Summary
Nitric oxide (NO) has been shown to exhibit significant anti-platelet activity and its release from polymer matrices has been already utilized to increase the biocompatibility of various blood-contacting devices. Herein, details of a new synthetic approach for preparing NO-releasing diazeniumdiolated polyurethanes (PU) are described. The method's utility is demonstrated by the incorporation of methoxymethyl- or sugar-protected pre-formed diazeniumdiolate moieties directly into chain extender diols which are then incorporated into the polyurethane backbone. This approach provides the ability to control the number of diazeniumdiolate groups incorporated into the polymer backbone, and hence the surface flux of NO that can ultimately be liberated from polymeric films prepared from the new PU materials. The method provides a means of covalently attaching diazeniumdiolate groups to polyurethanes in a form that resists dissociation of NO during processing but can be activated for spontaneous NO release via hydrolysis of the carbohydrate or methoxymethyl moieties under basic and acidic conditions, respectively.
Introduction
Implantable, blood-contacting materials are prone to thrombus formation on their surfaces, which can potentially limit their biomedical use.1,2 Nitric oxide (NO) release from such materials has been shown to be an effective method to prevent such undesirable interactions and create more biocompatible polymer surfaces.3-6 Prior studies of NO-releasing polyethyleneimines,7 silicone rubbers,8 and polymethacrylates9 have demonstrated the feasibility of covalently bonding NO-releasing moieties (i.e., diazeniumdiolates) to hydrophobic polymer backbones. These polymers, when exposed to physiological conditions, release NO with fluxes at or above the level of normal endothelial cells (ca. 0.5 − 4 × 10-10 mol cm-2 min-1),10 the only truly thromboresistant surface. Furthermore, when employed as coatings for implantable devices such as vascular grafts and catheters, these NO-releasing polymers have exhibited decreased clot formation on their surfaces compared to control materials that did not release NO.11,12
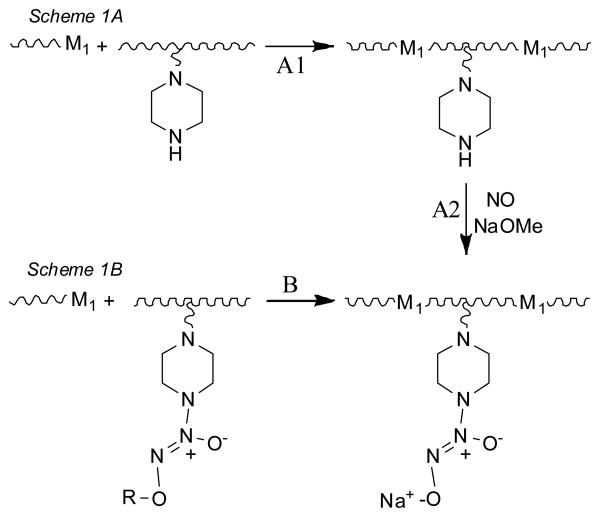
The current generalized methodology used to synthesize such diazeniumdiolated polymers is outlined in Scheme 1A, and involves initially forming the desired precursor polymer from monomers containing reactive secondary amine sites. These pre-formed polymers can then be loaded with NO at high pressures under trial and error conditions to yield diazeniumdiolated materials. An alternative method of synthesis, depicted in Scheme 1B, is to covalently attach the diazeniumdiolate group directly to the monomer prior to forming the polymer. Such pre-formed diazeniumdiolated monomers can then be reacted with other monomers, cross-linking agents or chain extenders to yield the desired polymer of interest.
Scheme 1.
Two general synthetic approaches to preparation of diazeniumdiolated polymers: (A) by incorporating amine sites pendent to the polymer backbone and then reacting them with NO in the presence of a base to form the ionic diazeniumdiolate groups; and (B) by incorporating diazeniumdiolate functionalities bearing protecting groups R pendent to the polymer backbone and then cleaving off the R groups to form the same ionic diazeniumdiolate-substituted polymer as produced in route A.
The literature suggests several Scheme 1A approaches for making PUs containing covalently bound diazeniumdiolate groups: a) placing secondary amine groups in the backbone by including relevant monomers in the diol chain extender before polymerization, followed by reacting the amines with NO;13 b) attaching secondary amines via linker to the amide nitrogen of preformed PUs followed by reacting these amine moieties with NO;13 and c) incorporating lysines into the chain extender followed by reaction with NO.14,15 While there are many previous reports of NO-releasing polymers being prepared using a Route A post-loading approach,7-9, 13-16 the method is not suitable for the controlled introduction of a defined number of diazeniumdiolates on the polymer backbone since the reaction yield for NO loading cannot be controlled or predicted. In contrast, the less often used process outlined in Scheme 1B, could potentially provide for enhanced control over the number of diazeniumdiolate groups (and hence NO loading) that can be incorporated pendent to the polymer backbone. Since the diazeniumdiolate group is attached to the monomer prior to forming the polymer, the amount of NO loading in the polymer is predetermined. This is in direct contrast to Scheme 1A, where the amount of NO-loading is dependent on the efficiency of the reaction of NO with the derivatized polymer and the extent of this reaction can only be determined by extensive chemical analysis post NO-loading.
This paper provides details on the alternative methodology for the synthesis of diazeniumdiolated polyurethanes that overcomes the limitations with currently used synthetic methods. The utility of the new method (Scheme 1B) is demonstrated in two ways. First, multiple chain extenders are used to create polyurethanes with designed loading, which demonstrates that the methodology is universal. Second, additional formulations of polymers were prepared using the same chain extender that we published on previously,17 in which O2-substituted diazeniumdiolate monomers are linked to the diol chain extender before polymerization, followed by deprotection to generate the spontaneous NO-releasing form of this new material. This demonstrates that the NO-release parameters can be tailor-built into the polymer by means of controlling the degree of chain extender incorporation.
Results and discussion
Design strategy for the synthesis of diazeniumdiolated polyurethanes
Owing to their excellent hydrolytic stability, physical strength, and high flexure endurance, polyurethanes are the most widely used and versatile polymers for preparing implantable medical devices such as heart valves, catheters, pacemaker leads and vascular grafts and patches.18,19 Thus, the additional function of NO release to provide anti-platelet action would further enhance the use of polyurethanes for preparing biomedical devices. In addition, the versatile nature of polyurethane synthesis allows for tailoring specific properties as demonstrated by several research groups.20-25 The synthesis of polyurethanes is well developed and provides various routes to prepare polymers containing diazeniumdiolate moieties. Thus, the use of polyurethanes as a model system for examining the two possible synthetic routes for incorporating NO-releasing moieties is attractive.
