Abstract
The study was designed to evaluate the hepatoprotective activity of ethanolic extract of Bacopa monnieri in acute experimental liver injury induced by Nitrobenzene in rats. The extract at the dose of 200 mg/kg body weight was administered orally once every day for 10 days. The increased serum marker enzymes, Aspartate transaminase, Alanine transaminase and alkaline phosphatase were restored towards normalization significantly by the extract. Significant increase in SOD, CAT and GPx was observed in extract treated liver injured experimental rats. Histopathological examination of the liver tissues supported the hepatoprotection. It is concluded that the ethanolic extract of Bacopa monieri plant possess good hepatoprotective activity.
Keywords: Hepatoprotective effect, Antioxidants, Liver marker enzymes
Introduction
Approximately 60–80% of the world’s population still rely on traditional medicines for the treatment of common illness [1, 2]. Ayurvedic medicine is essentially promotive and preventive in therapeutic approach. Many ayurvedic medicines are used for treating liver disorders. Thus search for crude drugs of the plant origin with antioxidant activity has become a central focus of study of hepatoprotection. Bacopamonnieri (L) Wettest belongs to Scrophulariacae family. It is commonly known as “Brahmi” and is a medical herb, found throughout the Indian subcontinent in wet and marshy places [3]. The plant is used especially for insanity, nervous breakdown, dermatitis and in memory enhancement [4] antiulcerogenic, adaptogenic activities and hepatoprotective effect against Morphine induced liver toxicity in rats [5]. It helps in the prevention of neurological diseases [6] and is also reported to possess anti-inflammatory, analgesic, antipyretic, sedative [7], free radical scavenging and lipid peroxidative activities [8]. The pharmacological effects of B. monnieri are mainly attributed to these saponins especially bacoside A and bacoside B, which are therefore, considered as bioactive marker compounds for this species [9].
NB is considered a hazardous air pollutant and has proven to be an animal carcinogen. It is classified as a group B2 chemical according to the 1986 Cancer guide lines [10] i.e. a likely human carcinogen. Metabolism of nitrobenzene produce intermediates such as Nitrosobenzene (NOB) and phenylhydroxylamine (PH) that play an important role in the process of NB carcinogenesis [11]. Following accidental nitrobenzene poisoning in humans, the highest concentration was found in the liver, brain, blood and stomach [12]. The objective of this study was to investigate the hepatoprotective effects of B. monnieri, on Nitrobenzene induced hepatic damage in rats.
Materials and Methods
Plant Material and Extraction
The whole plant of B. monnieri (Scrophulariaceae) was collected from areas in and around Coimbatore, India. The materials were identified and authenticated by Dr. G.V.S. Moorthy, Botanical Survey of India Coimbatore. Voucher No: BSI/SC/5/23/08-09/Tech-769 dated 14.08.2008. The collected materials were thoroughly washed in water, chopped, air dried at 35–40°C for a week and pulverized in electric grinder. 1 g of powder was then extracted in 5 ml of ethanol. The ethanol extract was then made to powder with the help of rotary evaporator under reduced pressure.
Experimental Animals
Adult albino rats of Wistar stain of both sexes of 12–16 weeks weighing between 160 and 180 g were used for the study. The animals were maintained in well-ventilated room temperature with natural day–night cycle in large polypropylene cages. They were fed with balanced rodent pellet diet and water ad libitum throughout the experimental period. The animals were quarantined for 1 week, prior to the experiments to acclimatize to laboratory conditions. The study protocol was approved by the IAEC (Institutional Animal Ethics Committee, Govt. of India).
Experimental Design
Animals were divided into four groups of six animals each.
Control, normal healthy rats.
Rats administered with nitrobenzene orally (50 mg/kg bw) as a single dose.
Rats subjected to induction and Treatment. Nitrobenzene was injected orally and treatment with ethanolic extract (200 mg/kg bw) orally was started a day after the injection for a period of 10 days.
Ethanolic extract alone was injected orally for 10 days.
Preparation of the Samples for Biochemical Studies
The animals were anesthetized using chloroform and sacrificed. Blood was collected directly from the heart of each animal and the clot was centrifuged for 15–20 min at 2,000 rpm to separate serum for biochemical analysis. The liver was dissected out for histopathological examinations.
