Abstract
The effect of Emblica officinalis fruit extract (EFE) against alcohol-induced hepatic damage in rats was investigated in the present study. In vitro studies showed that EFE possesses antioxidant as well nitric oxide (NO) scavenging activity. In vivo administration of alcohol (5 g/kg b.wt/day) for 60 days resulted increased liver lipid peroxidation, protein carbonyls, nitrite plus nitrate levels. Alcohol administration also significantly lowers the activities of superoxide dismutase, catalase, glutathione peroxidase, glutathione S-transferase and reduced glutathione as compared with control rats. Administration of EFE (250 mg/kg body weight) to alcoholic rats significantly brought the plasma enzymes towards near normal level and also significantly reduced the levels of lipid peroxidation, protein carbonyls and restored the enzymic and non-enzymatic antioxidants level. This observation was supplemented by histopathological examination in liver. Our data indicate that the tannoid, flavonoid and NO scavenging compounds present in EFE may offer protection against free radical mediated oxidative stress in rat hepatocytes of animals with alcohol-induced liver injury.
Keywords: Alcohol, Antioxidant enzymes, Emblica officinalis, Hepatotoxicity, Oxidative stress
Introduction
Enhanced oxidative stress, decreased antioxidant status and nitrosative stress appear to be chiefly responsible for alcoholic liver damage [1]. The close relation between ethanol and liver damage is mainly due to the fact that 80% of ingested alcohol is metabolized in the liver. During ethanol metabolism many reactive oxygen species are generated via cytochrome P450 2E1 [2]. Nitric oxide (NO) is an important mediator of many physiological functions, and its role in the pathogenesis of many diseases is gaining recognition [3]. Chronic alcohol consumption increases NO level and may lead to toxicity by peroxynitrite, a potent oxidant [4]. Overproduction of reactive nitrogen species/reactive oxygen species (RNS/ROS) may occur when its generation in a system exceeds the systems ability to neutralize and eliminate them.
In general ethanol toxicity, with its many complications at a time, affects different organs causing several disorders. The multiple pathogenicity of alcoholism demands multiple modes of therapeutic approach to fight against such problems by modulating enzyme activities, metabolism, receptor functioning, signal transduction machinery and scavenging free radicals at various levels [5]. The phytochemicals in plant extracts from different traditional plants exhibit such multiple activities. It has been found that compounds in their natural formulations are more active than their isolated form [6]. Medicinal plants that are commonly included in Ayurvedic recipes for liver ailments have recently drawn much attention, as they seem to have hepatoprotective activities and there is no reliable hepatoprotective drug available in modern medicine. Hence there has been a search for safer natural dietary supplementation of native plant extracts containing several principles for therapeutic purpose with multiple targets for treating multiple pathologies of alcoholism.
Emblica officinalis Gaertn (commonly known in India as Amla, Syn. Phyllanthus emblica L.; Family: Euphorbiaceae) is regarded as “one of the best rejuvenating herbs” in the Ayurveda: an Indian traditional medicinal science. Emblica officinalis extract contains several antioxidants such as emblicanin A and B, gallic acid, ellagic acid, ascorbic acid that possesses strong antioxidative activity [7, 8]. The fruit extract has many pharmacological activities for the treatment of a number of diseases and is a constituent of many hepatoprotective formulations [9]. Several recent reports revealed that fruit extract of Emblica officinalis protect against radiation [10], antiatherosclerosis [11], possess antidiabetic activity [12, 13], inhibits aging process [14], gastroprotective [15], cytoprotective and immunomodulatory [16]. Despite its extensive medicinal use no information is available related to its use on alcohol-induced hepatotoxicity. Hence the present work investigates the therapeutic efficacy of Emblica officinalis fruit extract (EFE) to treat alcohol-induced hepatic damage with an emphasis on its mechanism.
Materials and Methods
Chemicals
Phenylmethylsulfonyl fluoride, 2,2′-azinobis-(3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS), NADH, Thiobarbituric acid, butylated hydroxyl toluene (BHT) and deoxycholate, used in the present study were procured from Sigma Chemical Co. (St. Louis, MO, USA). Ethanol used for administration to rats was obtained by re-distillation. An aqueous EFE dry powder (90.8% water soluble extractives including 49.5% tannins) was obtained from Chemiloids Ltd, Vijayawada, India (Manufacturers and exporters of herbal extracts). A 5% EFE solution was prepared in water prior to administration.
