Abstract
Although the nucleus accumbens (NAc) has long been hypothesized to act as an interface between limbic and motor regions, direct evidence for this modulatory role on behavior is lacking. Using a disconnection procedure in rats, we found that basolateral amygdala (BLA) input to the core and medial shell of the NAc separately mediate two distinct incentive processes controlling the performance of goal-directed, instrumental actions, respectively: (1) the sensitivity of instrumental responding to changes in the experienced value of the goal or outcome, produced by specific satiety-induced outcome devaluation, and (2) the effect of reward-related cues on action selection, observed in outcome-specific Pavlovian-instrumental transfer. These results reveal, therefore, that dissociable neural circuits involving BLA inputs to the NAc core and medial shell mediate distinct components of the incentive motivational processes controlling choice and decision-making in instrumental conditioning.
Keywords: reward, transfer, devaluation, instrumental, Pavlovian, rat
Introduction
The nucleus accumbens (NAc) has been hypothesized to function as a limbic-motor interface, with the role of this interface acting to set the gain on behavioral output (Mogenson et al., 1980; Alexander et al., 1986). However, more recent anatomical and functional findings have identified specialized sub-regions within the NAc, suggesting that this characterization is too general (Groenewegen et al., 1999; Zahm, 2000; Balleine, 2005; Berridge et al., 2009; Wheeler & Carelli, 2009). Indeed, important distinctions have been made between core and shell sub-regions of the NAc based on their involvement in emotional and motivational processes (Parkinson et al., 1999; Di Chiara, 2002; Floresco et al., 2006; Murphy et al., 2008; Chaudhri et al., 2010). In the context of instrumental conditioning, the NAc core and shell have been shown to mediate dissociable incentive processes controlling instrumental goal-directed action (Corbit et al., 2001). Lesions of the NAc core impair sensitivity of instrumental responses to changes in outcome value, as shown by impaired performance on a choice test following outcome devaluation. In contrast, medial shell lesions have no effect on devaluation, but impair the ability of outcome-associated cues to bias action selection, as shown during outcome-specific Pavlovian-instrumental transfer (PIT) (Corbit et al., 2001). Thus, in contrast to a general modulatory role, the NAc may contain functionally separable sub-regions that serve as part of a broader set of cortico-limbic striatal circuits mediating distinct learning and decision-making processes.
The NAc receives excitatory inputs from the basolateral amygdala (BLA), a structure known to be important for associative learning (Everitt et al., 2003; Balleine & Killcross, 2006; Murray, 2007). BLA lesions impair performance on outcome devaluation tests and outcome-specific PIT (Corbit & Balleine, 2005; Ostlund & Balleine, 2008; Johnson et al., 2009). This suggests that BLA inputs to the NAc may be required for the operation of incentive processes in guiding instrumental behavior. To assess this possibility, we examined instrumental performance in rats that underwent a “disconnection” procedure, in which we performed excitotoxic lesions of the BLA in one hemisphere and the caudal core or medial shell of the NAc in the opposite hemisphere. Because the connections between BLA and NAc are ipsilateral, this procedure results in groups of rats with disrupted BLA-NAc circuitry in both hemispheres (McDonald, 1991; Brog et al., 1993). Following surgery, we used selective-satiety outcome devaluation and outcome-specific Pavlovian-instrumental transfer to determine whether BLA inputs to the NAc core and shell control the influence of distinct incentive processes on the performance of instrumental actions.
Materials and Methods
Subjects and Apparatus
A total of 62 adult male Long Evans rats (Harlan Laboratories, Indianapolis, IN) were used in this study. Rats arrived weighing 250-275 g and were housed individually in Plexiglas tubs located in a temperature- and humidity-controlled vivarium. Behavioral training and testing was conducted during the light phase of the 12 h light/dark cycle. Rats were fed 10-15 g of home chow following each daily training session, which was sufficient to maintain them at approximately 90% of their free-feeding body weight. Rats had free access to water in their home cage. All procedures were approved by the UCLA Animal Research Committee.
Behavioral testing took place in 24 operant chambers enclosed in sound- and light-attenuating shells (Med Associates, St. Albans VT). Each chamber was equipped with two retractable levers that could be extended to the left and right of a recessed food magazine. A pellet dispenser was attached to the magazine and was used to deliver a 45-mg grain pellet (Bio-Serv, Frenchtown NJ). A syringe pump was also attached to the magazine and was used to deliver 0.1 ml of 20% sucrose solution. An infrared photobeam crossed the magazine opening, allowing for the detection of head entries. Each chamber was equipped with two auditory stimulus generators (3 KHz 80-db tone and 80-db white noise). Illumination of the operant chamber was provided by a 3W 24V house light. A set of 3 microcomputers running Med Associates proprietary software (Med-PC) controlled all experimental events and recorded lever presses and magazine entries.
Behavioral procedures
General
Following surgery, rats underwent Pavlovian training in which two auditory stimuli were paired with delivery of two unique food outcomes (grain pellet or sucrose solution). Rats then underwent instrumental training in which two distinct actions (left or right lever press) were paired with delivery of grain pellets or sucrose solution. Sensitivity of instrumental responding to changes in outcome value was then assessed in choice tests following devaluation of one of the instrumental outcomes. Following these tests, rats were given one additional instrumental and Pavlovian training session and then tested in an outcome-specific PIT paradigm.
Pavlovian training
During Pavlovian training, two auditory stimuli (3 Khz tone and white noise, each 80 dB) were used as conditioned stimuli (CS's). Each stimulus was paired with delivery of grain pellets or sucrose solution. For half the rats in each group, white noise presentations were paired with delivery of grain pellets and tone presentations were paired with delivery of sucrose solution, whereas the remaining subjects received the opposite stimulus-outcome relationships. Each stimulus was 2 min in duration, during which the appropriate outcome was delivered according to a random-time 30-sec schedule. During each training session each stimulus type was presented 4 times, with a random interval averaging 5 min between stimulus presentations. The number of head entries into the food magazine was measured during CS presentations and during a 2-min interval preceding CS presentation. Rats received 6 Pavlovian training sessions, one per day.
Instrumental training
During instrumental training rats were trained to associate actions on each of the two levers with a unique outcome. For half of the subjects in each group, pressing the left lever delivered 45-mg grain pellets and pressing the right lever delivered 20% sucrose solution, whereas the remaining subjects received the opposite action–outcome relationship. Rats were first trained under continuous reinforcement for two days, and then shifted to a random ratio (RR) schedule, consisting of 2 days of RR-5 (each action delivered an outcome with a probability of 0.2), and 4 days of RR-10 (probability of 0.1). The session terminated when rats earned 20 outcomes or 30 min had elapsed since the start of the session. Rats were trained daily on each action-outcome pair in separate training sessions, with a 30-min interval between sessions.
