Abstract
The objective of the present study was to evaluate hypolipidemic effect of methanolic extract of Celastrus paniculatus in experimentally induced hypercholesterolemic rats. Hypercholesterolemia was induced by feeding the animals with high fat diet. Oral administration of methanolic seed extract (50%) of Celastrus paniculatus at the optimized dose of 65 mg/kg body weight, substantially reduced the plasma total cholesterol, triglycerides and LDL cholesterol in comparison with induced hypercholesterolemic animal group and the results were comparable with the standard hypocholesterolemic drug and almost similar to the control group. Atherogenic index and liver weight of treated animals also showed significant decrease compared to the hypercholesterolemic animals. It substantially increased the HDL cholesterol level as compared to control group. A significant increase in the activities of lipoprotein lipase and plasma LCAT enhanced hepatic bile acid synthesis and thereby, increased degradation of cholesterol to neutral sterols. Furthermore, the activities of HMG-CoA reductase, glucose 6-phosphate dehydrogenase and malate dehydrogenase were significantly reduced. Histological studies showed less cholesterol deposits in the aorta of animals fed with seed extract of C. paniculatus compared to the induced hypercholesterolemic animals not given C. paniculatus supplement.
Keywords: Atherogenic index, Celastrus paniculatus, Lipid profile, LDL, VLDL, Cardio protective, Lipid lowering, Neutral sterols, Plasma LCAT
Introduction
Coronary heart diseases (CHD) are the main cause of death in western countries and Asia. Among CHDs, ischemic heart disease (IHD) leads to the highest mortality rate. The number of heart patients suffering from IHD worldwide is gradually increasing [1]. Several epidemiological studies have demonstrated the relationship between plasma cholesterol levels and the development of IHD. Hypercholesterolemia is generally, associated with an increase in plasma concentration of LDL and VLDL. Lowering of elevated levels of LDL cholesterol can slower the progression of atherosclerotic lesions [2, 3]. About 70% of total cholesterol in human is synthesized de novo and the remaining is also supplied by absorption from diet (0.3–0.5 g/day in human). Several methods are presently practised to control blood cholesterol levels. These include balance of dietary fats; bile acids sequester and use of HMG-CoA reductase inhibitors (statins). HMG-CoA reductase is the key enzyme in the cholesterol biosynthesis pathway. Inhibition of this enzyme has proven to be the most efficient therapy for managing hypercholesterolemia [3].
Many plants in the Indian system of medicine have been reported to be beneficial in hypercholesterolemia. Allium sativum (garlic) [4], Asparagus racemosus (shatavari) [5], Cesalpinia bonucella [6], Terminalia arjuna [7], Emblica officinalis (amla) [8] and Picrorhiza kurroa [9] are known to have hypolipidemic activities. C. paniculatus (Celastraceae) commonly known as Malkangoni is a shrub used in Ayurvedic medicines in India. It is found all over India, Sri Lanka and Maldives [10]. Various medicinal properties are attributed to the aerial parts and seeds of this plant. The seeds of Celastrus paniculatus have been recommended and are being used in the treatment of rheumatism, gout and paralysis in the Ayurvedic system of medicine. Seed oil is also known to stimulate memory and improves intellect. C. paniculatus has also been reported for its antidiarrheal and anti haemorrhagic activities [2]. In the present study, the methanolic seed extract of C. paniculatus was further investigated for its hypolipidemic activity in experimental animals and some possible mechanisms of its lipid lowering properties are discussed.
Materials and Methods
Plant Material
Celastrus paniculatus seeds were obtained from the Toranmal forest of Nandurbar district of Maharashatra and authenticated. The voucher specimen was deposited in the Department of Botany, Jai Hind College, Dhule. The seeds were sun dried for 2 weeks and 100 g of seeds were ground to coarse powder of 20–65 mesh size and then refluxed with various solvents like 50% v/v methanol, ethanol and water for 3 h each using Soxhlet apparatus. The extracts were filtered and evaporated in a vaccum evaporator to obtain brown residue. The amount of residue remained was measured and stored in glass bottle at 4°C and used directly. In our preliminary studies with different extracts, the best results were obtained with 50% methanolic extract and hence the same has been used as extraction medium in our study.