Experimental Design
Two month old male albino Wistar rats, weighing about 120–140 g, were maintained in our animal house. They were fed with commercial pellet diet (Hindustan Lever Ltd, Bangalore, India) and tap water ad libitum. The animals were divided into four groups of eight rats in each group. Group I control rats (C), which received glucose instead of alcohol (i.e., caloric equivalent to alcohol), group II alcohol treated rats (A), which received 20% (v/v) alcohol in water, administered through stomach tube (5 g/kg body weight/day), group III (A + E) rats received aqueous Emblica fruit extract (250 mg/kg body weight) and then 20% (v/v) alcohol in water (5 g/kg body weight/day) after 8 h, group IV (E) rats received aqueous Emblica fruit extract (250 mg/kg body weight) plus glucose (iso-caloric equivalent to ethanol). The dose of the EFE in the present study was fixed based on our earlier studies [17, 18]. The extract and alcohol was administered as aqueous solution using intragastric tube daily for 60 days. Food and water intake of all the animals was recorded daily and weight of rats was followed on alternate days. At the end of the experimental period, the rats in each group were fasted overnight and then killed by cervical dislocation.
In Vitro Assays
Antiradical Activity Against ABTS+
The total antioxidant capacity was assessed based on the ability of a compound to scavenge the stable ABTS radical (decolourisation assay) was performed using procedure of Miller et al. [19]. ABTS+ generated by oxidation of ABTS+ (1.8 mM) with potassium persulfate (2 mM). The reaction mixture contained ABTS (0.002 M), EFE (50–1000 μg) and buffer in a total volume of 3.5 ml. The absorbance was measured at 734 nm in a UV–visible spectrophotometer. IC50 value in the concentration of sample required scavenging 50% ABTS free radical.
Nitric Oxide Scavenging Activity
NO generated from sodium nitroprusside was measured by the Greiss reaction [20]. Scavengers of NO compete with oxygen leading to reduced production of NO. Sodium nitroprusside (5 mM) in phosphate buffered saline was mixed with different concentrations of EFE (25–1000 μg) and incubated at 25°C for 150 min. The samples from the above were reacted with Greiss reagent (1% sulfanilamide, 5% o-phosphoric acid and 0.01% naphthylethylene diamine). The absorbance of the chromophore formed during diazotization of nitrite with sulfanilamide and subsequent coupling with napthylethylene diamine was read at 546 nm in a UV–visible spectrophotometer.
In Vivo Assays
The liver tissues were dissected out, weighed and washed using ice cold saline solution. Tissues were minced and homogenized (10% w/v) in Tris–HCl buffer (0.1 M; pH 7.4) and centrifuged at 10,000×g for 20 min at 4°C. The resulting supernatant was used for various biochemical assays.
Determination of Thiobarbituric Acid Reactive Substances (TBARS) and Protein Carbonyls
TBARS was measured by the formation of Malondialdehyde (MDA) by using the method of Ohkawa et al. [21]. The concentration of protein carbonyls was determined using 2,4-dinitrophenylhydrazine (DNPH) assay as described previously [22].
Determination of Antioxidant Status, Nitrite and Nitrate Analyses
Catalase (CAT) was assayed as described by Abei [23]. The CAT activity was expressed as nmol H2O2 decreased/mg protein/min. Superoxide dismutase (SOD) was assayed utilized the technique of Mishra and Fridovich [24]. A single unite of enzyme was expressed as 50% inhibition of NBT (nitro blue tetrazolium) reduction/min/mg protein. Glutathione peroxidase (GPx) activity was measured by the method described by Rotruk [25]. GPx activity was expressed μmol GSH oxidized/min/mg protein. Glutathione S-transferase (GST) activity was measured according to the method of Habig et al. [26]. GST activity expressed as μmol CDNB utilized/min/mg protein. Total reduced glutathione (GSH) content was measured by the method described by Ellman’s [27]. Nitrite and nitrates were determined by the method adopted by Sastry et al. [28]. Protein concentration was determined by the method of Lowry et al. [29].