Because the diazeniumdiolate group is labile under the conditions required for polymerization, it requires temporary protection. Carbohydrate,17,26 methoxymethyl (MOM),27 and other27, 28 protecting groups have been suggested. These protecting groups make diazeniumdiolates more hydrolytically stable. In principle, the chosen protective group in any polymer assembly strategy should be robust enough to withstand the conditions required for preparing the desired NO-releasing polyurethanes. Two examples demonstrating the use of Route 1B to prepare tailored NO-releasing polyurethanes are presented.
Example 1: use of a methoxymethyl (MOM) protecting group
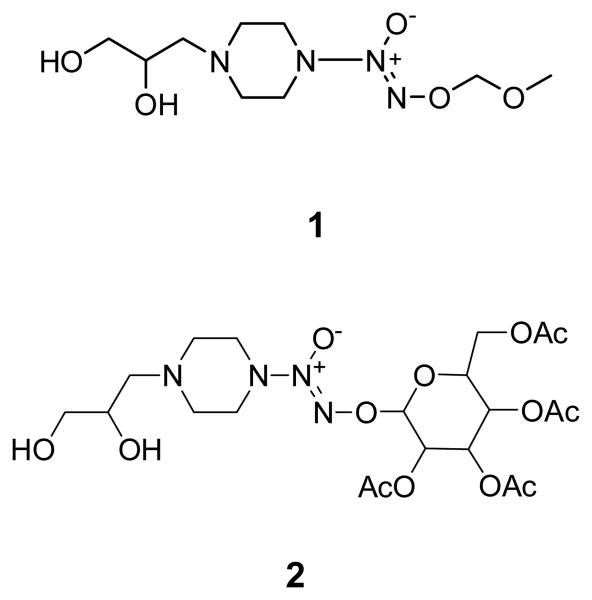
The first protected chain extender considered in this work in order to demonstrate utility in the synthetic methodology was O2-methoxymethyl 1-[4-(2,3-dihydroxypropyl)piperazin-1-yl]diazen-1-ium-1,2-diolate (1) (see Figure 1 for structure).
Fig. 1.
O2-Protected diazeniumdiolated chain extenders used to prepare diazeniumdiolated polyurethanes.
Compound 1 was thoroughly characterized using UV, combustion analysis, and NMR before polymerization to ensure the presence of the diazeniumdiolate moiety. The UV spectrum of 1 has a maximum absorbance at 232 nm in ethanol. This is in the range of previously reported MOM-protected diazeniumdiolate functionalities covalently bonded to the nitrogen of piperazine, which possess maximum absorbances between 225-245 nm.29 In addition, before 1 could be used to synthesize a polyurethane, test conditions were explored to determine the requirements for removal of the MOM protecting group. Methoxymethyl groups show superb stability in basic media, but readily hydrolyze under acidic conditions. To this end, a solution of 1 in THF was treated with 6 M HCl. The reaction course was monitored via UV spectroscopy. The λmax shifted from 232 nm to 244 nm with additional small peaks observed around 335 nm. These bands correspond to nitrosamines and strongly suggest that the diazeniumdiolate decomposed. When the diazeniumdiolate group decomposes in air under highly acidic conditions, it releases NO at a rapid rate. The oxidative products of NO (specifically N2O3) can then react with the secondary amine nitrogen in piperazine to form a nitrosamine according to equation 1:
| (1) |
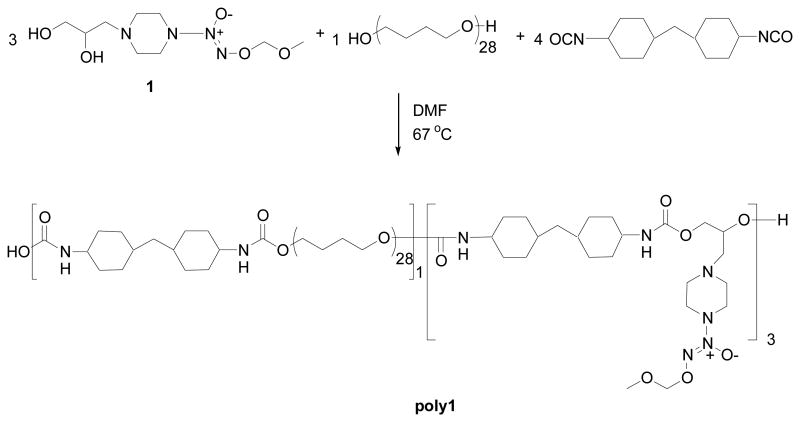
A polyurethane (poly1) was synthesized using compound 1 as the chain extender, an ether-based macroglycol and 4,4′-methylene-bis-cyclohexyl-diisocyanate (MDI) as the diisocyanate according to Scheme 2. The experimental mole ratio between the chain extender, macroglycol and diisocyanate was 3:1:4. The 1H NMR spectra of poly1 exhibit no resonances for unreacted monomers and confirmed the 3:1:4 incorporation of the components into the polymer via urethane linkages. Specifically, the reasonance at δ 5.2 ppm (corresponding to the −CH2- of the methoxymethyl protecting group) verified the expected loading of the diazeniumdiolate into the polymer backbone. The 1H-NMR spectrum also contained similar resonances to previously prepared polyurethanes demonstrating the expected connectivity.
Scheme 2.
Reaction scheme using a MOM-protected diazeniumdiolated chain extender, a macroglycol and a diisocyanate to yield a protected diazeniumdiolated polyurethane poly1.
