Abstract
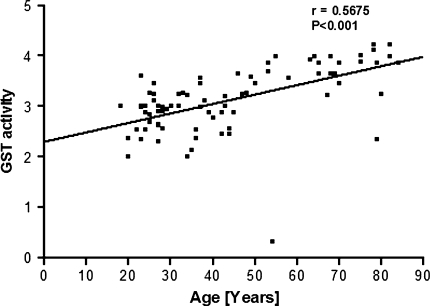
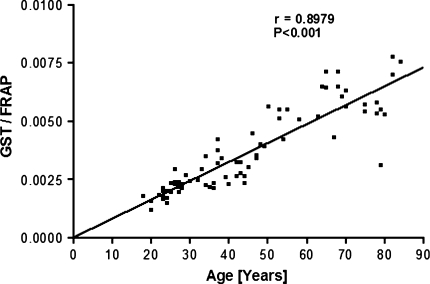
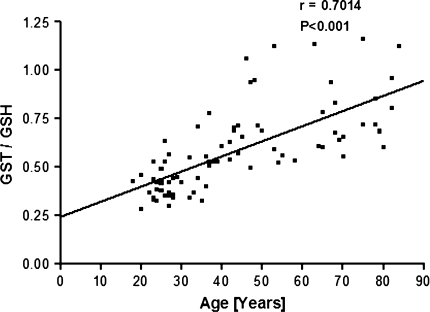
The correlation between antioxidant capacity and oxidative damage during aging has been reported in several tissues in different species. Glutathione-S-transferases (GST) can metabolise endogenous and exogenous toxins and carcinogens by catalysing the conjugation of diverse electrophiles with reduced glutathione (GSH). We observe a significant (P < 0.001) increase in plasma GST activity as a function of human age (r = 0.5675). A significant (P < 0.001) positive correlation (r = 0.8979) is observed between GST activity and total plasma antioxidant potential measured as ferric reducing ability of the plasma (FRAP). GST activity and red cell intracellular GSH also show a significant positive correlation (r = 0.7014). We hypothesize that the increased activity of plasma GST is a manifestation of increased generation of ROS and a concomitant decrease in the level of plasma antioxidant capacity during aging.
Keywords: Glutathione-S-transferase, Human aging, Oxidative stress
Introduction
Oxidative stress results when production of reactive oxidative species (ROS) exceeds the capacity of cellular antioxidant defenses to remove these toxic species [1, 2]. The correlation between antioxidant capacity and oxidative damage during aging has been reported in several tissues in different species [3–5], however data on changes of oxidative stress markers in plasma and erythrocytes of healthy populations during aging are few and sometimes contradictory [6–8]. Recently we have reported a significant age dependent decline in plasma antioxidant capacity, measured in terms of Ferric Reducing Ability of the Plasma (FRAP) values [9]. Since decrease in oxidative damage is related to dietary intake of antioxidants [10], it is important to study the correlation between antioxidant capacity of the plasma and markers of oxidative stress in different populations.
The glutathione-S-transferases (GST) can metabolise endogenous and exogenous toxins and carcinogens by catalysing the conjugation of diverse electrophiles with reduced glutathione (GSH). In the present study we report the age-dependent alteration in the activity of plasma GST in Indian population, we also correlate this parameter with total plasma antioxidant potential measured in terms of FRAP values [9] and with red cell reduced glutathione (GSH) which has already been reported to decrease as a function of human age [11].
Materials and Methods
The study was carried out on 80 normal healthy subjects of both sexes between the ages of 18–85 years. The subjects were screened for diabetes mellitus, asthma, tuberculosis or any other major illness. None of the subjects were smokers or were taking any medication. All persons gave their informed consent for the use of their blood samples for the study. The protocol of study was in conformity with the guidelines of the Institutional Ethical Committee.
Human venous blood from different healthy volunteers was obtained by venipuncture in heparin. The blood was centrifuged at 1,800 × g for 10 min at 4°C. After removal of plasma, buffy coat and upper 15% of the packed red blood cells, the RBC were washed twice with cold PBS (0.9% NaCl, 10 mM Na2HPO4, pH 7.4).