Histopathological Examination
A portion of the median lobe of the liver was dissected and fixed in 10% neutral buffered formalin solution for 24 h. The remaining livers were frozen quickly in dry ice and stored at −80°C for biochemical analysis. The fixed tissues were processed routinely, and were then embedded in paraffin, sectioned to 3–5 μm thickness, deparaffinized, and rehydrated using standard techniques. The extent of alcohol-induced necrosis and steatosis was evaluated by assessing morphological changes in liver sections stained with hematoxylin and eosin (H&E), using standard techniques.
Statistical Analysis
Results were expressed as mean ± SD of eight rats in each group. The data were analyzed by an analysis of variance (ANOVA) followed by Duncan’s multiple range test. Significance was evaluated at P < 0.05.
Results
The total antioxidant activity was measured using the ABTS assay. Inhibition of the ABTS radical showed dose-dependent (50–1000 μg) scavenging activity, similarly, EFE also showed dose-dependent scavenging of NO up to 500 μg, after which the elevation in NO scavenging almost reached a steady state. However, the highest scavenging activity was observed at 500 μg (Table 1).
Table 1.
In vitro ABTS radical and nitric oxide scavenging activity of EFE
| EFE concentration (μg) | ABTS (%) | Nitric oxide (%) |
|---|---|---|
| 25 | nd | 2.90 ± 0.4 |
| 50 | 4.8 ± 0.5 | 6.1 ± 1.3 |
| 100 | 17.2 ± 3.1 | nd |
| 150 | nd | 11.5 ± 1.8 |
| 250 | 33.5 ± 3.4 | 19.3 ± 2.1 |
| 500 | 52.9 ± 3.8 | 24.5 ± 2.1 |
| 750 | 72.1 ± 2.8 | nd |
| 1000 | 84.3 ± 3.1 | 25.1 ± 2.2 |
Data are mean ± SD of three independent experiments
nd not determined
Table 2 shows the levels of TBARS, protein carbonyls and NO levels in liver of control and experimental rats. A significant (P < 0.05) elevation in the levels of TBARS and protein carbonyls were observed in alcoholic rats. Administration of EFE along with ethanol caused a significant (P < 0.05) decreased in the level TBARS and protein carbonyls when compared with alcohol-administered rats. Nitrite and nitrate levels are considered to be reliable indicators of NO generation. Increased concentrations of nitrite and nitrate levels of alcoholic group rats suggested overproduction of NO when compared to other groups. Administration of EFE and ethanol decreased the levels of nitrite and nitrate significantly (P < 0.05).
Table 2.
Effect of EFE administration on hepatic lipid peroxidation, protein carbonyls and nitrite/nitrate levels in alcohol treated rats
| Groups | TBARS | Protein carbonyls | Nitrite/nitrates |
|---|---|---|---|
| Controls | 1.31 ± 0.18c | 0.64 ± 0.07c | 3.42 ± 0.21b |
| Alcohol | 2.81 ± 0.29a | 1.53 ± 0.12a | 6.62 ± 0.53a |
| A + EFE | 1.7 ± 0.22b | 0.97 ± 0.14b | 3.7 ± 0.34b |
| EFE | 1.29 ± 0.2c | 0.59 ± 0.08c | 3.26 ± 0.25b |
Data are mean ± SD values of eight rats per group. Means in the same row not sharing a common superscript are significantly different (P < 0.05). All the values are expressed as nmol/mg protein
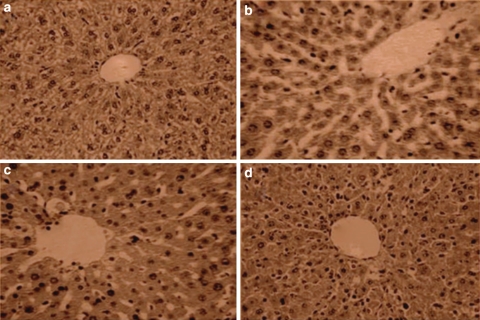
The liver samples of alcohol-administered rats showed the focal hepatocytes damage and degeneration (Fig. 1b). The administration of alcohol along with EFE showed near normal appearance of hepatocytes (Fig. 1c). The control (Fig. 1a) and EFE (Fig. 1d) alone treated rats showed normal appearance of liver.
Fig. 1.