Outcome devaluation test
One of the two outcomes was devalued by specific satiety and rats’ choice performance was tested. Rats were provided with 50 g of grain pellets or 50 ml of sucrose solution in their home cage for 1 hr. Rats were then placed in the operant chamber, the house light illuminated and both levers were inserted. Rats could respond on either of the two levers, which produced no outcomes during the session. The session terminated after 10 min and rats were returned to their home cage. The following day the opposite outcome from the previous session was devalued following the same procedure, after which performance on the choice test was examined.
Pavlovian-instrumental transfer test
In this test, rats were placed in the operant chamber, the house light was illuminated and both levers were inserted. Rats could freely respond on either lever; however, no pellets were delivered during the test. After 6 min, each of the two CS's was presented 4 times. The CS's were 2 min in duration, separated by a 2-min interval with no stimulus presentation. The stimuli were presented in the following order: (T=tone; N=noise) T-NN-T-N-T-T-N. Responses on each lever were recorded during stimulus presentations and during intervals with no stimulus. The total test time was 36 min.
Surgical procedures
Rats were anesthetized with an intraperitoneal injection of sodium pentobarbital (Nembutal, Abbot Labs; 50 mg/kg body weight). Rats were placed in a stereotaxic instrument, the scalp retracted and holes were drilled in the skull to allow passage of a 26 g infusion needle. To lesion the NAc core, 0.5 μl of 0.12 M NMDA (Sigma-Aldrich) was infused at the following location (all locations are expressed relative to Bregma, except for the dorsoventral coordinate, which was measured relative to the surface of the brain. A/P = anteroposterior; M/L = mediolateral; D/V = dorsoventral): +1.2 A/P; ± 2.1 M/L; -7.0 D/V. To lesion the medial shell, 0.2 μl of 0.015 M AMPA hydrobromide (Sigma-Aldrich) was infused in two locations: +1.6 A/P; ± 0.8 M/L; -6.8, -6.0 D/V. To lesion the BLA, 0.25 μl of 0.12 M NMDA was infused at two locations: -2.3, -3.0 A/P; ± 5.2 M/L; -7.6 D/V. The use of different neurotoxins was based on previous studies which showed differential effects of these substances in producing neural cell loss in the core and shell (Parkinson et al., 1999; Corbit et al., 2001). Infusate was delivered at approximately 0.1 μl per min via a 1 μl Hamilton syringe. Following infusion the scalp was closed with surgical staples and rats were returned to their home cage for 2 weeks recovery.
For half of the animals in this study, BLA lesions were made in the left hemisphere while for the remaining animals, BLA lesions were made in the right hemisphere. For the disconnection condition, NAc lesions were made in the opposite hemisphere from the BLA lesion, whereas for the ipsilateral control condition BLA and NAc lesions were made in the same hemisphere.
Histology
Following completion of the experiment, rats were perfused transcardially with 0.1 M phosphate-buffered saline followed by 10% formalin. Brains were removed and prepared for microscopic analysis following standard histological techniques. Sections were stained with thionin and examined at 100x magnification for lesions, which were identified by neuron loss and changes in structure shape.
Procedures for ipsilateral control experiment
An additional study was carried which further examined whether ipsilateral BLA and NAc lesions on PIT and devaluation. Rats underwent ipsilateral lesions of the BLA and NAc core or BLA and NAc shell or sham lesions using identical procedures to those described above. The behavioral procedures were in all ways identical to the procedures described in the main methods section, except for small differences in the Pavlovian training procedure. This procedure consisted of two phases. In the first phase rats were trained to associate 3 CS's with 3 unique outcomes. In addition to the tone and white noise, a click stimulus and was paired with delivery of 20% polycose. Rats underwent 8 trials with the 3 CS's. In the second phase, rats underwent an additional 8 trials with the tone and white noise CS's. After Pavlovian training was complete, a PIT test was administered.
Results
Lesion placement
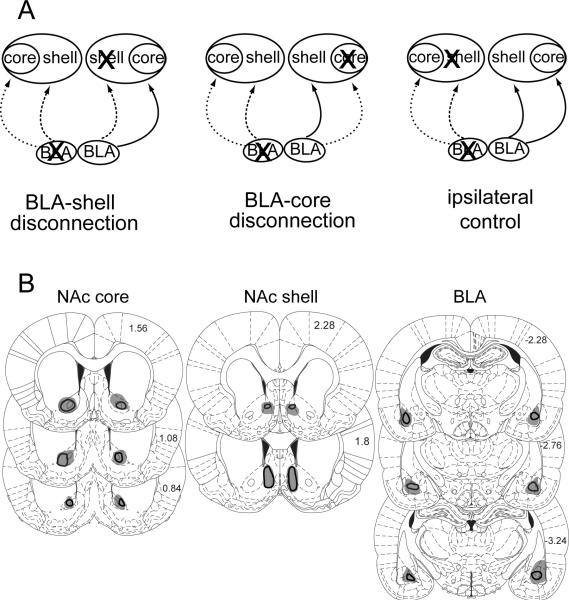
Rats in the disconnection group were administered BLA lesions in one hemisphere and NAc core (n=7) or shell (n=7) lesions in the opposite hemisphere. Rats in the ipsilateral control group had BLA and NAc lesions in the same hemisphere (n=9). As a further control, one group of rats was given unilateral BLA lesions and sham lesions of the contralateral NAc (n=9). The ipsilateral control condition consisted on animals with lesions of the BLA and NAc core (n=5) or BLA and NAc shell (n=4). We decided to combine these two sub-groups into a single ipsilateral control group based on the results of a preliminary study, in which we found that ipsilateral BLA-core and BLA-shell lesions had no effect on performance in the PIT or devaluation tasks. Because of small differences in the training procedures used in these animals, the results from this preliminary study are presented separately as Supplemental Material.
Lesion placement is depicted in Figure 1. BLA lesions generally spanned the rostrocaudal extent of this structure and, in some animals, included portions of lateral amygdala. NAc shell lesions were confined to the rostral and medial portion of the shell, and generally spanned its dorso-ventral extent. We restricted our lesion placement to the medial shell, as any attempt to lesion lateral portions of the shell may have resulted in damage the NAc core. NAc core lesions were confined to the medial and caudal portion of the NAc core. No systematic differences in lesion placement were observed across treatment groups.