Determination of Serum Marker Enzymes
The following serum marker enzymes were analyzed. ALT [13], AST [13], and ALP [14].
Determination of Antioxidant Enzyme Activities
The homogenate was prepared in 0.1 M Tris-HCl buffer (pH 7.4). The homogenate was centrifuged at 1,000 rpm for 5 min at 4°C and the supernatant was collected and used for the estimation of superoxide dismutase (SOD) [15], catalase (CAT) [16], glutathione peroxidase (GPx) [17] and lipid peroxidation [18].
Histopathological Studies
A portion of the liver sample from each group was fixed in 10% formaldehyde and stained with hematoxylin and eosin for histopathological observations.
Statistical Analysis
All biochemical values were illustrated as Mean ± standard deviation (SD) for both control and experimental animals. The statistical significance of values between the four groups was analyzed using student’s “t” test.
Result and Discussion
Determination of Serum Marker Enzymes
The activity of serum marker enzymes such as alanine aminotransferase (ALT), aspartate aminotransferase (AST) and Alkaline phosphatase (ALP) analyzed in serum samples of different groups of rats are shown in Table 1. In group II there was a significant increase (P < 0.01) in serum levels of ALT, AST and ALP. But when the ethanolic extract of Baccopa monneriwas given to group III, there was a significant decrease in the value, which tends to reach the normal values. In group IV, when the plant extract alone was given, the level approached the normal values. The liver weight and activity of serum marker enzymes (aspartate transaminase, alanine transaminase, lactate dehydrogenase, alkaline phosphatase, and gamma-glutamyl transpeptidase) were markedly increased in carcinogen-administered rats, whereas the activities of marker enzymes were near normal in bacoside A-pretreated rats [19].
Table 1.
ALT, AST and ALP levels in serum control and experimental groups of rats
| Groups treatment | AST (U/l) | ALT (U/l) | ALP (IU/l) | |
|---|---|---|---|---|
| I | Normal | 48.25 ± 1.361 | 50.2 ± 1.43 | 125.42 ± 1.51 |
| II | NB control | 140.51 ± 5.33a* | 96.8 ± 3.72a* | 180.21 ± 4.26a* |
| III | NB + ethanolic extract | 55.32 ± 4.24b* | 64.3 ± 3.64b* | 132.42 ± 5.32b* |
| IV | Ethanolic extract | 47.42 ± 1.23cNs | 52.2 ± 1.32cNs | 122.43 ± 1.34cNs |
* P < 0.01
a—Group-I compared with Group-II
b—Group-II compared with Group-III
c—Group-I compared with Group-IV
Determination of Antioxidant Enzymes and Lipid Peroxidation
The activity of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and lipid peroxidation analysed in liver homogenate of different groups of rats are shown in Table 2. Antioxidant enzymes such as SOD, CAT, GPx were analyzed in different groups of rat. In group II, there was a significant decrease (P < 0.01) in the antioxidant enzyme and the lipid peroxidation levels were found to be increased. But when the ethanolic extract of B. monnieri was given to group III, there was a significant increase in the antioxidant levels and lipid peroxidation levels were decreased. In group IV, when the plant extract alone was given, the level approached the normal values.
Table 2.