Animal Groups and Treatment
Male albino Wistar rats, 12 week old, weighing 150–160 g were used for the present study. The experimental study was approved by the Institutional Animal Ethics Committee of R.C. Patel College of Pharmacy, Shirpur, were the study was conducted. All animals were maintained at 22 ± 1°C temperature with 12 h light and dark cycle. The animals were fed with standard diet and water ad libitum. Rats were divided into four groups and each group contained six animals (n = 6). Group A rats served as untreated control and received standard diet plus 1 ml saline for 6 weeks. Group B received high fat diet (athero diet) for 6 weeks whereas group C rats received high fat diet plus 25, 45, 65 and 75 mg/kg body weight seed extract of Celastrus paniculatus for 6 weeks. Group D rats received athero diet plus standard atrovastatin tablet at 5 mg dose [1]. The extract was administered orally through gastric intubations. High fat diet composed of hydrogenated sunflower oil, egg yolk and cholesterol at previously used concentrations [11].
Biochemical Analysis
After 6 weeks’ treatment, blood samples were collected by retro orbital route and serum was separated. Analysis of serum for total cholesterol [12], triglycerides and HDL cholesterol [13] was performed by micro titer plate reader using standard kits (Span Diagnostics, Surat, India). Serum LDL cholesterol concentration was determined using Friedwald formula [14].
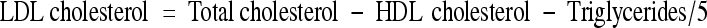 |
The atherogenic index was calculated by Friedwald formula.
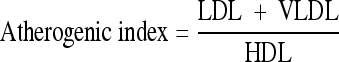 |
Bile acids and fecal sterols were extracted as per the previously known procedures [15]. Total bile acids [16], neutral sterols [17] and serum lipoproteins [17, 18] were also estimated. The activity of plasma lecithin cholesterol acyl transferase (LCAT) (EC 2.3.1.43) and lipoprotein lipase was estimated as described earlier [3]. The extent of increase in the esterified/unesterified cholesterol ratio was taken as a measure of LCAT activity. Activity of glucose 6-phosphate dehydrogenase and malic enzyme was determined as per Annie and Kurup [3]. β hydroxy β methyl glutaryl CoA reductase (HMG CoA reductase, EC 1.1.1.34) activity was assayed using the method described earlier [19]. The ratio of HMG CoA to mevalonate was taken as an index of enzyme activity which catalyzes the conversion of HMG to mevalonate. The lower the ratio, the higher the enzyme activity [19, 20].
Collection of Aorta
After 6 weeks of diet treatment, the animals were fasted overnight in preparation for serum and aorta collection. At 8.00 in the morning, the animals were weighed, anesthetized with chloroform and the thoracic abdominal cavity was opened. The heart together with the aorta (2–3 cm length) was excised from each animal. The aorta was cut at the origin and removed from the heart.
Histopathological Studies
A 2 mm section of the aorta of each animal was soaked in 10% (v/v) formal saline solution for hematoxylin and eosin staining and another 2 mm section was placed in kriomatrix and kept at −20°C for frozen sections [21]. The remaining aorta was soaked in deionized water and homogenized for biochemical analyses. The aorta sections that were soaked in 10% formal saline solution were processed for normal histological section. The tissue samples were ultrasectioned (5–6 μm thickness), stained with hematoxylin and eosin and examined under a light microscope for observation of structural abnormality. Ultra-thin sections of the frozen aorta (8 μm thickness) were stained with Schultz stain [21, 22] and examined for cholesterol deposits.
Statistical Analysis
Statistical significance of data was analyzed using one-way analysis of variance (ANOVA) in Microsoft Excel. Each data value is expressed as the mean ± SD of six animals. Post hoc test was also conducted to determine level of significance between the treated and control groups using Student–Newman–Keuls test and considered significant at P < 0.05.
Results and Discussion
Preliminary studies with different doses of the methanol extract (25, 45, 65 and 75 mg/kg body weight of rats) indicated a dose dependent decrease in total cholesterol in group C rats till 65 mg/kg body weight dose. The decline in the total cholesterol beyond this does became independent of the dose and was not significant and hence all other parameters were evaluated for animals treated with this dose.