Figure 1. Histological results.
A) Schematic of disconnection design. The ipsilateral control consisted of animals with either BLA-shell or BLA-core lesions B) Representation of the minimum (black outline) and maximum (gray shading) extent of tissue damage resulting from NAc core and shell lesions and BLA lesions. The left hemisphere represents lesions from control animals (ipsilateral BLA-NAc and unilateral BLA lesions). Lesions in the right hemisphere depict animals with disconnection of the core or shell from the BLA. Values indicate mm from Bregma. Reproduced from Paxinos and Watson.
Instrumental training and outcome devaluation test
In the instrumental task, rats learned in separate sessions to respond on the left or right lever to earn unique outcomes (grain pellets or 20% sucrose solution). There was no difference in the rate of responding for the two types of outcomes. For statistical analyses, response rates from each training session were averaged to derive an overall response rate measure for each training day, and training days were combined into four two-day blocks. A two-factor ANOVA with training block and lesion type revealed an overall effect of training block and a significant interaction between lesion type and training block on instrumental response rates (ANOVA: effect of training block; F3, 84 = 232, P < 0.01; interaction F9, 84 = 3.93, p < 0.01) (Figure 2 A). Post-hoc comparisons revealed that during the third and fourth training block, rats with BLA-shell disconnection responded at a significantly greater rate compared to the performance of rats with unilateral BLA lesions (significantly different at p < 0.05, independent samples t-test with Bonferroni correction). Overall, disconnection of the BLA from the NAc did not impair acquisition of the instrumental task.
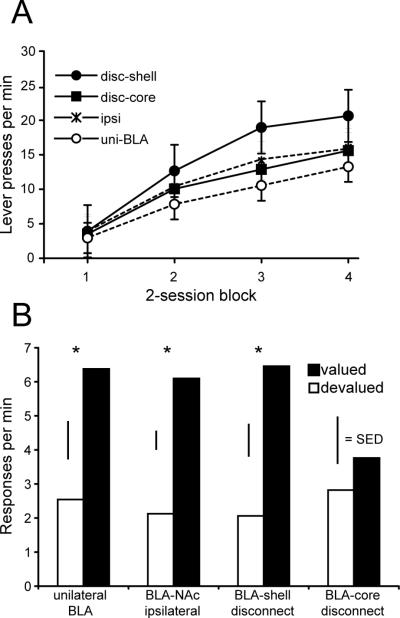
Figure 2. BLA-Nac core disconnection impairs rats’ sensitivity to outcome value during instrumental performance.
We trained rats over 8 sessions to bar press for food rewards and recorded the rate of responding across training. A) Rats from all groups acquired an instrumental response, as shown by an increase in response rates across training sessions. B) After one of the two instrumental outcomes was devalued by specific satiety, rats performed a choice test with both levers available for responding in extinction. Rats with unilateral BLA lesions (uni-BLA), ipsilateral NAc and BLA lesions (ipsi), and lesions that disconnect the NAc shell from the BLA bilaterally (disc-shell), made significantly fewer responses on the lever that, during training, resulted in delivery of the now devalued outcome. In contrast, disconnection of the NAc core from the BLA (disc-core) impaired rats’ ability to use outcome value to guide actions, as shown by the lack of a devaluation effect. * = non-devalued greater than devalued at P < 0.05, paired t-test with Bonferroni correction. Error bars = ± 1 SEM (panel A) or 1 standard error of the difference (SED) (panel B), a measure of within-subjects variability.
Following training, we examined how BLA-NAc disconnection affects sensitivity of instrumental responding to changes in outcome value. Normally, changes in the value of an instrumental outcome modify both the rate of performance of instrumental actions and choice between actions (Balleine & Dickinson, 1998). We used a specific-satiety devaluation procedure, by providing ad lib access to one of the outcomes in the animal's home cage for 1 h prior to a choice test in which they could freely respond on both levers, although responses during test did not produce any outcomes. The following day the opposite outcome was devalued following the same procedure, after which performance on the choice test was examined. Lesion type had no effect on the amount of pellets or sucrose solution consumed prior to the choice test. The volume of sucrose solution consumed was converted to grams (1 ml = 1 g). The average consumption for the groups was as follows: unilateral BLA = 12.2 ± 5.2; BLA-NAc shell disconnection 10 ± 4.6, ipsilateral = 14.4 ± 4.3, BLA NAc core disconnection 13 ± 4.1 (mean ± 1 S.D.).
Response rates on the “valued” and “devalued” levers from the two tests were averaged for each animal and statistical analyses performed on these averages. We found that control lesions as well as BLA-NAc shell disconnection spared sensitivity to changes in outcome value, as shown by reduced responding on the lever that, during training, resulted in the now devalued outcome (Figure 2 B). In contrast, BLA-NAc core disconnection impaired rats’ ability to use outcome value to guide instrumental performance in the choice test, as shown by equivalent responding on both levers. A two-factor ANOVA revealed a significant effect of outcome value on instrumental response rate (F1, 28 = 46.34, P < 0.01). Significantly greater rates of responding were observed on the valued compared to the devalued lever in all groups except rats with BLA-NAc core disconnection (paired t-tests with Bonforroni correction, all groups P < 0.01 except BLA-NAc core t6 = 1.67, P = 0.14). There was no simple effect of lesion on instrumental response rates independent of outcome value (F < 1), indicating that response rates by themselves were not affected by the disconnection condition.
Pavlovian training and Pavlovian-instrumental transfer test
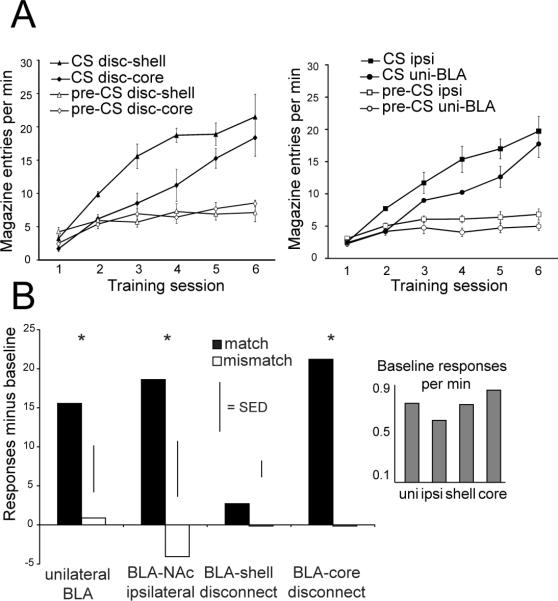
In the Pavlovian task, we used two different auditory cues, a tone or white noise, as conditioned stimuli (CSs) and we paired their presentation with delivery of either grain pellets or sucrose solution. We measured head entries into the magazine during the CS and during an equivalent period prior to CS onset (pre-CS). A three-factor ANOVA with stimulus (CS or pre-CS), training session, and lesion type revealed a significant effect of training session (F5, 140 = 96.91, P < 0.01) and stimulus (F1, 140 = 224.06, P < 0.01) on magazine approach, and a significant interaction involving these factors (F5, 140 = 66.80, P < 0.01). There was no main effect of lesion; however, there was a significant interaction of stimulus and lesion type on magazine entries (F3, 140 = 4.98 P < 0.01) (Figure 3 A). Post-hoc tests revealed that rats with disconnection of the BLA from the NAc shell made significantly more head insertions during CS presentation compared to rats with unilateral BLA lesions (significantly different at P < 0.05, independent samples t-test with Bonferroni correction). By the final training session there was no difference in conditioned magazine approach between the lesion groups.
