Mey J, Goossens J. OKT3: a monoclonal anti-human T lymphocyte antibody with potent mitogenic properties. J Immun. 1980;124:2708–2713. [PubMed] [Google Scholar]
- 46.Welte K, Platzer E, Wang C, et al. OKT8 antibody inhibits OKT3-induced IL-2 production and proliferation in OKT8+ cells. J Immun. 1983;131:2356–2361. [PubMed] [Google Scholar]
- 47.Van Wauwe J, Goossens J, Beverly P. Human T lymphocyte activation by monoclonal antibody: OKT3, but not UCHT 1, triggers mitogenesis via an interleukin 2-dependent mechanism. J Immun. 1984;133:129–132. [PubMed] [Google Scholar]
- 48.Rubin R, Cosimi A, Hirsch M, et al. Effects of antithymocyte globulin on cytomegalovirus infection in renal transplant recipients. Transplantation. 1981;31:143–145. doi: 10.1097/00007890-198102000-00016. [DOI] [PubMed] [Google Scholar]
- 49.Gratama J, Jansen J, Lipovich R, et al. Treatment of acute graft-versus-host disease with monoclonal antibody, OKT3. Transplantation. 1985;38:469–474. doi: 10.1097/00007890-198411000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Thistlethwaite J, Cosimi A, Delmonico F, et al. Evolving use of OKT3 monoclonal antibody for treatment of renal allograft rejection. Transplantation. 1984;38:695–701. doi: 10.1097/00007890-198412000-00029. [DOI] [PubMed] [Google Scholar]
- 51.Calne R, White D, Thiru S, et al. Cyclosporin A in patients receiving renal allografts from cadaveric donors. Lancet. 1978;ii:1323–1327. doi: 10.1016/s0140-6736(78)91970-0. [DOI] [PubMed] [Google Scholar]
- 52.Starzl TE, Weil R, III, Iwatsuki S. The use of cyclosporine and prednisone in cadaver kidney transplantation. Surgery Gynec Obstet. 1980;151:17–26. [PMC free article] [PubMed] [Google Scholar]
- 53.Starzl TE, Marchioro TL, Waddell WR. The reversal of rejection in human renal homografts with subsequent development of homograft tolerance. Surgery Gynec Obstet. 1963;117:385–395. [PMC free article] [PubMed] [Google Scholar]


