Abstract
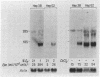
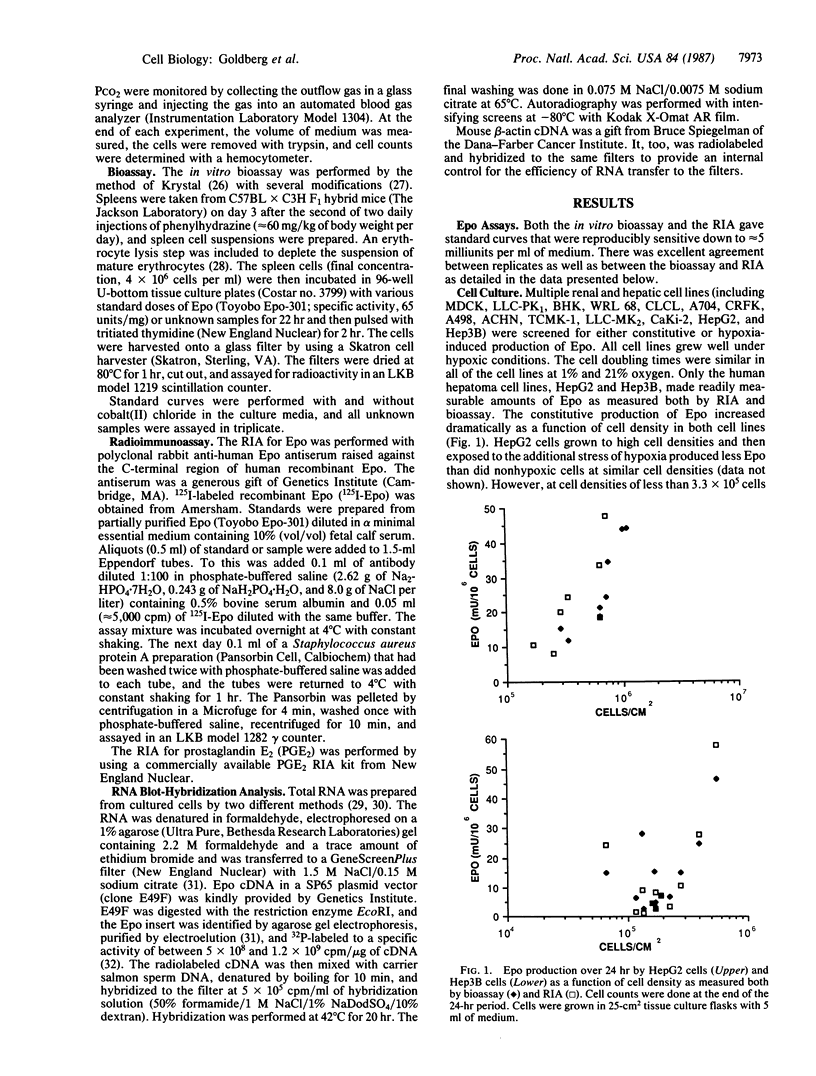
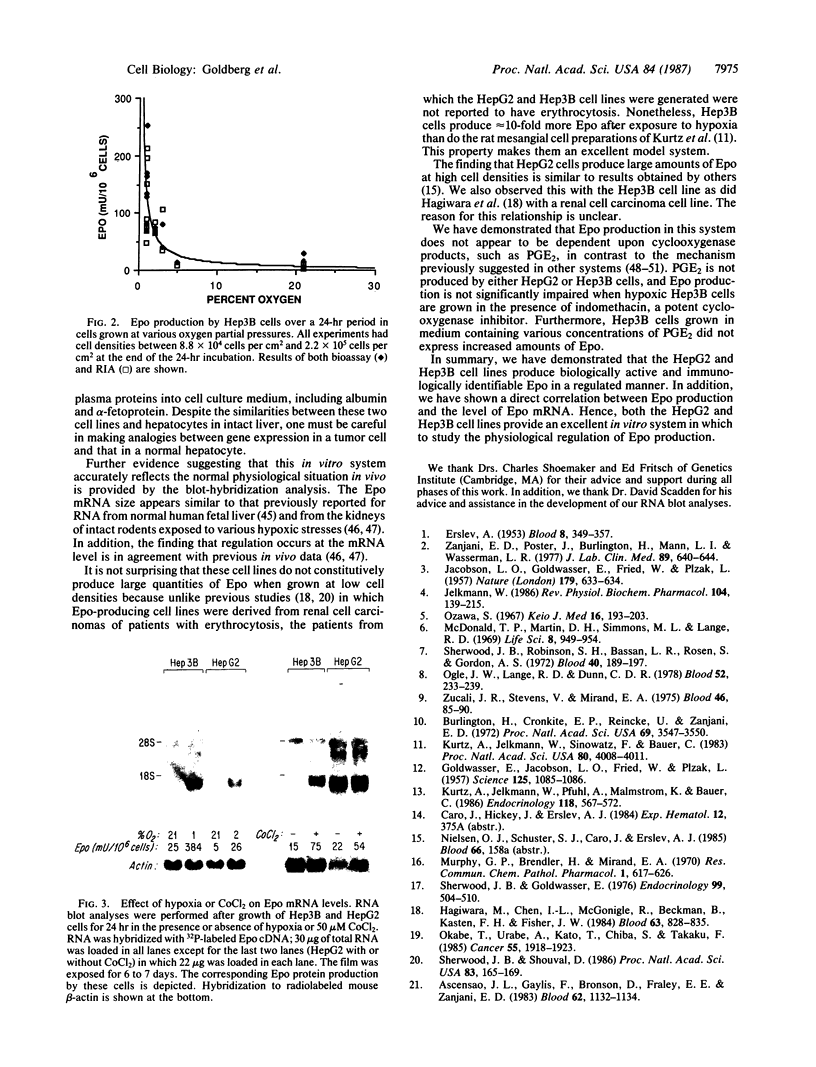
The development of a cell culture system that produces erythropoietin (Epo) in a regulated manner has been the focus of much effort. We have screened multiple renal and hepatic cell lines (including MDCK, LLC-PK1, BHK, WRL 68, CLCL, A704, CRFK, A498, ACHN, TCMK-1, LLC-MK2, CaKi-2, HepG2, and Hep3B) for either constitutive or regulated expression of Epo. Only the human hepatoma cell lines, Hep3B and HepG2, made significant amounts of Epo as measured both by radioimmunoassay and in vitro bioassay (as much as 330 milliunits per 10(6) cells in 24 hr). The constitutive production of Epo increased dramatically as a function of cell density in both cell lines. At cell densities less than 3.3 X 10(5) cells per cm2, there was little constitutive release of Epo in the medium (less than 30 milliunits per 10(6) cells in 24 hr). With Hep3B cells grown at low cell densities, a mean 18-fold increase in Epo expression was seen in response to hypoxia and a 6-fold increase was observed in response to incubation in medium containing 50 microM cobalt(II) chloride. At similar low cell densities, Epo production in HepG2 cells could be enhanced an average of about 3-fold by stimulation with either hypoxia or cobalt(II) chloride. Upon such stimulation, both cell lines demonstrated markedly elevated levels of Epo mRNA. Hence, both Hep3B and HepG2 cell lines provide an excellent in vitro system in which to study the physiological regulation of Epo expression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aden D. P., Fogel A., Plotkin S., Damjanov I., Knowles B. B. Controlled synthesis of HBsAg in a differentiated human liver carcinoma-derived cell line. Nature. 1979 Dec 6;282(5739):615–616. doi: 10.1038/282615a0. [DOI] [PubMed] [Google Scholar]
- Ascensao J. L., Gaylis F., Bronson D., Fraley E. E., Zanjani E. D. Erythropoietin production by a human testicular germ cell line. Blood. 1983 Nov;62(5):1132–1134. [PubMed] [Google Scholar]
- Beru N., McDonald J., Lacombe C., Goldwasser E. Expression of the erythropoietin gene. Mol Cell Biol. 1986 Jul;6(7):2571–2575. doi: 10.1128/mcb.6.7.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondurant M. C., Koury M. J. Anemia induces accumulation of erythropoietin mRNA in the kidney and liver. Mol Cell Biol. 1986 Jul;6(7):2731–2733. doi: 10.1128/mcb.6.7.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlington H., Cronkite E. P., Reincke U., Zanjani E. D. Erythropoietin production in cultures of goat renal glomeruli. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3547–3550. doi: 10.1073/pnas.69.12.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro J., Erslev A. J. Biologic and immunologic erythropoietin in extracts from hypoxic whole rat kidneys and in their glomerular and tubular fractions. J Lab Clin Med. 1984 Jun;103(6):922–931. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clemons G. K., Fitzsimmons S. L., DeManincor D. Immunoreactive erythropoietin concentrations in fetal and neonatal rats and the effects of hypoxia. Blood. 1986 Oct;68(4):892–899. [PubMed] [Google Scholar]