GST activity in plasma was assayed using 1-chloro-2,4-dinitrobenzene (CDNB) as substrate [12]. The Ferric Reducing Ability of Plasma (FRAP) values were determined following the method of Benzie and Strain [13]. Erythrocyte MDA was measured according to the method of Esterbauer and Cheeseman [14].
Plasma protein was estimated following the method of Lowry et al. [15].
Statistical analyses were performed using the software PRISM 4. Relationships between various parameters were assessed using Pearson correlation coefficient (r).
Results and Discussion
Glutathione-S-transferases (GSTs) are crucial enzymes in the cell detoxification process catalyzing the nucleophilic attack of glutathione (GSH) on toxic electrophilic substrates and producing a less dangerous compounds. Studies on GSTs are of great importance since they have been implicated in the development of drug resistance in tumoral cells and are related to human age related diseases such as Parkinson’s, Alzheimer’s, atherosclerosis, liver cirrhosis, aging and cataract formation [16, 17]. We have previously reported increased activity of superoxide dismutase (SOD) and catalse (CAT) and decrease in GSH as a function of human age which have been explained on the basis of increased oxidative stress in higher age groups [11, 18].
We observe a significant (P < 0.001) positive correlation (r = 0.5675) between the activity of plasma glutathione-S-transferase (GST) and human age (Fig. 1). As already reported earlier, we observed a decrease in the antioxidant capacity of plasma measured in terms of FRAP values and a decrease in erythrocyte intracellular GSH as a function of human age [9, 11]. To analyse the correlation of plasma GST activity with plasma antioxidant capacity, we plot a quotient: GST activity/FRAP as a function of human age (Fig. 2). The plot shows a strong positive correlation (r = 0.8979) between GST activity and total plasma antioxidant potential. We also report strong positive correlation (r = 0.7014) between GST and red cell reduced glutathione by plotting quotient of GST/GSH as a function of human age (Fig. 3). Our results thus confirm that human aging is associated with increase in the activity of GST which correlates with decrease in antioxidant potential and GSH. The increased activity of GST could be due to a compensatory mechanism in response to increased oxidative stress. The increased activity of GST is a manifestation of increased generation of ROS as a function of age in humans.
Fig. 1.
Glutathione-S-transferase (GST) activity plotted as a function of human age. GST activity is expressed as U/mg protein
Fig. 2.
Plot of quotient (GST/FRAP values) as a function of human age. FRAP (Ferric Reducing Ability of the Plasma) values expressed as μmol Fe(II) per liter of plasma. GST activity is expressed as U/mg protein
Fig. 3.
Plot of quotient (GST/GSH values) as a function of human age. GSH is expressed as mg/ml PRBC (packed red blood cells). GST activity is expressed as U/mg protein
GST shows many important functions in mammals including detoxification, catalysis, metabolic associated functions, drug resistance relation and inhibition and in age related diseases [19, 20]. It has been reported that GST activity increase in conditions of stress [21]. Viewed in conjunction with our previous findings of age-dependent increase in activity of plasma SOD and CAT, and decreased antioxidant potential and GSH, we hypothesize that the human body has inherent compensatory mechanisms against oxidative stress, however, this capacity gets overwhelmed during aging. Our findings also emphasize the need to establish age-dependent reference values for GST involving their role in age related and different disease conditions.
Acknowledgments
This research work was supported by the University Grants Commission, New Delhi through a research grant (F 31-292/2005 SR).