Representative photomicrographs of livers in different experimental groups. Liver sections were fixed in 10% buffered formalin, embedded in paraffin, and sectioned and stained with hematoxylin–eosin. Liver sections from a control rats showed no signs of pathological changes in hepatocytes. b Alcohol-administered rat hepatocytes showed fibrosis, atrophy and nucleus is degenerated, whereas d EFE alone administered rat hepatocytes showed control rat signs (original magnification, ×100)
The activities of antioxidant enzymes namely SOD, CAT, GPx, GST and the content of GSH in liver were given in Table 3. A significant (P < 0.05) decrease in the activities of enzymatic antioxidants was observed in alcohol treated rats. Administration of EFE to ethanol treated rats significantly (P < 0.05) increased the activities of enzymatic antioxidants when compared to ethanol treated rats (Table 3).
Table 3.
Effect of EEE administration on hepatic CAT, SOD, GPx, GST activities and GSH content in rats
| Group | CAT (U/mg protein/min) | SOD (U/mg protein/min) | GPx (μmol of GSH oxidized/min/protein) | GST (μmol CDNB utilized/min/mg protein) | GSH (μmol/mg protein) |
|---|---|---|---|---|---|
| Controls | 39.64 ± 1.17a | 22.02 ± 1.46a | 9.44 ± 0.92a | 1.26 ± 0.06a | 6.47 ± 0.24a |
| Alcohol | 26.42 ± 2.51c | 14.14 ± 1.57b | 4.41 ± 0.51b | 0.71 ± 0.08c | 3.38 ± 0.19b |
| A + EFE | 35.11 ± 1.35b | 21.27 ± 1.07a | 8.58 ± 0.56a | 1.07 ± 0.07b | 6.20 ± 0.24a |
| EEE | 40.51 ± 1.13a | 23.15 ± 1.36a | 8.97 ± 0.67a | 1.21 ± 0.06a | 6.53 ± 0.17a |
Values are mean ± SD of eight rats per group. Means in the same column not sharing a common superscript letter are significantly different at P < 0.05 (DMRT)
Discussion
The aqueous Emblica fruit extract used in the present study contain considerable amount of tannoids and polyphenolic compounds. The antioxidant activity of the extract was assessed on the basis of the radical scavenging effect on the stable ABTS+ (cation radical) free radical. A wide variety of in vitro methods used to assess radical scavenging ability of certain agents from natural and synthetic source [30]. It can be observed that the content of flavonoids and tannoid compounds of EFE could be responsible for the radical scavenging activity. Numerous studies indicate that free radicals or reactive oxygen species such as a hydroxy ethyl radical, superoxide and hydroxy radicals are responsible for ethanol induced oxidative stress. All these radicals formed from the ethanol mediated process have a great potential to react rapidly with lipids and proteins, which in turn leads to peroxidation of lipids and proteins [31]. In the present study, scavenging of free radicals may be one of the important mechanisms of protection against alcohol-induced hepatic damage by EFE, which is evident from the dose-dependent in vitro scavenging of ABTS radicals. It is known that tannoid compounds found in Emblica officinalis fruit had inhibitory effect of lipid peroxidation by its free radical scavenging nature [32]. The tannoids found in EFE such as emblicanin A and B might have possess the capability to prevent the Fe2+, Cu2+ induced lipid peroxidation. Since the transition metals play a central role in lipid peroxidation process [33], our results obliviously indicate that EFE has radical scavenging activity and inhibit the lipid and protein oxidation in alcohol toxicity.
The increased formation of lipid peroxides and associated reactive oxygen species leads to liver damage, membrane integrity and other pathological changes. Our previous findings revealed increased levels of serum enzymes such as AST, ALT, ALP and γGT in alcohol treated rats [17], indicate that increased permeability and damage and/or necrosis of hepatocytes as evidenced by histological observations [34]. Administration of EFE to alcohol treated rats improved the histomorphology of the liver near to normal. The histological changes were also in correlation with the biochemical findings. The EFE administrated rats indicate the hepatoprotective effect as well preserves the structural integrity of the liver from the adverse effects of alcohol.