Figure 3. BLA-NAc shell disconnection impairs outcome-specific Pavlovian-instrumental transfer.
A) We trained rats over 6 sessions to associate auditory stimuli with delivery of food pellets. During the session we measured the number of food cup entries during stimulus presentation (CS, filled symbols) and during an equivalent period prior to stimulus onset (pre-CS, open symbols). The graph depicts the number of food cup entries per min for each of the 6 training sessions. Data from the two disconnection groups are presented in the left panel and data for the two control groups in the right panel. We observed an increase in food cup entries across training sessions. In addition, rats with BLA-shell disconnection showed a greater number of head entries during stimulus presentations compared to the unilateral BLA lesion control condition. B) During the transfer test, two cues were presented while rats had access to both levers in extinction. Control rats (ipsi and uni-BLA) and rats with BLA-NAc core disconnection (disc-core) increased their rate of responding above baseline on the lever whose outcome during training matched the cue, compared to when cue and lever outcome were mismatched. In contrast, rats with BLA-NAc shell disconnection (disc-shell) showed no increase in instrumental performance above baseline during cue presentation. Inset depicts baseline responses per min measured during intervals between cue presentations. * = match greater than mismatch at P < 0.05, paired t-test with Bonferroni correction. Error bars = ± 1 SEM (panel A) or 1 SED (panel B).
We next assessed the effects of BLA-NAc disconnection on instrumental responding during presentation of outcome-associated stimuli. Cues can elicit goal-directed instrumental behavior by aiding in the retrieval of actions with which the cue shares an outcome. To assess this process, we used an outcome-specific PIT procedure, in which we presented each of the two previously learned cues while rats made instrumental responses on both levers in extinction. Responses during the intervals between cue presentations (baseline) were subtracted from responses during cue presentations, and are plotted in Figure 3 B. Responses were classified as a “match” when the lever response and cue predicted the same outcome, and “mismatch” when the lever and cue predicted opposite outcomes. We found that control rats and rats with BLA-NAc core disconnection demonstrated outcome-specific transfer, as shown by an increase in responses on the lever that, during training, delivered the same outcome as was predicted by the cue (match), as compared to mismatched responses. In contrast, rats with BLA-NAc shell disconnection showed no enhancement of instrumental response rates during cue presentation on the appropriately matched lever (Figure 3 B). A two factor ANOVA revealed a significant main effect of stimulus (match versus mismatch) (F1, 28 = 21.16, P < 0.01). Significantly greater rates of responding were observed when the cue and lever outcome matched compared to when they mismatched for all groups except BLA-NAc shell disconnection (paired t-test with Bonferroni correction, P's < 0.01 for all groups except for NAc-shell disconnection group, t6 = 1.08, P = 0.32). The lack of outcome-specific PIT in the BLA-NAc shell disconnection group is not a result of an overall response suppression in these animals, as no differences in baseline responding was observed between groups (F < 1). This suggests that the lack of outcome-specific transfer is the result of an impaired ability to use cue-outcome associations to guide instrumental behavior.
Ipsilateral NAc-BLA disconnection does not impair performance during PIT and devaluation
We performed an additional experiment confirming the lack of an effect of ipsilateral BLA-NAc lesions on devaluation and PIT. Thirty rats (10 per group) underwent ipsilateral lesions of the BLA and NAc core or BLA and NAc shell or sham lesions using identical procedures to those described in the methods section. Rats were trained using Pavlovian and instrumental procedures as described above and then tested under devaluation and outcome-specific PIT tests. Performance on PIT and devaluation tests was analyzed using a 2-factor ANOVA with both within- and between-subjects effects. Lesions did not impair performance on either test. During the PIT test, rats made a significantly greater number of responses on the matched compared to the mismatched lever (ANOVA: effect of stimulus [match or mismatch] F1, 27 = 27.99, p < 0.01) (Figure 4). There was no effect of lesion or interaction of lesion and stimulus on instrumental performance (p's > 0.1). All rats regardless of group demonstrated greater response rates for the matched lever compared to the mismatched lever (paired t-test; BLA-NAc shell lesions t9 = 2.51, p < 0.05; BLA-NAc core lesions t9 = 3.64, p < 0.01; sham lesions t9 = 3.31, p < 0.01).
Figure 4. Ipsilateral lesions do not disrupt choice performance following outcome devaluation.
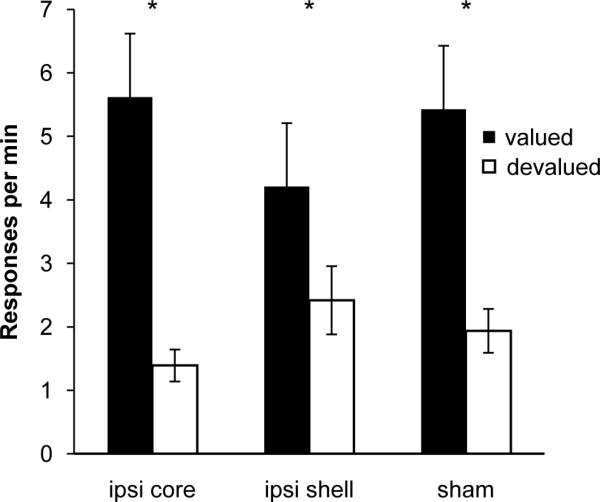
Rats respond at a greater rate on the lever that during training delivered the valued outcome compared to the devalued outcome. * = non-devalued greater than devalued at P < 0.05, paired t-test with Bonferroni correction. Error bars = ± 1 SEM
During the devaluation test, rats made a significantly greater number of responses on the lever that during training resulted in the valued outcome compared to the devalued outcome (ANOVA: effect of value F1, 27 = 35.54, p < 0.01) (Figure 5). There was no significant effect of lesion, or an interaction between lesion and value on instrumental response rates (p's > 0.1). All rats demonstrated greater response rates for the valued lever compared to the devalued lever regardless of group (paired t-test; BLA-NAc shell lesions t9 = 2.75, p < 0.05; BLA-NAc core lesions t9 = 4.04, p < 0.01; sham lesions t9 = 3.44 p < 0.01).