- ERSLEV A. Humoral regulation of red cell production. Blood. 1953 Apr;8(4):349–357. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- GOLDWASSER E., JACOBSON L. O., FRIED W., PLZAK L. Mechanism of the erythropoietic effect of cobalt. Science. 1957 May 31;125(3257):1085–1086. doi: 10.1126/science.125.3257.1085. [DOI] [PubMed] [Google Scholar]
- Gross D. M., Brookins J., Fink G. D., Fisher J. W. Effects of prostaglandins A2, E2 and F2 alpha on erythropoietin production. J Pharmacol Exp Ther. 1976 Aug;198(2):489–496. [PubMed] [Google Scholar]
- Gross D. M., Mujovic V. M., Jubiz W., Fisher J. W. Enhanced erythropoietin and prostaglandin E production in the dog following renal artery constriction. Proc Soc Exp Biol Med. 1976 Mar;151(3):498–501. doi: 10.3181/00379727-151-39244. [DOI] [PubMed] [Google Scholar]
- Gruber D. F., Zucali J. R., Mirand E. A. Identification of erythropoietin producing cells in fetal mouse liver cultures. Exp Hematol. 1977 Sep;5(5):392–398. [PubMed] [Google Scholar]
- Hagiwara M., Chen I. L., McGonigle R., Beckman B., Kasten F. H., Fisher J. W. Erythropoietin production in a primary culture of human renal carcinoma cells maintained in nude mice. Blood. 1984 Apr;63(4):828–835. [PubMed] [Google Scholar]
- Hagiwara M., McNamara D. B., Chen I. L., Fisher J. W. Role of endogenous prostaglandin E2 in erythropoietin production and dome formation by human renal carcinoma cells in culture. J Clin Invest. 1984 Oct;74(4):1252–1261. doi: 10.1172/JCI111535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankins W. D., Schooley J., Eastment C. Erythropoietin, an autocrine regulator? Serum-free production of erythropoietin by cloned erythroid cell lines. Blood. 1986 Jul;68(1):263–268. [PubMed] [Google Scholar]
- JACOBSON L. O., GOLDWASSER E., FRIED W., PLZAK L. Role of the kidney in erythropoiesis. Nature. 1957 Mar 23;179(4560):633–634. doi: 10.1038/179633a0. [DOI] [PubMed] [Google Scholar]
- Jacobs K., Shoemaker C., Rudersdorf R., Neill S. D., Kaufman R. J., Mufson A., Seehra J., Jones S. S., Hewick R., Fritsch E. F. Isolation and characterization of genomic and cDNA clones of human erythropoietin. 1985 Feb 28-Mar 6Nature. 313(6005):806–810. doi: 10.1038/313806a0. [DOI] [PubMed] [Google Scholar]
- Jelkmann W. Renal erythropoietin: properties and production. Rev Physiol Biochem Pharmacol. 1986;104:139–215. doi: 10.1007/BFb0031014. [DOI] [PubMed] [Google Scholar]