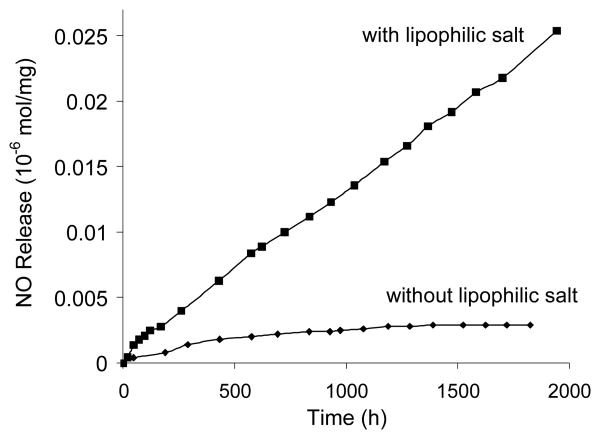
The NO release behavior of poly1 was found by soaking 1-cm disks of material in PBS at 37 °C and using the Griess assay (see Experimental Section) to detect NO release indirectly via nitrite formation, which is a valid assumption here given the previously reported stability of the MOM group.29 As shown in Figure 2, the release ceases after ∼ 500 h unless a lipophilic salt is added that can buffer the organic phase with respect to proton activity, as previously reported.30 The data is normalized per mg of poly1 in order to compare the relative release rates as a function of NO-releasing polymer. When the lipophilic salt is added, the NO release rate from poly1 is very slow and linear. After 67 d, only 0.067% of theoretical NO was detected. The extremely slow rate of release may be attributed to the very slow hydrolysis of the MOM group at pH 7.4.
Fig. 2.
NO release curve for poly1 in PBS buffer at 37 °C Each data point is an average of 3 separate measurements. A table summarizing the average values and standard deviations can be found in the supporting information.
Example 2: use of a carbohydrate protecting group
In an ideal situation, a protecting group that could be cleaved under basic conditions and produce benign by-products is desired. Both our group17 and Wu et al.26 have reported on the protection of diazeniumdiolates using carbohydrate chemistry. Glucose groups can be placed on the terminal oxygen of the diazeniumdiolate. It has been demonstrated that hydrolysis of the carbohydrate motif to yield the NO-releasing diazeniumdiolate and the free carbohydrate unit can take place under basic conditions.17 An interesting facet of this approach is the observation that a benign by-product, in the form of the monosaccharide, is released upon hydrolysis.
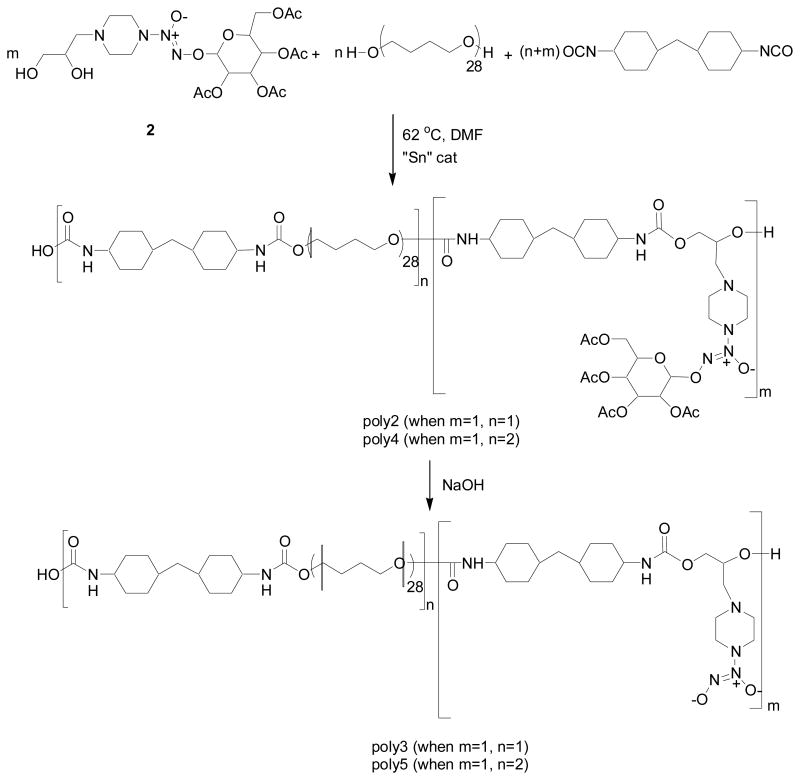
A polyurethane (poly2) was synthesized using 2 as the chain extender, terathane as the ether-based macroglycol and MDI as the diisocyanate linker to give poly2 according to Scheme 3. The mole ratio of the chain extender to macroglycol to diisocyanate used was 1:1:2. Proton NMR and UV spectroscopy were employed to characterize the resulting polymer. Proton NMR signals verified both the connectivity of the components to form urethane linkages and 1:1:2 incorporation of the chain extender to terathane to MDI (see Supporting Information for spectrum) in the resulting polymer. The NMR spectrum also showed no unreacted monomers further supporting full incorporation of the diazeniumdiolated monomer. The UV spectrum of poly2 in THF had a λ max at 232 nm, which shifted to 250 nm upon treatment with 0.01 M NaOH demonstrating both the presence of the sugar protected diazeniumdioate group as well as the ability to deprotect the sugar to give the sodium ion stabilized diazendiumdiolate. Taken together, this data shows that using a sugar-protected diazeniumdiolated chain extender known amounts of diazeniumdiolates can certainly be introduced into the polymer backbone.
Scheme 3.
Polymerization reaction of sugar-protected diazeniumdiolated chain extenders with the macroglycol and diisocyanate required to synthesize polyurethanes poly2 and poly4 followed by deprotection to generate spontaneous NO-releasers poly3 and poly5.
Although the carbohydrate protecting groups are most easily removed under highly basic conditions, they have been reported to undergo slow hydrolysis at pH 7.4.26 It was anticipated, therefore, that under physiological conditions the acetyl glucose group of poly2 would be cleaved. Using UV spectroscopy and chemiluminescence to probe the reaction, however, the removal of the acetyl glucose group was found to be difficult even with prolonged incubation at pH 7.4 at 37 °C. This is probably due to the fact that the acetyl groups were still present since excess hydroxide is required for fully cleaving the sugar residue. In fact, Showalter et al. demonstrated previously that basic conditions are required for efficient cleavage of the sugar protecting group.17 The acetyl glucose protecting group can therefore be removed by treating a solution of poly2 in THF with 1 mM NaOH for 5 h to yield poly3 (Scheme 3). This was determined by monitoring the UV spectrum of poly2 after treatment with NaOH. The λmax at 232 nm decreased and λmax at 250 nm increased as a function of time.