Figure 5. Ipsilateral BLA-NAc lesions do not impair performance during outcome-specific PIT.
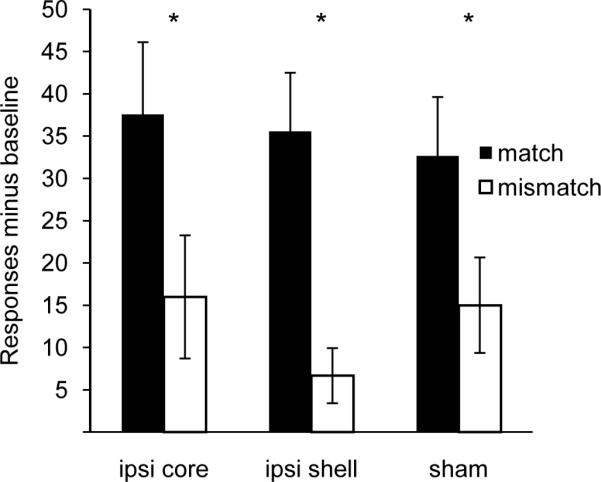
Rats respond at a greater rate on the lever whose outcome matches the cue (match) than on the mismatched lever regardless of lesion type. * = match greater than mismatch at P < 0.05, paired t-test with Bonferroni correction. Error bars = ± 1 SEM.
Discussion
Our results show that neural circuits comprised of the BLA and NAc sub-regions underlie distinct components of incentive behavior. Using asymmetrical lesions to “disconnect” the BLA from the caudal core or medial shell of the NAc, we found that BLA-NAc core disconnection impaired rats’ ability to use outcome value to guide instrumental actions, but had no effect on outcome-specific PIT. In contrast, BLA-NAc shell disconnection impaired the ability of outcome-associated stimuli to bias instrumental actions during PIT, but had no effect on choice following devaluation. Thus, NAc core and shell sub-regions differentially mediate the effects of instrumental and Pavlovian incentive processes respectively, and these effects depend on input from the BLA. These results have important implications for understanding the function of the BLA-NAc circuitry during reward-seeking behavior.
The BLA and NAc form an important neural circuit underlying reward-seeking behavior. The NAc core and shell receive excitatory afferent input from the BLA that is topographically organized (Krettek & Price, 1978; Christie et al., 1987; McDonald, 1991; Brog et al., 1993; Wright et al., 1996). The importance of BLA input to the NAc in reward-seeking behavior has been shown in tasks involving the association of environmental stimuli with a rewarding outcome, such as food or access to mates. In these tasks, asymmetrical BLA-NAc disconnection lesions prevent the development of conditioned appetitive responses to presentations of reward-associated stimuli (Cador et al., 1989; Everitt et al., 1989; Robbins et al., 1989; Everitt et al., 1991; Setlow et al., 2002; Di Ciano & Everitt, 2004; Ambroggi et al., 2008). More recently, the BLA has been shown to be necessary for the development of neural responses in the NAc to presentation of a reward-associated stimulus (Ambroggi et al., 2008; Popescu et al., 2009). However, the existence of dissociable functional ensembles within this circuit has not been identified. Our results provide evidence for separate functional circuits consisting of BLA inputs to the caudomedial core and medial shell subregions of the NAc. Furthermore, our results suggest that during reward-seeking behavior the BLA supplies outcome information to the different NAc sub-regions, which use this information to modulate instrumental actions depending on whether the task involves a Pavlovian or instrumental incentive process.
The BLA has been recognized as having a role in forming and maintaining representations of outcomes associated with actions or stimuli (Schoenbaum et al., 1998; Balleine & Killcross, 2006; Paton et al., 2006; Belova et al., 2008). Bilateral BLA lesions impair performance on tasks that require the use of sensory-specific outcome representations to guide instrumental performance, including outcome-specific PIT and devaluation tasks (Hatfield et al., 1996; Blundell et al., 2001; Corbit & Balleine, 2005; Dwyer & Killcross, 2006; Ostlund & Balleine, 2008; Johnson et al., 2009). Accurate performance during outcome-selective PIT and choice tests following devaluation requires that animals use outcome representations to guide action selection. Thus, the impaired performance that we observed on these tasks following BLA-NAc disconnection may reflect an inability of subjects to use outcome information associated with an action or stimulus to direct instrumental responding. Based on this interpretation, we suggest that the BLA conveys sensory-specific outcome information to the NAc where it is used to direct outcome-appropriate instrumental responding. The BLA may convey this information through direct excitatory glutamatergic projections to the NAc or by modulating dopamine release in the NAc (Floresco et al., 2001a; b; Ahn & Phillips, 2002; Simmons & Neill, 2009; Jones et al., 2010).
Our results suggest that the NAc integrates BLA inputs representing outcome information with information about the animal's motivational state to direct motor action. NAc sub-regions appear to be differentially involved depending on whether the task relies on an instrumental incentive process that mediates the effects of outcome value on instrumental behavior (such as following devaluation), or a Pavlovian incentive process that mediates the effects of reward-related cues on instrumental behavior (such as during outcome-selective PIT). Indeed, previous research has shown that bilateral lesions of the medial shell impair PIT and leave outcome devaluation intact, whereas the opposite result is observed following lesions of the NAc core (Corbit et al., 2001). Although it is possible that the BLA-core disconnection rendered rats unable to discriminate the two instrumental outcomes we think this unlikely. Both their performance in the PIT test as well as previous findings from our lab using similar training procedures suggest that rats with bilateral lesions of either the BLA or accumbens core can discriminate the sensory properties of pellet and sucrose outcomes normally (Balleine & Killcross, 1994) Balleine, 1993; (Corbit et al., 2001; Corbit & Balleine, 2005).