SOD, CAT, GPx and LPO levels in liver of control and experimental groups of rats groups treatment
| SOD | CAT | GPx | LPO | ||
|---|---|---|---|---|---|
| I | Normal | 23.34 ± 0.37 | 50.85 ± 4.63 | 26.20 ± 0.35 | 0.96 ± 0.07 |
| II | NB control | 17.21 ± 0.25a* | 26.43 ± 2.25a* | 20.32 ± 0.52a* | 3.82 ± 0.25a* |
| III | NB + ethanolic extract | 20.93 ± 0.39b* | 46.75 ± 4.83b* | 23.45 ± 0.45b* | 1.86 ± 0.15b* |
| IV | Ethanolic extract | 22.72 ± 0.41cNs | 51.83 ± 4.93cNs | 25.62 ± 0.53cNs | 1.05 ± 0.09cNs |
Catalase (μmol of H2O2 utilized per minute per mg protein)
Superoxide dismutase (units per mg protein)
Glutathione peroxidase (μmol of GSH utilized per minute mg per protein)
a—Group-I compared with Group-II; b—Group-II compared with Group-III; c—Group-II compared with Group-IV; * P < 0.01
Nitrobenzene carcinogenicity is considered to correlate with its metabolic activation. It forms a number of phenolic compounds by oxidation and nitroxides by reduction [20]. Reduction of nitro group plays a more potent role in NB carcinogenicity. Nitroreduction, which is driven by microsomal P-450s and NAD(P)H, can produce reactive nitroxide intermediates aromatic nitroso- and hydroxylamine compounds, e.g. NOB and PH, associated with their reactive free radicals, e.g., the nitroanion free radical and superoxide free radical [21]. Activities of antioxidant enzymes (superoxide dismutase, catalase, glutathione peroxidase, glutathione reductase, glutathione-S-transferase, and reduced glutathione) in liver were also decreased in carcinogen-administered rats, which were significantly elevated in bacoside A-pretreated rats. It is concluded that pretreatment of bacoside A prevents the elevation of LPO and activity of serum marker enzymes and maintains the antioxidant system and thus protects the rats from Diethylnitrosamine induced hepatotoxicity [19].
Histopathological Studies in Liver
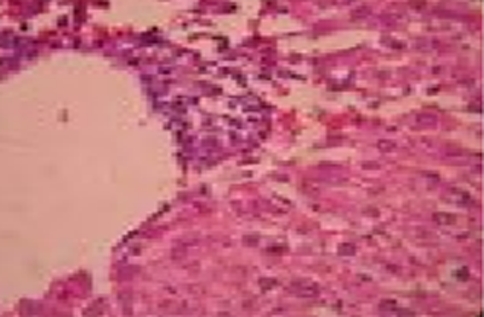
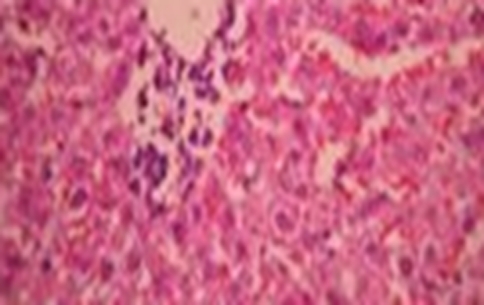
Histopathological studies of sections of liver of control and experimental rats were carried out to test the hepatoprotective effect of the ethanolic extract of Baccopa moneri. In group I (normal control) rats, liver showed normal histological architecture (Fig. 1). In group II (NB control), liver showed marked inflammatory changes associated with fatty changes and confluent hepatic necrosis (Fig. 2). Group III (NB + B. monnieri) which is NB damaged and treated with the ethanolic extract showed normal architecture with lesser degrees of inflammation (Fig. 3).Group IV (B. monnieri only) showed normal liver (Fig. 4).
Fig. 1.
Normal
Fig. 2.
NB
Fig. 3.
NB + B. monnieri
Fig. 4.
B. monnieri
In conclusion, the ethanolic extract of B. monnieri showed good hepatoprotective activity in NB induced liver damage. The hepatoprotective activity may be due to the antioxidant effect of the plants.
Acknowledgments
We, the authors are thankful to our Chancellor, Chief Executive Officer, Vice Chancellor and Registrar of Karpagam University for providing facilities and encouragement.