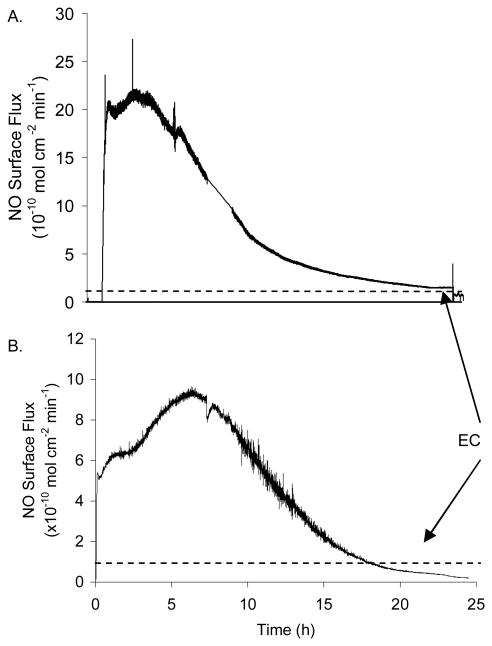
Nitric oxide release measurements were made from thin films (ca. 150 μm) of poly3 under physiological conditions (pH 7.4, 37 °C) using chemiluminescence. A representative NO surface flux curve is shown in Figure 3A. The NO release profile from films prepared with poly3 shows a higher level of initial NO release that slows with time. This polyurethane film releases levels of NO greater than or equal to that of the endothelium over several hours. The release of NO from these materials can be extended with the addition of lipophilic sites into the polymer membrane. Integration of the amount of NO released after the film no longer releases NO yields a nearly theoretical amount of NO given the amount of chain extender incorporated into the polymer.
Fig. 3.
Representative NO surface flux of poly3 (A) and poly5 (B) for coatings (150-μm thickness) on 8 mm diameter tubings. NO release was measured directly via chemiluminescence in PBS (pH 7.4) at 37 °C. The dotted horizontal lines denote the levels that are produced by the normal endothelium (EC).
To demonstrate the ability to control the level of NO release via this synthetic strategy, a second polyurethane was synthesized as in Scheme 1B, and Scheme 3. The ratio of chain extender to ether-based macroglycol to diisocyanate, in this case, was 1:2:3. The resulting polyurethane (poly4) had similar structural characteristics as poly2 with signature glucose 1H-NMR signals between δ 5 – 6 ppm.
As shown in Figure 3B, the NO release profile of the deprotected polymer 4 (poly5) is slower than that observed for poly3 as expected. The rate of NO release from this polymer composition would be predicted to be slower than that of poly3 given the lower amount of chain extender utilized to prepare this material. The removal of the diazeniumdiolate group is proton-dependent, and, therefore, relies upon water uptake into the polymer. In any given volume of polymer matrix, there are fewer diazeniumdiolate groups per unit volume. In addition, poly5 releases proportionally less NO than poly3 as expected based on the mole ratio of diazeniumdiolated monomer utilized in the synthesis. Thus, using Scheme 1B, two polyurethanes were synthesized with varying diazeniumdiolate loading in a controlled manner.
NO-addition reaction to form a polyurethane containing a pendent diamine chain extender using Scheme 1A
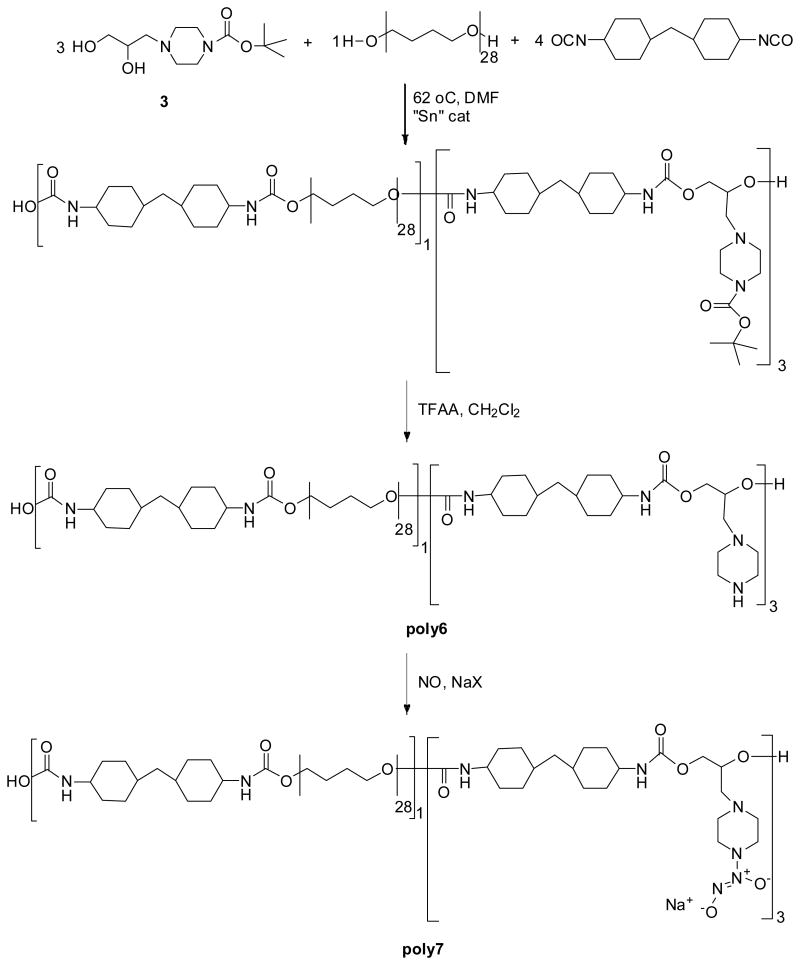
To compare the effect of NO loading onto the same polyurethane backbone, but altering the step at which NO is added (Scheme 1B, post-loading), a chain extender (see Compound 3, Scheme 4 for structure) containing a pendent piperazine was synthesized and utilized in the preparation of a piperazine-containing polyurethane (poly6). This approach takes into account the increased reactivity of the piperazine nitrogen to NO as well as the need for a pendent diamine group. As early as 1962, Drago showed that a high pressure reaction of piperazine with NO could produce various salts of diazeniumdiolated piperazine.31 Hrabie et al. further expanded this to isolate the piperazine diazeniumdiolate as a sodium salt in its pure form.32 More recently, Saavedra et al. used piperazine as a linker for incorporating diazeniumdiolates into other biomedical functional molecules.29
Scheme 4.