Dissociations between the core and medial shell have been observed in behavioral responses to reward-associated stimuli, and support the functional division described here (Parkinson et al., 1999; Di Chiara, 2002; Di Ciano et al., 2007). This division may be due to differences in connectivity between the core and shell. In particular, the NAc core's afferent and efferent projections are consistent with the general pattern of corticostriatal circuitry, and in this context the core is in a position to influence motor cortical activity through its connections with pallido-thalamic circuits (Zahm & Brog, 1992; Heimer et al., 1997; Stratford & Kelley, 1999; Zahm, 2000). Unlike the core, the NAc shell projects to hypothalamus and other regions of the extended amygdala and therefore may influence behavior through a different circuit than the NAc core (Zahm & Brog, 1992; Heimer et al., 1997; Stratford & Kelley, 1999; Zahm, 2000). Alternatively, our results could be due to differences in the rostro-caudal placement of our NAc lesions, since lesions of medial shell were primarily located rostral to core lesions. Positive and negative affective responses to stimuli have been shown to be differentially mediated across a rostrocaudal extent of the medial shell (Reynolds & Berridge, 2002; Reynolds & Berridge, 2003); however, it is not entirely clear how this rostrocaudal organization of the medial shell can explain the effects we observed on incentive behavior. Indeed, whether specialized regions exist within rostral or caudal regions of the medial shell (or core) for processing incentive information is not known.
We observed normal acquisition of instrumental actions and of Pavlovian conditioned approach responses after BLA-NAc disconnection suggesting, consistent with previous findings (e.g. Corbit et al, 2001), that the disconnection treatment did not affect learning. However, we did observe a tendency in rats with BLA-shell disconnection for elevated response rates during instrumental and Pavlovian training. One possibility is that BLA-NAc shell disconnection lesions enhance general locomotor activity, which may increase the instrumental and Pavlovian response rates during training. Some evidence supports this conclusion. For example, (Jongen-Relo et al., 2003) reported that, relative to core lesions and controls, shell lesions induced locomotor hyperactivity. Furthermore, NAc shell inactivation increased cue-induced reinstatement of food-seeking and cocaine-seeking responses (Di Ciano et al., 2007; Floresco et al., 2008). Certainly, the NAc shell participates in the ventral indirect pathway through the basal ganglia, which is generally inhibitory on motor activity, and so these lesions may have resulted in a degree of disinhibition that increased general locomotor activity and facilitated cue-induced reinstatement of reward-seeking responses. We believe that these general arousing effects are likely to occur independent of the disruptive effects that these lesions have on performance during outcome-specific PIT used in our study; no effect of BLA-NAc shell disconnection was observed in overall response rates during the PIT and devaluation tests. Rather, BLA-NAc disconnection caused specific deficits during tests that required the use of acquired associations to guide subsequent choice performance. Overall response rates during the devaluation test were unaffected by disconnection, although BLA-NAc core disconnection did appear to reduce response rates for the valued outcome during the devaluation test. The relatively low response rates observed on the valued action in these animals were most likely a consequence of interference during the choice test; i.e. when two spatially distinct actions are equally valued, the choice between the actions must necessarily interfere with the performance of both of them.
One limitation of the disconnection procedure used in this study is that it does not eliminate the possibility that BLA-NAc disconnection lesions impair behavior by interrupting indirect pathways involving prefrontal (prelimbic and orbital) cortical regions that receive BLA projections that in turn project to the NAc. Consistent with this alternative, prefrontal lesions impair performance on reward-related tasks, including PIT and devaluation (Schoenbaum et al., 1998; Schoenbaum et al., 2003; Ostlund & Balleine, 2005; Floresco & Ghods-Sharifi, 2007; Ostlund & Balleine, 2007), and BLA control over NAc neural activity has been shown to depend on an intact prefrontal cortex (Jackson & Moghaddam, 2001; Ishikawa et al., 2008; McGinty & Grace, 2008). However, disconnection of the BLA from the prelimbic cortex appears to have no effect on goal-directed responding, suggesting that BLA-NAc connectivity may be sufficient without PFC input to mediate performance of goal-directed actions. (Coutureau et al., 2009). Another possibility is that disconnection impairs the ability of the NAc to influence BLA activity via subcortical neuromodulatory centers (Woolf et al., 1986; Luiten et al., 1987; Zaborszky & Cullinan, 1992), or deprives BLA and NAc input to a common target such as the hypothalamus (Petrovich et al., 2002; Petrovich et al., 2005). Nevertheless, whether the connections are direct or indirect, our results show that interactions between BLA and NAc are required for incentive motivational processes to act on goal-directed behavior.
Conclusions
Overall, our findings contribute to a growing understanding of the functional role that specific cortico-limbic striatal circuits play in the control of distinct components of goal-directed behavior. Our findings support the hypothesis that the BLA functions to associate outcomes with stimuli or actions and then relays this information to NAc sub-regions where it interacts with motivational information to mediate goal-directed behavior. Generally, therefore, our results indicate that the limbic-motor interface, involving the BLA and NAc core and shell mediates two distinct incentive processes. Differences in the pattern of afferent and efferent connectivity between the BLA and the NAc core and shell may be responsible for this parcellation of function.
Acknowledgements
This research was supported by grant HD59257 to B.W.B from the National Institute of Child Health and Human Development and DA026559 to M.W.S. from the National Institute on Drug Abuse.