It was observed that keeping the animals on high fat diet (group B) significantly increased the total cholesterol, triglycerides, LDL and VLDL cholesterol as compared to the rats on normal diet (group A, Table 1). When the seed extract of Celastrus paniculatus is co-administered with high fat diet, total cholesterol, triglycerides, LDL and VLDL cholesterol level decreased considerably (Newman–Keuls, P < 0.05). The seed extract of C. paniculatus at the dose of 65 mg/kg body weight lowered the plasma TC, TG and LDL as compared to the hypercholesterolemic group (group B). The liver weight, atherogenic index and body weight in different groups is presented in Table 2. The liver weight in treated group (group C) was significantly lower (Newman–Keuls, P < 0.05) than that in hypercholesterolemic group (group B) indicating decrease in cholesterol and fat deposition in liver (Table 2). Atherogenic index in group C was three times less than group B and was comparable to group A and D (Table 2). The body weight of the group B animals showed significant gain when fed with high fat diet over the group A animals (normal controls). Treatment with seed extract and standard drug (Atrovastatin) in group C and group D, respectively reduced body weight by approximately 20% (Newman–Keuls, P < 0.05) compared to group B animals (Table 2).
Table 1.
Effect of 50% methanolic extract of Celastrus paniculatus on lipid profile of hypercholesterolemic rats
| Groups | TC (mg/dl) | TG (mg/dl) | HDL (mg/dl) | LDL (mg/dl) | VLDL (mg/dl) |
|---|---|---|---|---|---|
| Group A Control |
66.20 ± 5.22 | 86.32 ± 3.14 | 34.82 ± 5.47 | 17.34 ± 2.56 | 15.40 ± 1.23 |
| Group B Hypercholesterolemic |
145.20 ± 9.36 | 204.10 ± 16.23 | 37.12 ± 7.58 | 70.26 ± 8.16 | 40.00 ± 7.63 |
| Group C Hypercholesterolemic plus (65 mg extract/kg BW) |
68.12 ± 5.95* | 95.02 ± 2.56* | 38.40 ± 4.25* | 16.68 ± 2.56* | 15.22 ± 3.10* |
| Group D Standard Atrovastatin (5 mg/kg BW) |
68.90 ± 5.21 | 87.32 ± 3.14 | 32.00 ± 3.89 | 17.24 ± 3.26 | 13.19 ± 3.44 |
Values are expressed as Mean ± SD of each group (n = 6). The results were analysed to be statistically significant at P < 0.05.* Significantly different from group A
Table 2.
Effect of 50% methanolic extract of Celastrus paniculatus on cholesterol deposition, body weight and atherogenic index of hypercholesterolemic rats
| Groups | Relative liver weight (g) | Atherogenic index | Body weight (g) |
|---|---|---|---|
| Group A Control |
4.12 ± 1.85 | 0.81 ± 0.09 | 280 |
| Group B Hypercholesterole-emic |
7.90 ± 1.10 | 2.90 ± 0.12 | 360 |
| Group C Hypercholesterolemic plus (65 mg extract/kg BW) |
5.17 ± 1.93* | 0.95 ± 0.08* | 270* |
| Group D Standard Atrovastatin (5 mg/kg BW) |
4.90 ± 1.23 | 0.89 ± 0.02 | 296 |
Values are expressed as Mean ± SD of each group (n = 6). The results were analysed to be statistically significant at P < 0.05. * Significantly different from group B
The activity of glucose 6-phosphate dehydrogenase and malate dehydrogenase was significantly reduced while activities of plasma LCAT and lipoprotein lipase were enhanced in the animal group fed with the extract (Table 3). The high concentrations of fecal neutral sterols and bile acids in the liver in the treatment group shows the enhanced rate of degradative processes and reduction in intestinal absorption of free cholesterol and other lipids. The significantly higher (Newman–Keuls, P < 0.05) levels of bile acids in the liver and feces and increased concentrations of fecal neutral sterols (Table 3) provide the evidence for higher rate of degradation. The decrease in the cholesterol levels of animals fed with extract may be attributed to increase in the level of serum HDL, increase in the activity of lipoprotein lipase and plasma LCAT, which are known to be involved in transport of tissue cholesterol to liver for its excretion. Hence the hypocholesterolemic effect of the extract seems to mediated through increased hepatic clearance of cholesterol and by down regulation of lipogenic enzymes like glucose 6-phosphate dehydrogenase and malate dehydrogenase.
Table 3.