Route used to prepare PUs containing pendent piperazine moieties that are reacted with NO at elevated pressures.
When poly6 was reacted homogeneously with NO for 24 h to yield poly7, it was found that the majority of the nitrogen oxide species generated was nitrite instead of NO. The ratio of NO/NO2- was only 0.2 for poly7. This was determined by measuring NO release with an electrochemical sensor33 and total nitric oxide and nitrite release via the Griess assay.34 Even when poly6 was reacted with NO in the presence of exogenous cations (i.e., sodium salts), the majority of nitrogen oxide species released from NO-reacted polymers using the approach outlined in Scheme 1A, was nitrite ion. Furthermore, under no reaction conditions employed thus far with these polymers has the amount of NO loaded ever equaled the amine content of the base polymer.
Comparison of scheme 1A and B, for preparing NO-releasing polymers
Two possible synthetic routes have been presented for preparing covalently bonded diazeniumdiolated polymers. The initial route (Scheme 1A) is based on incorporating pendent amine sites into the polymer via the chain extender and subsequently reacting the resulting polymer with NO. The second route (Scheme 1B) involves directly incorporating pre-formed diazeniumdiolates into the polymer backbone. Evidence has been presented that suggests that both methods are useful for synthesizing NO-releasing polyurethanes.
The prevailing challenge, however, associated with preparing NO-releasing polyurethanes via Route A is the destabilization of the diazeniumdiolate group upon exposure to air during the work-up of the polymer. This destabilization is thought to arise from the rigidity of the polymer backbone, preventing hydrogen bonding between the hydrogen of an ammonium nitrogen and the negatively charged diazeniumdiolate formed on a secondary nitrogen. Incorporating exogenous cationic sites in the presence of a base during the NO addition process, however, can lead to an increase in stability of the diazeniumdiolate moieties, and consequently, higher NO-release values.35
Although NO-releasing polyurethanes were successfully prepared using Scheme 1A, the method relies on NO's ability to react with amine sites within the polymer structure. It has been demonstrated that there is limited control over the number of amine sites that will react with NO and form stable NO-adducts even when exogenous cationic sites are added. In addition to this work, Zhang et al. also reported incomplete NO loading onto the backbone of silicone rubbers possessing secondary amine sites.8 For example, only 15% of the amine sites incorporated into DACA-SR reacted with NO and of these 60% did not form stable diazeniumdiolates.8 In a similar manner, Parzuchowski et al. reported that significant amounts of non-reacted amine sites remained on polymethacrylates possessing pendent secondary amine sites after the NO reaction, regardless of the time the material was exposed to NO.9 For materials reported thus far, no significant additional NO loading has been achieved after 72 h exposure to NO. Therefore, external factors such as NO addition time have not proven successful in increasing the NO-loading.
To date, the extent of NO-loading for any hydrophobic polymer synthesized using Scheme 1A, can be determined only after extensive chemical analysis is performed on the NO-reacted polymer. Thus, the idea of being able to tailor or control the NO-releasing properties of the material via its synthesis has not yet been fully realized. The second method of synthesizing NO-releasing polyurethanes presented here (Scheme 1B) is a significant step forward toward true tailored-design synthesis of NO-releasing polymers. This synthetic scheme enables known quantities of diazeniumdiolates to be incorporated onto the polymer backbone. This has dramatically improved the control over the amount of NO-loading achieved. For example, NO was not able to be loaded at significant levels to the base polyurethane polymer (poly6) using Scheme 1A. In contrast, the use of Scheme 1B, enabled controlled quantities of diazeniumdiolates to be loaded into the same base polymer. These results are summarized in Table I.
Table I. Comparison of Loading Amines with Diazeniumioldates.
| Polymer | Scheme | Loading of NONOate group as a function of amine sites | % NO release over time (h) |
|---|---|---|---|
| poly1 | 1B | 100% | 0.67% (67 d) |
| poly2 → poly3 | 1B | 100% | 16% (23 h)* |
| poly4→ poly5 | 1B | 100% | 3.1% (17 h)* |
| poly7 | 1A | <1% | nitrite release |
| DACA/N2O28 | 1A | 40-60% of amines | NR |
| PM/N2O29 | 1A | <50% of amines | 23-29% (15 h) |
NR = not reported
still releasing and organic phase not buffered
In addition to controlling NO-loading by rational design of polymer structures, the ability to manufacture the NO-releasing polymer for use in biomedical applications using current manufacturing processes is of interest. Nitric oxide-releasing polymer prepared by Method A cannot undergo traditional manufacturing methods (extrusion, moisture conditions, etc.) which has limited their entry into the market. Therefore, the addition of a protecting group as used in Method B provides additional stability during fabrication that allows for the NO moieties to be maintained. Continued efforts towards this end could include investigations into coating the protected polyurethane material onto medical devices and then determining conditions that can hydrolyze the protecting groups in situ. One such method would be to treat the acetylated glucose groups with catalytic amounts of sodium methoxide followed by treatment with NaOH. The end result would be an attractive processing method for the preparation of NO-releasing medical devices. Other studies might include designing additional protecting groups that are cleaved under controlled conditions such as light.
Experimental
Reagents
Compound 2 was prepared and characterized as previously described in the literature.17 NMR data obtained from the compounds were verified with the previously published procedures. Di-tert-butyldicarbonate, dibutyltin dilaurate, 4,4′-methylene-bis-cyclohexyl-diisocyanate (MDI), trifluoroacetic acid, sulfanilic acid, N-(naphthyl)ethylene diamine dihydrochloride, and terathane (MW 2000) were purchased from Aldrich (Milwaukee, WI) and used as received. Phosphate buffered saline ((PBS), pH 7.4, 0.01 M phosphate containing 138 mM NaCl and 2.7 mM KCl) was obtained from Sigma (St. Louis, MO). Potassium tetrakis(4-chlorophenyl)borate (KTpClPB) is a product of Fluka (St. Louis, MO). Dimethylformamide, methanol, tetrahydrofuran, hexanes, dichloromethane, chloroform, acetonitrile, and concentrated hydrochloric acid were products of Fisher (Fair Lawn, NJ). DMF was dried by distillation over CaH2 under reduced pressure and then stored over 4-Å molecular sieves. THF was distilled over sodium using a benzophenone indicator. Other reagents were purchased and used as received.