Higher liver nitrite/nitrate levels in this study indicating increased production of NO in response to chronic alcohol administration via induction of inducible nitric oxide synthase (iNOS) and agreement with earlier studies [35, 36]. NO and its metabolite peroxynitrite (ONOO−) cross cell membranes through anion channels, that produces nitration of tyrosine and inactivation of biologically important proteins and enzymes [3]. EFE administration to ethanolic rats decreased the levels of nitrite/nitrate significantly compared to ethanolic group. We observed decreased levels of nitrites/nitrates in alcoholic rats receiving EFE, might be mainly due to inhibition of the hepatic cytosolic iNOS enzyme activity by EFE [14]. It is evident from earlier studies that inhibition of nitric oxide synthase can be employed as a potential therapeutic target [37]. Amelioration of the nitrosative stress by EFE fruit extract is due to the various therapeutic compounds present in EFE, which has NO scavenging effects as well inhibitory effects of iNOS activity.
Free radical scavenging enzymes such as SOD, CAT, GPx and GST are the first line of defense against oxidative injury. SOD scavenges superoxide ions and catalase converts H2O2 to water. In this study decreased activities of CAT, SOD and GPx as well GSH in alcohol treated rats were observed. The decreased SOD activity could be due to the oxidative inactivation of the enzyme as a result of excessive reactive oxygen species generation [38]. The GSTs are a multigene family of isozymes that catalyze the conjugation of GSH to a variety of electrophilic compounds, and thereby exert a critical role in cellular protection against ROS [39, 40]. The detoxification of 4-hydroxynonenal, a toxic aldehyde and a product of lipid peroxidation is compromised when GST activity is reduced. Thus, ethanol or its metabolic products might specifically target GST isoenzymes and the reduction in enzyme activity or expression may contribute to ethanol hepatotoxicity [41]. GSH is a major non-protein thiol and plays a central role in coordinating the antioxidant defense process. It is involved in the maintenance of normal cell structure and function through its redox and detoxification reactions [42]. GSH in association with GPx metabolizes hydrogen peroxide to water, thereby protecting mammalian cells against oxidative damage. Peroxynitrite interacts with biomolecules causing well-known peroxynitrite-dependent cytotoxicity, which is more severe in GSH depletion and decreased antioxidant status [3]. Surprisingly activities of all these defense enzymes and level of GSH in liver were markedly elevated in ethanolic rats receiving EFE. As reported by Bhattacharya et al., the major tannoid principles emblicanin A, emblicanin B, punigluconin and pedunculagin present in Emblica might have contributed largely for the observed hike in antioxidant status [43].
In conclusion, our study revealed that EFE has shown protective action against alcohol-induced hepatic injury by ameliorating oxidative stress in rats as evidenced by liver histopathological studies, lowered lipid peroxidation, NO levels and elevated antioxidant status. The tannins, flavonoids present in EFE could be contributed for the above mechanism.
References
- 1.Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol Med. 2008;44:723–738. doi: 10.1016/j.freeradbiomed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tuma DJ, Casey CA. Dangerous byproducts of alcohol breakdown—focus on adducts. Alcohol Res Health. 2003;27:285–290. [PMC free article] [PubMed] [Google Scholar]
- 3.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Venkatraman A, Shiva S, Wigley A, Ulasova E, Shhieng D, Bailey SM, et al. The role of iNOS in alcohol-dependent hepatotoxicity and mitochondrial dysfunction in mice. Hepatology. 2004;40:565–573. doi: 10.1002/hep.20326. [DOI] [PubMed] [Google Scholar]