Concentration of hepatic and fecal bile acids, sterols and activities of lipogenic and lipolytic enzymes in rats fed with methanolic seed extract (50%) of Celastrus paniculatus
| Parameters | Animal groups | ||
|---|---|---|---|
| Group A (Control) |
Group B Hypercholesterolemic |
Group C Hypercholesterolemic plus 50% extract |
|
| Hepatic bile acids (mg/100 g) | 36 ± 2.3 | 40.6 ± 3.3 | 70.6 ± 1.5# |
| Hepatic neutral sterols (mg/100 g) | 100.3 ± 3.5 | 105.6 ± 4.5 | 135.2 ± 3.2# |
| Fecal bile acids (mg/rat/day) | 35.9 ± 1.3 | 38.3 ± 2.5 | 51.3 ± 2.1# |
| Fecal neutral sterols (mg/rat/day) | 90.5 ± 2.6 | 98.8 ± 3.1 | 123.5 ± 3.2# |
| Plasma LCATa | 28.5 ± 0.6 | 27.9 ± 0.3 | 39.4 ± 0.8# |
| Lipoprotein lipaseb | 40.6 ± 1.2 | 35.3 ± 1.2 | 56.6 ± 1.6# |
| Glucose 6-phosphate dehydrogenasec | 64.2 ± 1.3 | 100.3 ± 3.2 | 62.2 ± 1.2# |
| Malate dehydrogennased | 501.3 ± 8.6 | 990.3 ± 9.6 | 453.2 ± 9.6# |
| HMG CoA reductase (ratio of HMG CoA to mevalonate)e | 4.1 ± 0.2 | 6.8 ± 03 | 3.9 ± 0.3# |
Control group (A) and group B compared with 50% ethanolic extract fed group. # P < 0.05
a% increase in the ratio of ester cholesterol to free cholesterol during incubation
bμ moles of glycerol liberated/h/g protein
cOne unit is defined as that amount of the enzyme that causes an increase of 0.01 in optical density/mg protein
dOne unit is defined as that amount of the enzyme that causes an increase of 1.0 in optical density/mg protein [3]
eDecreased ratio indicates increased activity [20]
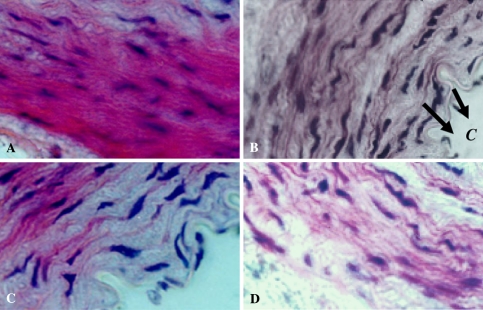
Apart from lowering serum lipid level, the seed extract also reduced the cholesterol deposition in the aorta of high cholesterol diet animals. These observations indicate that animals fed with high fat diet and seed extract prevented a build up of lipids in the aorta. The aorta of the animals fed with the extract showed normal physiology (Fig. 1a). However, aorta of high cholesterol diet animals showed spaces within the intima tunica and media tunica. These spaces originally contained fat droplets which were dissolved during the staining (Fig. 1b). In the high cholesterol diet animals, cholesterol deposits were concentrated in the media tunica and intima tunica regions, mainly in the endothelial layer. Thus the hypocholesterolemic effects of C. panicualtus seed extract can be probably explained by its known properties to stimulate bile fluid secretion as well as biliary cholesterol secretion and enhance excretion of bile acids in feces.
Fig. 1.
Sections of aorta of animals fed with high fat diet and methanolic seed extract (a). Arrow indicates lipid depositions within intima tunica and media tunica in animals fed with high cholesterol diet (b). Aorta section of animal fed with standard drug (c) and normal control (d). C: cholesterol
It is known that for being effective antihyperlipidemic agent the compound should reduce the plasma levels of LDL cholesterol, as it transports 70% of plasma cholesterol in humans [23]. Epidemiological and clinical studies have demonstrated positive correlation in LDL cholesterol concentration in serum and risk of coronary heart diseases. This report shows significant decrease in plasma LDL cholesterol level as a function of treatment with seed extract of C. paniculatus. The reduced triglyceride level in treated animals could be co-related to elevated lipoprotein lipase activity in agreement with the previous report on a mushroom extracted exo-biopolymer [23, 24]. Animal study also reveals negative correlation in plasma HDL cholesterol level and incidence of coronary heart diseases [23]. In the present study, the methanolic extract of Celastrus paniculatus in experimental group (group C) showed significant increase in HDL cholesterol compared to the group B. The result of our study showed that liver weight of the rats fed with high fat plus seed extract were significantly lower than that of control group. The liver weight reducing effect may be partly attributed to inhibition of cholesterol deposition in liver tissues or inhibition of HMG CoA reductase activity which is the key enzyme in cholesterol biosynthesis pathway [1, 2].