Instrumentation
1H-NMR spectra were collected on a Varian Mercury 300 or Inova 400 and were referenced to the residual proton solvent resonances. UV-vis spectra were recorded on a Beckman DU 640B spectrophotometer. Nitrite determinations, using the Griess assay, were carried out using a Labsystems MultiscanRC microtiter plate reader at 540 nm. Elemental analyses were performed by the University of Michigan Microanalysis Laboratory (Ann Arbor, MI), Galbraith Laboratories (Knoxville, TN) or Atlantic Microlab, Inc. (Norcross, GA). Gel permeation chromatography was performed to determine the molecular weight of the polymers using a UV and RI detector, polystyrene calibration standards, and THF as the eluent. The columns were the Waters Styrogel Models HT2, HT3, and HT4.
Preparation of polymer films
For polymer films without additions, films were prepared by dissolving, dispersing, or swelling ∼200 mg of polymer components in 2 mL THF. For films with lipophilic additives, KTpClPB was dissolved into the polymer solution after the polymer was dissolved in a 1:1 mole ratio between chain extender and KTpClPB. For the films prepared with poly1 and lipophilic additive, both an additional plasticizer and PVC were added to the film to maintain film integrity as reported by Yun et al.35 In this case, polymer blended films were prepared by dissolving 27 wt. % poly1, 39 wt. % DOS, 14 wt. % PVC and 20 wt. % KTpClPB in THF. The resulting polymer cocktails were then cast into a 2.5 cm diameter Teflon molds and allowed to cure under ambient conditions overnight. A 1 cm diameter circular punch was cut from the 2.5 cm film and used for analysis.
NO release measurements by chemiluminescence
NO measurements were performed using a Sievers Nitric Oxide Analyzer (NOA), model 280 or 280i. The instrument was calibrated before each experiment with house nitrogen as the zero gas. The flow rate was set to 200 mL/min with a cell pressure of 5.4 torr and an oxygen pressure of 6.0 psig. The measurement was performed by inserting the NO-adducts of the PUs into a clean, dry, NOA measurement cell, sealing the cell with a rubber septum, and collecting a baseline level of NO. Purged PBS buffer was then injected via syringe through a septum into the NOA measurement cell. The NO produced from the sample was flushed from the solution into the chemiluminescence chamber via a constant nitrogen purge. The data were recorded on an internal RAM card and processed using Excel.
NO release measurements by an NO selective sensor
An NO-reacted polymer sample was placed into 70 mL deoxygenated PBS buffer and the amount of NO released was measured via an amperometric NO-selective electrochemical sensor.33 Briefly, the sensor consisted of glass encapsulated Pt (76 μm dia.) working electrode and a Ag/AgCl reference electrode (with 0.3 mM HCl/30 mM NaCl inner sensor solution) covered by a gas permeable membrane (expanded polytetrafluoroethylene), that provides high selectivity for detection of NO. A potential of + 900 mV vs. Ag/AgCl was applied to the platinum working electrode (WE) to achieve the complete oxidation of NO. Sensor was calibrated with NO saturated solution in deoxygenated PBS solution prior to measurement. The measured current was directly proportional to the concentration of NO.
Nitrite release measurements via colorimetric Griess assay
Aqueous solutions of PBS (pH 7.4) obtained from the NOA cell (after removal of the NO-reacted polymers and after purging of the solution for 15 min with N2) were analyzed via the Griess assay for nitrite release from the polymer.34 The Griess assay is a two-step process: diazotization followed by azo coupling. Briefly, 200- μL samples were pipetted into a 96-well microtiter plate and chilled to 4 °C. Forty μL of a 1:1 mixture of 6 M HCl and 12.5 mM sulfanilic acid was added to each well and the samples were chilled for an additional 10 min at 4 °C. Twenty μL of 12.5 mM N-(naphthyl)ethylene diamine dihydrochloride (NEDA) was then added to make an azo compound which has an absorbance maximum at 540 nm. The samples were analyzed with a microtiter plate reader. The absorbance at 540 nm is directly proportional to the concentration of nitrite in the sample.
O2-Methoxymethyl 1-[4-(2,3-Dihydroxypropyl)piperazin-1-yl]diazen-1-ium-1,2-diolate (1)
This compound was prepared by two different procedures. (A) A slurry of 798 mg (3.3 mmol) of sodium 1-[4-(2,3-dihydroxypropyl)piperazin-1-yl-]diazen-1-ium-1,2-diolate17 and 400 mg of anhydrous sodium carbonate in 20 mL of tetrahydrofuran was cooled to 0 °C. To the stirred mixture was added 0.306 mL (4 mmol) of chloromethyl methyl ether. After warming to room temperature and stirring overnight, the reaction mixture was concentrated to dryness on a rotary evaporator. After extracting with acetonitrile and without any further washing, the slurry was filtered through a layer of anhydrous magnesium sulfate. Evaporation of the solvent gave 386 mg of an amber oil. Purification was carried out by Flash 40 chromatography using a 4- × 7-cm KP-Sil column and 10:1 dichloromethane:methanol as the eluent, giving 193 mg of pure compound as a pale yellow oil. (B) A solution of 2.00 g (11 mmol) of O2-methoxymethyl 1-(piperazin-1-yl)diazen-1-ium-1,2-diolate29 and 0.80 mL (0.89 g, 12 mmol) of glycidol in 50 mL of ethanol was heated to reflux for 4 h. The reaction mixture was allowed to cool to room temperature and the ethanol was removed on the rotary evaporator to obtain 3.73 g of pale yellow oil. Purification was carried out as above except that a 4- × 15-cm column was used. UV (water) λmax (ε) 232 nm (8.7 mM-1 cm-1); UV (ethanol) λmax (ε) 236 nm (6.6 mM-1 cm-1); 1H-NMR (CDCl3) δ δ2.39-2.47 (m, 2H), 2.59-2.62 (m, 2H), 2.81-2.89 (m, 2H), 3.50 (s, 3H), 3.47-3.54 (m, 4H), 3.72-3.74 (m, 2H), 3.78-3.90 (m, 1H), 5.22 (s, 2H). Anal. Calcd for C9H20N4O5 (prepared by route A): C, 40.90; H, 7.63; N, 21.20. Found: C, 41.50; H, 7.80; N, 20.85; Calcd for C9H20N4O5·0.5 H2O (prepared by route B): C, 39.50; H, 7.69; N, 20.50. Found: C, 39.81; H, 7.78; N, 20.30.