References
- 1.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 2.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 3.Inal ME, Kanbak G, Sunal E. Antioxidant enzyme activities and malondialdehyde levels related to aging. Clin Chim Acta. 2001;305:75–80. doi: 10.1016/S0009-8981(00)00422-8. [DOI] [PubMed] [Google Scholar]
- 4.Melov S. Animals models of oxidative stress, aging, and therapeutic antioxidant interventions. Int J Biochem Cell Biol. 2002;34:1395–1400. doi: 10.1016/S1357-2725(02)00086-9. [DOI] [PubMed] [Google Scholar]
- 5.Barja G. Rate of generation of oxidative stress-related damage and animal longevity. Free Radic Biol Med. 2002;33:1167–1172. doi: 10.1016/S0891-5849(02)00910-3. [DOI] [PubMed] [Google Scholar]
- 6.Bonnefont-Rousselot D, Therond P, Beaudeux JL, Peynet J, Legrand A, Delattre J. Aging and oxidative stress. Which potential markers? Ann Biol Clin. 2001;59:453–459. [PubMed] [Google Scholar]
- 7.Stadtman ER. Importance of individuality in oxidative stress and aging. Free Radic Biol Med. 2002;33:597–604. doi: 10.1016/S0891-5849(02)00904-8. [DOI] [PubMed] [Google Scholar]
- 8.Winklhofer-Roob BM, Meinitzer A, Maritschnegg M, Roob JM, Khoschsorur G, Ribalta J, Sundl I, Wuga S, Wonisch W, Tiran B, Rock E, VITAGE Study Group Effects of vitamin E depletion/repletion on biomarkers of oxidative stress in health aging. Ann NY Acad Sci. 2004;1031:361–364. doi: 10.1196/annals.1331.045. [DOI] [PubMed] [Google Scholar]
- 9.Rizvi SI, Jha R, Maurya PK. Erythrocyte plasma membrane redox system in human aging. Rejuvenation Res. 2006;9:474–490. doi: 10.1089/rej.2006.9.470. [DOI] [PubMed] [Google Scholar]
- 10.Maurya PK, Rizvi SI. Protective role of tea catechins on erythrocytes subjected to oxidative stress during human aging. Nat Prod Res. 2008;14:1–8. doi: 10.1080/14786410802267643. [DOI] [PubMed] [Google Scholar]
- 11.Rizvi SI, Maurya PK. Markers of oxidative stress in erythrocytes during aging in humans. Ann NY Acad Sci. 2007;1100:373–382. doi: 10.1196/annals.1395.041. [DOI] [PubMed] [Google Scholar]
- 12.Habig WH, Pabst MN, Jakoby WB. Glutathione-S-transferase. The first enzymatic step in mercaptopunc acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 13.Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: the FRAP assay. Anal Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 14.Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malondialdehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407–413. doi: 10.1016/0076-6879(90)86134-H. [DOI] [PubMed] [Google Scholar]
- 15.Lowry OH, Rosenbrough NJ, Farr AL, et al. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 16.Perez-Pastene C, Graumann R, Díaz-Grez F, Miranda M, Venegas P, Godoy OT, Layson L, Villagra R, Matamala JM, Herrera L, Segura-Aguilar J. Association of GST M1 null polymorphism with Parkinson’s disease in a Chilean population with a strong Amerindian genetic component. Neurosci Lett. 2007;418(2):181–185. doi: 10.1016/j.neulet.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 17.Tchaikovskaya T, Fraifeld V, Urphanishvili T, Andorfer JH, Davies P, Listowsky I. Glutathione S-transferase hGSTM3 and ageing-associated neurodegeneration: relationship to Alzheimer’s disease. Mech Ageing Dev. 2005;126(2):309–315. doi: 10.1016/j.mad.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 18.Rizvi SI, Maurya PK. Alterations in antioxidant enzymes during aging in humans. Mol Biotechnol. 2007;27:58–61. doi: 10.1007/s12033-007-0048-7. [DOI] [PubMed] [Google Scholar]
- 19.Zimniak P. Detoxification reactions: relevance to aging. Ageing Res Rev. 2008;7(4):281–300. doi: 10.1016/j.arr.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dourado DF, Fernandes PA, Ramos MJ. Mammalian cytosolic glutathione transferases. Curr Protein Pept Sci. 2008;9(4):325–337. doi: 10.2174/138920308785132677. [DOI] [PubMed] [Google Scholar]
- 21.Goncharova ND, Shmaliy AV, Marenin VY, Smelkova SA. Hypothalamic-pituitary-adrenal system and enzymes of the glutathione-dependent antioxidant system during stress and aging. Bull Exp Biol Med. 2007;144(5):730–733. doi: 10.1007/s10517-007-0418-y. [DOI] [PubMed] [Google Scholar]