- 5.Xu BJ, Zheng YN, Sung CK. Natural medicines for alcoholism treatment: a review. Drug Alcohol Rev. 2005;24:525–536. doi: 10.1080/09595230500293795. [DOI] [PubMed] [Google Scholar]
- 6.Khopde SM, Indira Priyadarsini K, Mohan H, Gawandi VB, Satav JG, Yakhmi JV, et al. Characterizing the antioxidant activity of amla (Phyllanthus emblica) extract. Curr Sci. 2001;81:185–190. [Google Scholar]
- 7.Pozharitskaya ON, Ivanova SA, Shikov AN, Makarov VG. Separation and evaluation of free radical-scavenging activity of phenol components of Emblica officinalis extract by using an HPTLC-DPPH* method. J Sep Sci. 2007;30:1250–1254. doi: 10.1002/jssc.200600532. [DOI] [PubMed] [Google Scholar]
- 8.Scartezzini P, Antognoni F, Raggi MA, Poli F, Sabbioni C. Vitamin C content and antioxidant activity of the fruit and of the Ayurvedic preparation Emblica officinalis Gaertn. J Ethnopharmacol. 2006;104:113–118. doi: 10.1016/j.jep.2005.08.065. [DOI] [PubMed] [Google Scholar]
- 9.De S, Ravishankar B, Bhavsar GC. Plants with hepatoprotective activity—a review. Indian Drugs. 1993;30:355–363. [Google Scholar]
- 10.Jindal A, Soyal D, Sharma A, Goyal PK. Protective effect of an extract of Emblica officinalis against radiation-induced damage in mice. Integr Cancer Ther. 2009;8:98–105. doi: 10.1177/1534735409331455. [DOI] [PubMed] [Google Scholar]
- 11.Kim HJ, Yokozawa T, Kim HY, Tohda C, Rao TP, Juneja LR. Influence of amla (Emblica officinalis Gaertn.) on hypercholesterolemia and lipid peroxidation in cholesterol-fed rats. J Nutr Sci Vitaminol Tokyo. 2005;51:413–418. doi: 10.3177/jnsv.51.413. [DOI] [PubMed] [Google Scholar]
- 12.Kusirisin W, Srichairatanakool S, Lerttrakarnnon P, Lailerd N, Suttajit M, Jaikang C, et al. Antioxidative activity, polyphenolic content and anti-glycation effect of some Thai medicinal plants traditionally used in diabetic patients. Med Chem. 2009;5:139–147. doi: 10.2174/157340609787582918. [DOI] [PubMed] [Google Scholar]
- 13.Suryanarayana P, Saraswat M, Petrash JM, Reddy GB. Emblica officinalis and its enriched tannoids delay streptozotocin-induced diabetic cataract in rats. Mol Vis. 2007;13:1291–1297. [PubMed] [Google Scholar]
- 14.Yokozawa T, Kim HY, Kim HJ, Okubo T, Chu DC, Juneja LR. Amla (Emblica officinalis Gaertn.) prevents dyslipidaemia and oxidative stress in the ageing process. Br J Nutr. 2007;97:1187–1195. doi: 10.1017/S0007114507691971. [DOI] [PubMed] [Google Scholar]
- 15.Al-Rehaily AJ, Al-Howiriny TA, Al-Sohaibani MO, Rafatullah S. Gastroprotective effects of ‘Amla’ Emblica officinalis on in vivo test models in rats. Phytomedicine. 2002;9:515–522. doi: 10.1078/09447110260573146. [DOI] [PubMed] [Google Scholar]
- 16.Sai Ram M, Neetu D, Yogesh B, Anju B, Dipti P, Pauline T, et al. Cyto-protective and immunomodulating properties of amla (Emblica officinalis) in lymphocytes: an in vitro study. J Ethnopharmacol. 2002;81:5–10. doi: 10.1016/S0378-8741(01)00421-4. [DOI] [PubMed] [Google Scholar]
- 17.Reddy VD, Padmavathi P, Varadacharyulu NC. Emblica officinalis protects against ethanol-induced liver mitochondrial dysfunction in rats. J Med Food. 2009;12:327–333. doi: 10.1089/jmf.2007.0694. [DOI] [PubMed] [Google Scholar]
- 18.Reddy VD, Padmavathi P, Paramahamsa M, Varadacharyulu NC, et al. Modulatory role of Emblica officinalis against alcohol induced biochemical and biophysical changes in rat erythrocyte membranes. Food Chem Toxicol. 2009;47:1958–1963. doi: 10.1016/j.fct.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 19.Miller NJ, Castelluccio C, Tijburg L, Rice-Evans C. The antioxidant properties of theaflavins and their gallate esters—radical scavengers or metal chelaters? FEBS Lett. 1996;392:40–44. doi: 10.1016/0014-5793(96)00780-6. [DOI] [PubMed] [Google Scholar]
- 20.Sreejayan N, Rao MNA. Nitric oxide scavenging by curcuminoids. J Pharm Pharmacol. 1997;49:105–107. doi: 10.1111/j.2042-7158.1997.tb06761.x. [DOI] [PubMed] [Google Scholar]