References
- Ahn S, Phillips AG. Modulation by Central and Basolateral Amygdalar Nuclei of Dopaminergic Correlates of Feeding to Satiety in the Rat Nucleus Accumbens and Medial Prefrontal Cortex. J. Neurosci. 2002;22:10958–10965. doi: 10.1523/JNEUROSCI.22-24-10958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, Delong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Ambroggi F, Ishikawa A, Fields HL, Nicola SM. Basolateral Amygdala Neurons Facilitate Reward-Seeking Behavior by Exciting Nucleus Accumbens Neurons. Neuron. 2008;59:648–661. doi: 10.1016/j.neuron.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine B, Killcross S. Effects of ibotenic acid lesions of the nucleus accumbens on instrumental action. Behav Brain Res. 1994;65:181–193. doi: 10.1016/0166-4328(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Balleine BW. Neural bases of food-seeking: affect, arousal and reward in corticostriatolimbic circuits. Physiol Behav. 2005;86:717–730. doi: 10.1016/j.physbeh.2005.08.061. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Dickinson A. The role of incentive learning in instrumental outcome revaluation by sensory-specific satiety. Anim Learn Behav. 1998;26:46–59. [Google Scholar]
- Balleine BW, Killcross S. Parallel incentive processing: an integrated view of amygdala function. Trends Neurosci. 2006;29:272–279. doi: 10.1016/j.tins.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Belova MA, Paton JJ, Salzman CD. Moment-to-Moment Tracking of State Value in the Amygdala. J Neurosci. 2008;28:10023–10030. doi: 10.1523/JNEUROSCI.1400-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: 'liking', 'wanting', and learning. Curr Opin Pharmacol. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell P, Hall G, Killcross S. Lesions of the Basolateral Amygdala Disrupt Selective Aspects of Reinforcer Representation in Rats. J. Neurosci. 2001;21:9018–9026. doi: 10.1523/JNEUROSCI.21-22-09018.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Cador M, Robbins TW, Everitt BJ. Involvement of the amygdala in stimulus-reward associations: Interaction with the ventral striatum. Neuroscience. 1989;30:77–86. doi: 10.1016/0306-4522(89)90354-0. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Schairer WW, Janak PH. Separable roles of the nucleus accumbens core and shell in context- and cue-induced alcohol-seeking. Neuropsychopharmacology. 2010;35:783–791. doi: 10.1038/npp.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie MJ, Summers RJ, Stephenson JA, Cook CJ, Beart PM. Excitatory amino acid projections to the nucleus accumbens septi in the rat: A retrograde transport study utilizingd[3H]aspartate and [3H]GABA. Neuroscience. 1987;22:425–439. doi: 10.1016/0306-4522(87)90345-9. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. Double Dissociation of Basolateral and Central Amygdala Lesions on the General and Outcome-Specific Forms of Pavlovian-Instrumental Transfer. J. Neurosci. 2005;25:962–970. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Muir JL, Balleine BW. The role of the nucleus accumbens in instrumental conditioning: Evidence of a functional dissociation between accumbens core and shell. J Neurosci. 2001;21:3251–3260. doi: 10.1523/JNEUROSCI.21-09-03251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutureau E, Marchand AR, Di Scala G. Goal-directed responding is sensitive to lesions to the prelimbic cortex or basolateral nucleus of the amygdala but not to their disconnection. Behav Neurosci. 2009;123:443–448. doi: 10.1037/a0014818. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Direct Interactions between the Basolateral Amygdala and Nucleus Accumbens Core Underlie Cocaine-Seeking Behavior by Rats. J. Neurosci. 2004;24:7167–7173. doi: 10.1523/JNEUROSCI.1581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Robbins TW, Everitt BJ. Differential Effects of Nucleus Accumbens Core, Shell, or Dorsal Striatal Inactivations on the Persistence, Reacquisition, or Reinstatement of Responding for a Drug-Paired Conditioned Reinforcer. Neuropsychopharmacology. 2007;33:1413–1425. doi: 10.1038/sj.npp.1301522. [DOI] [PubMed] [Google Scholar]
- Dwyer DM, Killcross S. Lesions of the basolateral amygdala disrupt conditioning based on the retrieved representations of motivationally significant events. J Neurosci. 2006;26:8305–8309. doi: 10.1523/JNEUROSCI.1647-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Cador M, Robbins TW. Interactions between the amygdala and ventral striatum in stimulus reward associations- studies using a 2nd-order schedule of sexual reinforcement. Neuroscience. 1989;30:63–75. doi: 10.1016/0306-4522(89)90353-9. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW. Appetitive Behavior: Impact of Amygdala-Dependent Mechanisms of Emotional Learning. Ann New York Acad Sci. 2003;985:233–250. [PubMed] [Google Scholar]
- Everitt BJ, Morris KA, O'Brien A, Robbins TW. The basolateral amygdala-ventral striatal system and conditioned place preference: Further evidence of limbic-striatal interactions underlying reward-related processes. Neuroscience. 1991;42:1–18. doi: 10.1016/0306-4522(91)90145-e. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Blaha CD, Yang CR, Phillips AG. Dopamine D1 and NMDA Receptors Mediate Potentiation of Basolateral Amygdala-Evoked Firing of Nucleus Accumbens Neurons. J. Neurosci. 2001a;21:6370–6376. doi: 10.1523/JNEUROSCI.21-16-06370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Blaha CD, Yang CR, Phillips AG. Modulation of Hippocampal and Amygdalar-Evoked Activity of Nucleus Accumbens Neurons by Dopamine: Cellular Mechanisms of Input Selection. J. Neurosci. 2001b;21:2851–2860. doi: 10.1523/JNEUROSCI.21-08-02851.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Ghods-Sharifi S. Amygdala-Prefrontal Cortical Circuitry Regulates Effort-Based Decision Making. Cereb. Cortex. 2007;17:251–260. doi: 10.1093/cercor/bhj143. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Ghods-Sharifi S, Vexelman C, Magyar O. Dissociable roles for the nucleus accumbens core and shell in regulating set shifting. J Neurosci. 2006;26:2449–2457. doi: 10.1523/JNEUROSCI.4431-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, McLaughlin RJ, Haluk DM. Opposing roles for the nucleus accumbens core and shell in cue-induced reinstatement of food-seeking behavior. Neuroscience. 2008;154:877–884. doi: 10.1016/j.neuroscience.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- Hatfield T, Han JS, Conley M, Gallagher M, Holland P. Neurotoxic lesions of basolateral, but not central, amygdala interfere with Pavlovian second-order conditioning and reinforcer devaluation effects. J Neurosci. 1996;16:5256–5265. doi: 10.1523/JNEUROSCI.16-16-05256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer L, Alheid GF, de Olmos JS, Groenewegen HJ, Haber SN, Harlan RE, Zahm DS. The accumbens: beyond the core-shell dichotomy. J Neuropsychiatry Clin Neurosci. 1997;9:354–381. doi: 10.1176/jnp.9.3.354. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Ambroggi F, Nicola SM, Fields HL. Dorsomedial prefrontal cortex contribution to behavioral and nucleus accumbens neuronal responses to incentive cues. J Neurosci. 