- Knowles B. B., Howe C. C., Aden D. P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980 Jul 25;209(4455):497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- Krystal G. A simple microassay for erythropoietin based on 3H-thymidine incorporation into spleen cells from phenylhydrazine treated mice. Exp Hematol. 1983 Aug;11(7):649–660. [PubMed] [Google Scholar]
- Kurtz A., Jelkmann W., Pfeilschifter J., Bauer C. Role of prostaglandins in hypoxia-stimulated erythropoietin production. Am J Physiol. 1985 Jul;249(1 Pt 1):C3–C8. doi: 10.1152/ajpcell.1985.249.1.C3. [DOI] [PubMed] [Google Scholar]
- Kurtz A., Jelkmann W., Pfuhl A., Malmström K., Bauer C. Erythropoietin production by fetal mouse liver cells in response to hypoxia and adenylate cyclase stimulation. Endocrinology. 1986 Feb;118(2):567–572. doi: 10.1210/endo-118-2-567. [DOI] [PubMed] [Google Scholar]
- Kurtz A., Jelkmann W., Sinowatz F., Bauer C. Renal mesangial cell cultures as a model for study of erythropoietin production. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4008–4011. doi: 10.1073/pnas.80.13.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappin T. R., Elder G. E., McKibbin S. H., McNamee P. T., McGeown M. G., Bridges J. M. The effect of transferrin saturation on the estimation of erythropoietin by the mouse spleen cell microassay. Exp Hematol. 1985 Nov;13(10):1007–1013. [PubMed] [Google Scholar]
- McDonald T. P., Martin D. H., Simmons M. L., Lange R. D. Preliminary results of erythropoietin production by bovine kidney cells in culture. Life Sci. 1969 Sep 15;8(18):949–954. doi: 10.1016/0024-3205(69)90199-4. [DOI] [PubMed] [Google Scholar]
- Murphy G. P., Brendler H., Mirand E. A. Erythropoietin release from renal cell carcinomas grown in tissue culture. Res Commun Chem Pathol Pharmacol. 1970 Sep;1(5):617–626. [PubMed] [Google Scholar]
- Naughton B. A., Birnbach D. J., Liu P., Kolks G. A., Tung M. Z., Piliero S. J., Gordon A. S. Erythropoietin (Ep) production and Kupffer cell alterations following nephrectomy, hypoxia, or combined nephrectomy and hypoxia. Proc Soc Exp Biol Med. 1979 Feb;160(2):170–174. doi: 10.3181/00379727-160-40413. [DOI] [PubMed] [Google Scholar]
- Naughton G. K., Naughton B. A., Gordon A. S. Erythropoietin production by macrophages in the regenerating liver. J Surg Oncol. 1985 Nov;30(3):184–197. doi: 10.1002/jso.2930300312. [DOI] [PubMed] [Google Scholar]
- Ogle J. W., Lange R. D., Dunn C. D. Erythropoiesis-stimulating factor production by rabbit kidney cultures from "programmed" rabbits. Blood. 1978 Jul;52(1):233–239. [PubMed] [Google Scholar]
- Okabe T., Urabe A., Kato T., Chiba S., Takaku F. Production of erythropoietin-like activity by human renal and hepatic carcinomas in cell culture. Cancer. 1985 May 1;55(9):1918–1923. doi: 10.1002/1097-0142(19850501)55:9<1918::aid-cncr2820550915>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Ozawa S. Erythropoietin from the kidney cells cultured in vitro. Keio J Med. 1967 Dec;16(4):193–203. doi: 10.2302/kjm.16.193. [DOI] [PubMed] [Google Scholar]