- 21.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 22.Reznick AZ, Packer L. Oxidative damage to proteins: spectroscopic method for carbonyl assay. Methods Enzymol. 1994;233:357–363. doi: 10.1016/S0076-6879(94)33041-7. [DOI] [PubMed] [Google Scholar]
- 23.Abei H. Catalase in vitro. Methods Enzymol. 1988;10:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 24.Mishra PH, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 25.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 26.Habig WH, Pabst MJ, Jakoby WB. Glutathione-S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 27.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 28.Sastry KVH, Moudgal RP, Mohan J, Tyagi JS, Rao GS. Spectrophotometric determination of serum nitrite and nitrate by copper-cadmium alloy. Anal Biochem. 2002;306:79–82. doi: 10.1006/abio.2002.5676. [DOI] [PubMed] [Google Scholar]
- 29.Lowry OH, Rosebrough NJ, Farr AL, Randall R. Protein measurement with the Folin-phenol reagent. J Biol Chem. 1951;193:263–275. [PubMed] [Google Scholar]
- 30.Nishizawa M, Kohno M, Nishimura M, Kitagawa A, Niwano Y. Non-reductive scavenging of 1, 1-diphenyl-2-picryl hydrazyl (DPPH) by peroxyradical: a useful method for quantitative analysis of peroxyradical. Chem Pharm Bull. 2005;53:714–716. doi: 10.1248/cpb.53.714. [DOI] [PubMed] [Google Scholar]
- 31.Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology. 2006;43:S63–S74. doi: 10.1002/hep.20957. [DOI] [PubMed] [Google Scholar]
- 32.Verma R, Chakraborty D. Emblica officinalis aqueous extract ameliorates ochratoxin-induced lipid peroxidation in the testis of mice. Acta Pol Pharm. 2008;65:187–194. [PubMed] [Google Scholar]
- 33.Angel C. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem Phys Lipids. 2009;157:1–11. doi: 10.1016/j.chemphyslip.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Sillanaukee P. Laboratory markers of alcohol abuse. Alcohol Alcohol. 1996;31:613–616. doi: 10.1093/oxfordjournals.alcalc.a008199. [DOI] [PubMed] [Google Scholar]
- 35.Wang JF, Greenberg SS, Spitzer JJ. Chronic alcohol administration stimulates nitric oxide formation in the rat liver with or without pretreatment by lipopolysaccharide. Alcohol Clin Exp Res. 1995;19:387–393. doi: 10.1111/j.1530-0277.1995.tb01520.x. [DOI] [PubMed] [Google Scholar]
- 36.Yuan GJ, Zhou XR, Gong ZJ, Zhang P, Sun XM, Zheng SH. Expression and activity of inducible nitric oxide synthase and endothelial nitric oxide synthase correlate with ethanol-induced liver injury. World J Gastroenterol. 2006;12:2375–2381. doi: 10.3748/wjg.v12.i15.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seitz HK, Salaspuro M, Savolainen M, Haber P, Ishii H, Teschke R, et al. From alcohol toxicity to treatment. Alcohol Clin Exp Res. 2005;29:1341–1350. doi: 10.1111/j.1530-0277.2005.tb03472.x. [DOI] [PubMed] [Google Scholar]
- 38.Pigeolot E, Corbisier P, Houbion A, Lambert D, Michiels C, Raes M. Glutathione peroxidase, superoxide dismutase and catalase inactivation by peroxide and oxygen derived radicals. Mech Ageing Dev. 1990;51:283–297. doi: 10.1016/0047-6374(90)90078-T. [DOI] [PubMed] [Google Scholar]
- 39.Wilce MC, Parker MW. Structure and function of glutathione s-transferases. Biochim Biophys Acta. 1994;1205:1–18. doi: 10.1016/0167-4838(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 40.Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- 41.Alin P, Danielson UH, Mannervik B. 4-Hydroxyalk-2-enals are substrates for glutathione transferase. FEBS Lett. 1985;179:267–270. doi: 10.1016/0014-5793(85)80532-9. [DOI] [PubMed] [Google Scholar]
- 42.Gueeri H. Influence on prolonged ethanol intake on the level and turnover of alcohol and aldehyde dehydrogenase and glutathione. Adv Exp Med Biol. 1995;23:12–14. doi: 10.1007/978-1-4684-3632-7_27. [DOI] [PubMed] [Google Scholar]
- 43.Bhattacharya A, Ghosal S, Bhattacharya SK. Antioxidant activity of tannoid principles of Emblica officinalis (amla) in chronic stress induced changes in rat brain. Ind J Exp Biol. 2000;38:877–880. [PubMed] [Google Scholar]