2008;28:5088–5098. doi: 10.1523/JNEUROSCI.0253-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson ME, Moghaddam B. Amygdala Regulation of Nucleus Accumbens Dopamine Output is Governed by the Prefrontal Cortex. J. Neurosci. 2001;21:676–681. doi: 10.1523/JNEUROSCI.21-02-00676.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AW, Gallagher M, Holland PC. The Basolateral Amygdala Is Critical to the Expression of Pavlovian and Instrumental Outcome-Specific Reinforcer Devaluation Effects. J. Neurosci. 2009;29:696–704. doi: 10.1523/JNEUROSCI.3758-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JL, Day JJ, Aragona BJ, Wheeler RA, Wightman RM, Carelli RM. Basolateral Amygdala Modulates Terminal Dopamine Release in the Nucleus Accumbens and Conditioned Responding. Biological Psychiatry. 2010;67:737–744. doi: 10.1016/j.biopsych.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongen-Relo AL, Kaufmann S, Feldon J. A differential involvement of the shell and core subterritories of the nucleus accumbens of rats in memory processes. Behav Neurosci. 2003;117:150–168. doi: 10.1037//0735-7044.117.1.150. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat. J Comp Neurol. 1978;178:225–254. doi: 10.1002/cne.901780204. [DOI] [PubMed] [Google Scholar]
- Luiten PG, Gaykema RP, Traber J, Spencer DG., Jr. Cortical projection patterns of magnocellular basal nucleus subdivisions as revealed by anterogradely transported Phaseolus vulgaris leucoagglutinin. Brain Res. 1987;413:229–250. doi: 10.1016/0006-8993(87)91014-6. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Topographical organization of amygdaloid projections to the caudatoputamen, nucleus accumbens, and related striatal-like areas of the rat brain. Neuroscience. 1991;44:15–33. doi: 10.1016/0306-4522(91)90248-m. [DOI] [PubMed] [Google Scholar]
- McGinty VB, Grace AA. Selective Activation of Medial Prefrontal-to-Accumbens Projection Neurons by Amygdala Stimulation and Pavlovian Conditioned Stimuli. Cereb. Cortex. 2008;18:1961–1972. doi: 10.1093/cercor/bhm223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog. Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Murphy ER, Robinson ESJ, Theobald DEH, Dalley JW, Robbins TW. Contrasting effects of selective lesions of nucleus accumbens core or shell on inhibitory control and amphetamine-induced impulsive behaviour. Eur J Neurosci. 2008;28:353–363. doi: 10.1111/j.1460-9568.2008.06309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA. The amygdala, reward and emotion. Trends Cog Sci. 2007;11:489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. Lesions of medial prefrontal cortex disrupt the acquisition but not the expression of goal-directed learning. J Neurosci. 2005;25:7763–7770. doi: 10.1523/JNEUROSCI.1921-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. Orbitofrontal Cortex Mediates Outcome Encoding in Pavlovian But Not Instrumental Conditioning. J. Neurosci. 2007;27:4819–4825. doi: 10.1523/JNEUROSCI.5443-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. Differential Involvement of the Basolateral Amygdala and Mediodorsal Thalamus in Instrumental Action Selection. J. Neurosci. 2008;28:4398–4405. doi: 10.1523/JNEUROSCI.5472-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JA, Olmstead MC, Burns LH, Robbins TW, Everitt BJ. Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by D-amphetamine. J Neurosci. 1999;19:2401–2411. doi: 10.1523/JNEUROSCI.19-06-02401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Holland PC, Gallagher M. Amygdalar and prefrontal pathways to the lateral hypothalamus are activated by a learned cue that stimulates eating. J Neurosci. 2005;25:8295–8302. doi: 10.1523/JNEUROSCI.2480-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Setlow B, Holland PC, Gallagher M. Amygdalo-Hypothalamic Circuit Allows Learned Cues to Override Satiety and Promote Eating. J. Neurosci. 2002;22:8748–8753. doi: 10.1523/JNEUROSCI.22-19-08748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu AT, Popa D, Pare D. Coherent gamma oscillations couple the amygdala and striatum during learning. Nat Neurosci. 2009;12:801–807. doi: 10.1038/nn.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Positive and Negative Motivation in Nucleus Accumbens Shell: Bivalent Rostrocaudal Gradients for GABA-Elicited Eating, Taste “Liking”/“Disliking” Reactions, Place Preference/Avoidance, and Fear. J. Neurosci. 2002;22:7308–7320. doi: 10.1523/JNEUROSCI.22-16-07308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Glutamate motivational ensembles in nucleus accumbens: rostrocaudal shell gradients of fear and feeding. Eur J Neurosci. 2003;17:2187–2200. doi: 10.1046/j.1460-9568.2003.02642.x. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Cador M, Taylor JR, Everitt BJ. Limbic-striatal interactions in reward-related processes. Neurosci Biobehav Rev. 1989;13:155–162. doi: 10.1016/s0149-7634(89)80025-9. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat Neurosci. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron. 2003;39:855–867. doi: 10.1016/s0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- Setlow B, Holland P, Gallagher M. Disconnection of the basolateral amygdala complex and nucleus accumbens impairs appetitive pavlovian second-order conditioned responses. Behav Neurosci. 2002;116:267–275. doi: 10.1037//0735-7044.116.2.267. [DOI] [PubMed] [Google Scholar]
- Simmons DA, Neill DB. Functional interaction between the basolateral amygdala and the nucleus accumbens underlies incentive motivation for food reward on a fixed ratio schedule. Neuroscience. 2009;159:1264–1273. doi: 10.1016/j.neuroscience.2009.01.026. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE. Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J Neurosci. 1999;19:11040–11048. doi: 10.1523/JNEUROSCI.19-24-11040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RA, Carelli RM. Dissecting motivational circuitry to understand substance abuse. Neuropharmacology. 2009;56:149–159. doi: 10.1016/j.neuropharm.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf NJ, Hernit MC, Butcher LL. Cholinergic and non-cholinergic projections from the rat basal forebrain revealed by combined choline acetyltransferase and Phaseolus vulgaris leucoagglutinin immunohistochemistry. Neurosci Lett. 1986;66:281–286. doi: 10.1016/0304-3940(86)90032-7. [DOI] [PubMed] [Google Scholar]
- Wright CI, Beijer AV, Groenewegen HJ. Basal amygdaloid complex afferents to the rat nucleus accumbens are compartmentally organized. J Neurosci. 1996;16:1877–1893. doi: 10.1523/JNEUROSCI.16-05-01877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, Cullinan WE. Projections from the nucleus accumbens to cholinergic neurons of the ventral pallidum: a correlated light and electron microscopic double-immunolabeling study in rat. Brain Res. 1992;570:92–101. doi: 10.1016/0006-8993(92)90568-t. [DOI] [PubMed] [Google Scholar]
- Zahm DS. An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neurosci Biobehav Rev. 2000;24:85–105. doi: 10.1016/s0149-7634(99)00065-2. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Brog JS. On the significance of subterritories in the “accumbens” part of the rat ventral striatum. Neuroscience. 1992;50:751–767. doi: 10.1016/0306-4522(92)90202-d. [DOI] [PubMed] [Google Scholar]