4-(2,3-Dihydroxypropyl)piperazin-1-carboxylic acid tert-butyl ester (3)
A solution of 1.02 g (5.48 mmol) of tert-butyl 1-piperazine carboxylate and 0.40 mL (0.446 g, 6.02 mmol) of glycidol in 50 mL of methanol was heated to reflux for 4 h. The reaction mixture was allowed to cool to room temperature and the methanol was removed on the rotary evaporator to obtain 2.15 g of pale yellow oil. The oil crystallized upon standing and was recrystallized from ether/petroleum ether to give an analytically pure sample: mp 62-63 °C; 1H-NMR (CDCl3) δ 1.47 (s, 9H), 2.33-2.44 (m, 4H), 2.54-2.65 (m, 2H), 3.41-3.57 (m, 6H), 3.74-3.87 (m, 1H); MS, exact mass calculated for C12H24N2O4, M+ 260.1736; measured M+ 260.1742. Anal. Calcd for C12H24N2O4·0.5 H2O: C, 54.35; H, 9.42; N, 10.14. Found: C, 53.99; H, 9.20; N, 9.81.
Synthesis of poly1
A dry 250-mL 3-neck round-bottom flask equipped with a condenser, stir bar, and an N2 inlet/outlet was charged with 1.17 mmol 1, 0.36 mmol terathane 2000, and 1.54 mmol MDI in dry THF. The ratio of 1 to terathane to HDMI was 3:1:4. The reaction mixture was refluxed overnight at 67 °C. After cooling, the mixture was precipitated into hexane, filtered and dried under high vacuum.
Synthesis of poly2
A dry 100-mL 3-neck flask equipped with a condenser, mechanical stirrer, and an N2 inlet/outlet was charged with terathane 2000 (4.00 g, 1.82 mmol), MDI (3.64 g, 0.95 mL, 3.64 mmol), and dibutyltin dilaurate (1 drop) in dry DMF (10 mL). The reaction mixture was heated to 65 °C. After 1.5 h, 2 (1.00 g, 1.82 mmol) was added with an additional 7 mL of dry DMF, giving a final molar ratio of 2 to terathane to HDMI of 1:1:2. The solution was allowed to stir overnight at 65 °C during which time the viscosity increased. The solution was poured into H2O and the solid was collected and dried under vacuum. UV (THF) λmax 232 nm. Mw 126,631 with PDI 1.89.
Synthesis of poly3
A 396.6-mg aliquot of poly2 was dissolved in 15 mL THF. After the sample had dissolved, 600 μL 2.5 M NaOH was added to the poly2 solution and the solution was allowed to stir overnight at room temperature. After 18 h, the polymer solution was precipitated into hexanes. The resulting polymer was collected and dried under vacuum. UV (THF) λmax 248 nm. Mw 92,317 with PDI 2.14.
Synthesis of poly4
A dry 100-mL 3-neck flask equipped with a condenser, mechanical stirrer, and an N2 inlet/outlet was charged with terathane 2000 (4.93 g, 2.48 mmol), MDI (0.97 mL, 3.73 mmol), and dibutyltin dilaurate (1 drop) in dry DMF (10 mL). The reaction mixture was heated to 65 °C. After 1.5 h, 2 (0.70 g, 1.27 mmol) was added with an additional 7 mL of dry DMF, giving a final molar ratio of 2 to terathane to HDMI was 1:2:3. The solution was allowed to stir overnight at 65 °C during which time the viscosity increased. The solution was poured into H2O and solid poly4 was collected and dried under vacuum. UV (THF) λmax 232 nm. Mw 157,269 with PDI 2.27.
Synthesis of poly5
A 571.2-mg aliquot of poly4 was dissolved in 15 mL THF. After the sample had dissolved, 500 μL 2.5 M NaOH was added to the poly4 solution and the solution was allowed to stir overnight at room temperature. After 24 h, the polymer solution was precipitated into hexanes. The resulting polymer was collected and dried under vacuum. UV (THF) λmax 248 nm. Mw 158,561 with PDI 1.81.
Synthesis of poly6
A dry 100-mL 3-neck flask equipped with a condenser, mechanical stirrer, and an N2 inlet/outlet was charged with terathane 2000 (3.84 g, 1.90 mmol), MDI (2.0 mL, 7.7 mmol), and dibutyltin dilaurate (1 drop) in dry DMF (5 mL). The reaction mixture was heated to 62 °C. After 2 h, 1.50 g (5.8 mmol) of 3 was added with an additional 7 mL of dry DMF, giving a final molar ratio of 8 to terathane to MDI of 3:1:4. The solution was allowed to stir overnight at 65 °C during which time the viscosity increased. The solution was poured into H2O and the solid was collected and dried under vacuum. The BOC-protected polymer was dissolved in 25% trifluoroacetic acid (TFAA)/CH2Cl2 (133 mL) and allowed to stir for 10 h. The solvent was removed to give a viscous oil. The oil was dissolved in DMF and the polymer was deprotonated and precipitated by pouring into NaHCO3 (saturated, 500 mL). The solid was collected, washed with H2O and dried under vacuum. Any residual water was removed by redissolving the polymer in THF (300 mL) and drying over MgSO4. The mixture was filtered through a ¼-inch plug of Celite and the polymer was precipitated by pouring the filtrate into hexanes (600 mL). The polymer was filtered and dried under vacuum. Yield of poly6 = 6.2 g.
Significance and conclusions
Because polyurethanes are the most widely used blood-contacting polymers for implantable devices and NO has been shown to have significant effect on biofouling, it is of great interest to develop methods that can yield NO-releasing polyurethanes in a controlled manner. Toward this end, in this paper, synthetic methods were presented for the first time that allows controlled incorporation of NO-releasing moieties into polyurethane backbones. This opens the door to designing polyurethanes that have both NO-releasing properties and the desired mechanical properties within their synthesis. For example, additional polyurethanes could be prepared by altering the identity and/or mole ratio of the components (i.e., chain extender, macroglycol or diisocyanate) used in the reaction mixture. From a broader perspective, as a result of the methodology presented herein, known amounts of NO-releasing groups can now be incorporated into polymer structures via their synthesis. Thus, it is envisioned that this methodology can be applied to multiple polymer structures to gain better control of NO moieties into polymer substrates. This would allow for more advanced materials to be developed that have the dose response required for a physiological response and the mechanical properties appropriate for the desired application.