Although the exact mechanism involved in the hypolipidemic activity of Celastrus paniculatus remains unidentified, it has been shown to protect against cardiovascular diseases by altering serum lipid profile [25]. The seed extract contains sesquiterpens like celapenin, celapangin and celapagin [26] and alkaloids like celastrin and paniculatin [27] which may be responsible for the observed multiple pharmacological activities [27, 28]. The alkaloids may mediate the hypolipidemic action either by up regulation of activities of lipolytic enzymes or by stimulating faecal file acid excretion in agreement with previous findings [10].
References
- 1.Alberts AW, Chen J, Curon G, Hunt V, Huff J, Hoffman C, Rothrock J, Lopez M, oshau H, Harris E, Patchett A, Monaghan R, Currie S, Stapley E, Albers-Schonberg G, Hensen O, Hirschfield J, Hoogsteen K, Liesch J, Springer J. Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and cholesterol-lowering agent. Proc Natl Acad Sci. 1980;77:3957. doi: 10.1073/pnas.77.7.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul R. Influence of nicotinic acid (Niacin) on hypercholesterolemia and hyperlipemia and on the course of atherosclerosis. In: Atlshul R, editor. Niacin in vascular disorder of and hyperlipemia. Springfield, IL: Charles C. Thomas; 1964. p. 135. [Google Scholar]
- 3.Annie A, Kurup PA. Dietary carbohydrates and regulation of the activity of HMGCoA reductase and cholesterol metabolism. Indian J Biochem Biophys. 1986;23:28. [PubMed] [Google Scholar]
- 4.Helen A, Rajasree R, Krishnakumar K, Augusti KT, Vijayammal PL. Antioxidant role of oils isolated from garlic (Allium sativum Linn), onion (Allium cepa Linn) on nicotine-induced lipid peroxidation. Vet Hum Toxieo. 1999;41:316. [PubMed] [Google Scholar]
- 5.Kamat JP, Boloor KK, Devasagayam TP, Venkatachalam SR. Antioxidant properties of Asparagus racemosus against damage induced by y-radiation in rat liver mitochondria. J Ethnopharmacol. 2000;71:425. doi: 10.1016/S0378-8741(00)00176-8. [DOI] [PubMed] [Google Scholar]
- 6.Chakrabarti S, Biswas TK, Seal T, Rokeya B, Ali L, Khan AK, Nahar N, Mosihuzzaman M, Mukherjee B. Antidiabetic activity of Caesalpinia bonducela F. in chronic type 2 diabetic model in Long-Evans rats and evaluation of insulin secretagogue property of its fractions on isolated islets. J Ethnopharmacol. 2005;97:117. doi: 10.1016/j.jep.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 7.Dwivedi S, Agarwal MP. Antianginal and cardioprotective effects of Terminalia arjuna, an indigenous drug in coronary artery disease. J Assoc Physicians India. 1994;42:287. [PubMed] [Google Scholar]
- 8.Bhattacharya A, Chatterjee A, Ghosal S, Bhattacharya SK. Antioxidant activity of active tannoid principles of Emblica officinalis (amla) lndian J Exp Biol. 1998;37:676. [PubMed] [Google Scholar]
- 9.Kirtikar KR, Basu BD. Indian medicinal plants, vol. I. In: Blatter E, Cauis R, Mhaskar KS, Basu LM, editors. Allahabad, India; 1984. p. 258.