- Paul P., Rothmann S. A., McMahon J. T., Gordon A. S. Erythropoietin secretion by isolated rat Kupffer cells. Exp Hematol. 1984 Dec;12(11):825–830. [PubMed] [Google Scholar]
- Peschle C., Marone G., Genovese A., Magli C., Condorelli M. Hepatic erythropoietin: enhanced production in anephric rats with hyperplasia of Kupffer cells. Br J Haematol. 1976 Jan;32(1):105–111. doi: 10.1111/j.1365-2141.1976.tb01880.x. [DOI] [PubMed] [Google Scholar]
- Peschle C., Marone G., Genovese A., Rappaport I. A., Condorelli M. Increased erythropoietin production in anephric rats with hyperplasia of the reticuloendothelial system induced by colloidal carbon or zymosan. Blood. 1976 Feb;47(2):325–337. [PubMed] [Google Scholar]
- Rich I. N., Heit W., Kubanek B. Extrarenal erythropoietin production by macrophages. Blood. 1982 Oct;60(4):1007–1018. [PubMed] [Google Scholar]
- Schuster S. J., Wilson J. H., Erslev A. J., Caro J. Physiologic regulation and tissue localization of renal erythropoietin messenger RNA. Blood. 1987 Jul;70(1):316–318. [PubMed] [Google Scholar]
- Sherwood J. B., Goldwasser E. Erythropoietin production by human renal carcinoma cells in culture. Endocrinology. 1976 Aug;99(2):504–510. doi: 10.1210/endo-99-2-504. [DOI] [PubMed] [Google Scholar]
- Sherwood J. B., Robinson S. H., Bassan L. R., Rosen S., Gordon A. S. Production of erythrogenin by organ cultures of rat kidney. Blood. 1972 Aug;40(2):189–197. [PubMed] [Google Scholar]
- Sherwood J. B., Shouval D. Continuous production of erythropoietin by an established human renal carcinoma cell line: development of the cell line. Proc Natl Acad Sci U S A. 1986 Jan;83(1):165–169. doi: 10.1073/pnas.83.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohman R. C., Moss P. S., Micou-Eastwood J., Spector D., Przybyla A., Paterson B. Messenger RNA for myosin polypeptides: isolation from single myogenic cell cultures. Cell. 1977 Feb;10(2):265–273. doi: 10.1016/0092-8674(77)90220-3. [DOI] [PubMed] [Google Scholar]
- Tambourin P., Casadevall N., Choppin J., Lacombe C., Heard J. M., Fichelson S., Wendling F., Varet B. Production of erythropoietin-like activity by a murine erythroleukemia cell line. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6269–6273. doi: 10.1073/pnas.80.20.6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanjani E. D., Poster J., Burlington H., Mann L. I., Wasserman L. R. Liver as the primary site of erythropoietin formation in the fetus. J Lab Clin Med. 1977 Mar;89(3):640–644. [PubMed] [Google Scholar]
- Zucali J. R., Stevens V., Mirand E. A. In vitro production of erythropoietin by mouse fetal liver. Blood. 1975 Jul;46(1):85–90. [PubMed] [Google Scholar]