References
- 1.WHO Traditional Medicine Strategy 2002–2005. Geneva: World Health Organization; 2002. [Google Scholar]
- 2.Zang X. Traditional medicine: its importance and protection. In: Twarog S, Kapoor P, editors. Protecting and promoting traditional knowledge: systems, national experiences and international dimensions. Part I. The role of traditional knowledge in health care and agriculture. New York: United Nations; 2004. pp. 3–6. [Google Scholar]
- 3.Kapoor LD. Handbook of ayurvedic medicinal plants. Baca Raton, FL: CRC Press; 1990. pp. 317–329. [Google Scholar]
- 4.Chopra RN, Chopra IC, Verma BS. Supplementary to glossary of Indian medicinal plants. New Delhi: National Institute of Science Communication, Council of Scientific and Industrial Research; 1998. pp. 10–11. [Google Scholar]
- 5.Russo A, Borelli F. Bacopa monniera, a reputed nootropic plant: an overview. Phytomedicine. 2005;12:305–317. doi: 10.1016/j.phymed.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Vohora D, Pal SN, Pillai KK. Protection from phenytoin-induced cognitive deficit by Bacopa monniera, a reputed Indian nootropic plant. J Ethnopharmacol. 2000;71:383–390. doi: 10.1016/S0378-8741(99)00213-5. [DOI] [PubMed] [Google Scholar]
- 7.Kishore K, Singh M. Effect of bacosides, a alcoholic extract of Bacopa monnieri Linn. (brahmi), on experimental amnesia in mice. Indian J Exp Biol. 2005;43:640–645. [PubMed] [Google Scholar]
- 8.Anbarasi K, Vani G, Balakrishna K, Desai CS. Creatine kinase isoenzyme patterns upon exposure to cigaratte smoke: protective effect of bacoside A. Vasc Pharmacol. 2005;42:57–61. doi: 10.1016/j.vph.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Deepak M, A Amit. The need for establishing identities of bacoside A, and B, the putative major bioactive saponins of Indian medicinal plant Bacopamonnieri. Phytomedicine. 2004;11:264–268. doi: 10.1078/0944-7113-00351. [DOI] [PubMed] [Google Scholar]
- 10.Cattley RC, Everitt JI, Gross EA. Carcinogenicity and toxicity of inhaled nitrobenzene in B6C3F1 mice and F344 and CD rats. Toxicol Sci. 1994;22:328–340. doi: 10.1093/toxsci/22.3.328. [DOI] [PubMed] [Google Scholar]
- 11.Howard PC, Beland FA, Cerniglia CE. Reduction of the carcinogen 1-nitropyrene to 1-aminopyrene by the rat intestinal bacteria. Carcinogenesis. 1983;4:985–990. doi: 10.1093/carcin/4.8.985. [DOI] [PubMed] [Google Scholar]
- 12.Nitrobenzene environmental health criteria 230. Geneva: WHO; 2003. [Google Scholar]
- 13.King EJ. Practical clinical Enzymol. London: D. Van Nostrand Co.; 1965. [Google Scholar]
- 14.King EJ, Armstrong AR. Calcium, magnesium, phosphorus and phosphatase. In: Varley B, Gowenlock AH, Bell M, editors. Practical clinical biochemistry. London: Heinemann; 1980. pp. 850–852. [Google Scholar]
- 15.Misra HP, Fridovich I. The role of superoxide anion in the antioxidation of epinephrine and simple assay of superoxide dismutase. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 16.Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 17.Rotruck JT, Pope AL, Ganther HE. Selenium, biochemical role as a component of glutathione peroxidase purification and assay. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 18.Hogberg J, Larson RE, Kristoferson A, Orrhenices S. NADPH-dependent reductase solubilised from microsomes by peroxidation and its activity. Biochem Biophys Res Commun. 1974;56:836–842. doi: 10.1016/0006-291X(74)90681-0. [DOI] [PubMed] [Google Scholar]
- 19.Janani P, Sivakumari K, Parthasarathy C. Hepatoprotective activity of bacoside A against N-nitrosodiethylamine-induced liver toxicity in adult rats. Cell Biol Toxicol. 2008;24:471–474. doi: 10.1007/s10565-008-9109-3. [DOI] [PubMed] [Google Scholar]
- 20.Holder JW. Nitrobenzene potential human cancer risk based on animal studies. Toxicol Ind Health. 1999;15:458–463. [PubMed] [Google Scholar]
- 21.Mason RP, Holtzman JL. The role of catalytic superoxide formation in O2 inhibition of nitroreductase. Biochem Biophys Res Commun. 1975;67:1267–1274. doi: 10.1016/0006-291X(75)90163-1. [DOI] [PubMed] [Google Scholar]