- 10.Mathur NT, Varma M, Dixit VP. Hypolipidemic and antiatherosclerotic effect of Celastrus paniculatus seed extract (50% E-OH) in cholesterol fed rabbits. Indian Drugs. 1993;30(2):76. [Google Scholar]
- 11.Burnstein M, Scholnic MR, Mortin R. Rapid method of estimation and isolation of lipoproteins from serum by precipitation of polyamines. J Lipid Res. 1970;11:583. [PubMed] [Google Scholar]
- 12.Zlatkis A, Zak B, Boyle AJ. A method for determination of serum cholesterol. J Lab Clin Med. 1953;41:486. [PubMed] [Google Scholar]
- 13.Selveraj S, Ramsundaram S, Sundaramahalingam M, Narayanaperumal JP, Rathinasamy SD. Hypolipidemic effect of Triphala in experimentally induced hypercholeseremic rats. Yakazaku zasshi. 2007;127(2):385. doi: 10.1248/yakushi.127.385. [DOI] [PubMed] [Google Scholar]
- 14.Friedwald WT, Levy RT, Fredrickson DS. Estimation of concentration of low density lipoproteins cholesterol in plasma without use of preoperative ultracentrifuge. Clin Chem. 1970;18:449. [PubMed] [Google Scholar]
- 15.Grundy SM, Ahrens EH, Miettinen TA. Quantitative isolation and gas-liquid chromatographic analysis of total dietary and neutral sterols. J Lipid Res. 1965;6:411. [PubMed] [Google Scholar]
- 16.Palmer H. The enzymatic assay of bile acids and related 3 hydroxy steroids: its application to serum and other biological fluids. Methods Enzymol. 1969;15:280. doi: 10.1016/S0076-6879(69)15008-9. [DOI] [Google Scholar]
- 17.Ghanbari Sissan MA, Leelamma S. Influence of components of oral contraceptive on lipid metabolism. Indian J Exp Biol. 1996;34:131–134. [PubMed] [Google Scholar]
- 18.Warnick GR, Albers JJ. A comprehensive evaluation of the heparin-anganese precipitation procedure for estimating high density lipoprotein cholesterol. J Lipid Res. 1978;19:65. [PubMed] [Google Scholar]
- 19.Venugopalan Rao A, Ramakrishnan S. Indirect assessment of hydroxymethyl glutaryl CoA reductase (NAOPH) activity in liver tissue. Clin Chem. 1975;21:1523. [PubMed] [Google Scholar]
- 20.Chitra V, Leelamma S. Hypolipidemic effect of coriander seeds (Coriandrumsativum): mechanism of action. Plant Foods Hum Nutr. 1997;51:167–172. doi: 10.1023/A:1007975430328. [DOI] [PubMed] [Google Scholar]
- 21.Ahmad-Raus R, Abdul-Latif ES, Mohammad J. Lowering of lipid composition in aorta of guinea pigs by Curcuma domestica. BMC Complement Altern Med. 2001;1:6. doi: 10.1186/1472-6882-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schultz A. Eine methode des mikrochemischen cholesterinnachweises am gewebsschnitt. Zentrabi Allgem Pathol. 1924;35:314–318. [Google Scholar]
- 23.Phil-Sun O, Sei-Jung L, Kye-Taek L. Hypolipidemic and antiobedetive of the plant glycoprotein (36 kDa) from the Rhus vernicufulia stokes fruit in Triton WR-1339 induced hyperlipidemic rats. Biosci Biotechnol Biochem. 2006;70(2):447. doi: 10.1271/bbb.70.447. [DOI] [PubMed] [Google Scholar]
- 24.Kannel WB, Castelli CW, Gordon T, McNamara PM. Serum cholesterol, lipoproteins, and the risk of coronary heart disease. Ann Intern Med. 1971;74:1. doi: 10.7326/0003-4819-74-1-1. [DOI] [PubMed] [Google Scholar]
- 25.Bordia A, Verma SK. Effect of Celastrus Paniculatus wild oil (Bravobol) on blood lipids in patients of coronary. Antiseptic. 1998;95(4):112. [Google Scholar]
- 26.Francesca A, Nicola B, Raffaele C, Domenico M, Angelo A, Simona D, Francesco A. A new sesquiterpenes with intestinal relaxant effect from Celastrus paniculatus. Planta Med. 2004;70:652. doi: 10.1055/s-2004-827190. [DOI] [PubMed] [Google Scholar]
- 27.Patil KS, Jayaprakash S. Effect of Celastrus paniculatus Willd. seed on adjuvant induced arthritis in rats. Pharmacogn Mag. 2007;3:177. [Google Scholar]
- 28.Nalina K, Aroor AR, Kumar KB, Rao A. Studies on biogenic amines and their metabolites in mentally retarded children in Celastrus oil therapy. Altern Med. 1986;1:355. [Google Scholar]