Supplementary Material
Acknowledgments
The authors are grateful for funding from the National Institutes of Health (NIH) through grant NIH EB-000783. Research was also supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, as well as National Cancer Institute contract NO1-CO-2008-00001 to SAIC.
Footnotes
Supplementary information. NMR Spectra for poly1 – poly6, UV spectra for compound 1, poly2, and poly3, as well as a table summarizing the NO release for poly1 are included. The material is available free of charge via the Internet.
Bibliographic references and notes
- 1.Wagner WR, Schaub RD, Sorensen EN, Synder TA, Wilhelm CR, Winowich S, Borovetz HS, Kormos RL. J Biomat Sci Polymer Ed. 2000;11:1239–1259. doi: 10.1163/156856200744183. [DOI] [PubMed] [Google Scholar]
- 2.Frost MC, Meyerhoff ME. Anal Chem. 2006;78:7370–7377. doi: 10.1021/ac069475k. [DOI] [PubMed] [Google Scholar]
- 3.Pulfer SK, Ott D, Smith DJ. J Biomed Mater Res. 1997;37:182–189. doi: 10.1002/(sici)1097-4636(199711)37:2<182::aid-jbm6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 4.Schoenfisch MH, Mowery KA, Rader MV, Baliga N, Wahr JA, Meyerhoff ME. Anal Chem. 2000;72:1119–1126. doi: 10.1021/ac991370c. [DOI] [PubMed] [Google Scholar]
- 5.Mowery KA, Schoenfisch MH, Baliga N, Wahr JA, Meyerhoff ME. Electroanalysis. 1999;11:681–686. [Google Scholar]
- 6.Skrzypchaki AM, Lafayette NG, Bartlett RH, Zhou Z, Frost MC, Meyerhoff ME, Reynolds MM, Annich GM. Perfusion. 2007;22:193–200. doi: 10.1177/0267659107080877. [DOI] [PubMed] [Google Scholar]
- 7.Smith DJ, Chakravarthy D, Pulfer S, Simmons ML, Hrabie JA, Citro ML, Saavedra JE, Davies KM, Hutsell TC, Mooradian DL, Hanson SR, Keefer LK. J Med Chem. 1996;39:1148–1156. doi: 10.1021/jm950652b. [DOI] [PubMed] [Google Scholar]
- 8.Zhang H, Annich GM, Miskulin J, Osterholzer K, Merz SI, Bartlett RH, Meyerhoff ME. Biomaterials. 2002;23:1485–1494. doi: 10.1016/s0142-9612(01)00274-5. [DOI] [PubMed] [Google Scholar]
- 9.Parzuchowski PG, Frost MC, Meyerhoff ME. J Am Chem Soc. 2002;124:12182–12191. doi: 10.1021/ja020268l. [DOI] [PubMed] [Google Scholar]
- 10.Vaughn MW, Kuo L, Liao JC. Am J Physiol. 1998;274:H2163–H2176. doi: 10.1152/ajpheart.1998.274.6.H2163. [DOI] [PubMed] [Google Scholar]
- 11.Fleser PS, Nuthakki VK, Malinzak LE, Callahan RE, Seymour ML, Reynolds MM, Merz SI, Meyerhoff ME, Bendick PJ, Zelenock GB, Shanley CJ. J Vasc Surg. 2004;40:803–811. doi: 10.1016/j.jvs.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Frost MC, Rudich SM, Zhang H, Maraschio MA, Meyerhoff ME. Anal Chem. 2002;74:5942–5947. doi: 10.1021/ac025944g. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds MM, Hrabie JA, Oh BK, Politis JK, Citro ML, Keefer LK, Meyerhoff ME. Biomacromolecules. 2006;7:987–994. doi: 10.1021/bm060028o. [DOI] [PubMed] [Google Scholar]
- 14.Jun HW, Taite LJ, West JL. Biomacromolecules. 2005;6:838–844. doi: 10.1021/bm049419y. [DOI] [PubMed] [Google Scholar]
- 15.Taite LJ, Yang P, Jun HW, West JL. J Biomed Mater Res Part B: Appl Biomater. 2008;84B:108–116. doi: 10.1002/jbm.b.30850. [DOI] [PubMed] [Google Scholar]
- 16.Robbins ME, Hopper ED, Schoenfisch MH. Langmuir. 2004;20:10296–10302. doi: 10.1021/la048368n. [DOI] [PubMed] [Google Scholar]
- 17.Showalter BM, Reynolds MM, Valdez CA, Saavedra JE, Davies KM, Klose JR, Chmurny GN, Citro ML, Barchi JJ, Jr, Merz SI, Meyerhoff ME, Keefer LK. J Am Chem Soc. 2005;127:14188–14189. doi: 10.1021/ja054510a. [DOI] [PubMed] [Google Scholar]
- 18.Szycher M. In: Blood Compatible Materials and Devices: Perspectives Towards the 21st Century. Sharma CP, Szycher M, editors. Technomic Publishing Co., Inc.; Lancaster: 1991. pp. 33–85. [Google Scholar]
- 19.Lamda MNK, Woodhouse KA, Cooper SL. Polyurethanes in Biomedical Applications. CRC Press; Boca Raton: 1998. [Google Scholar]
- 20.Park Y, Ryu E, Kim H, Jeong J, Kim J, Shim J, Jeon S, Jo Y, Kim W, Min B. Artificial Organs. 1999;23:210–214. doi: 10.1046/j.1525-1594.1999.06013.x. [DOI] [PubMed] [Google Scholar]
- 21.Shibuta R, Tanaka M, Sisido M, Imanishi Y. J Biomed Mater Res. 1986;20:971–987. doi: 10.1002/jbm.820200712. [DOI] [PubMed] [Google Scholar]
- 22.Chen JH, Wei J, Chang CY, Laiw RF, Lee YD. J Biomed Mater Res. 1998;41:633–648. doi: 10.1002/(sici)1097-4636(19980915)41:4<633::aid-jbm16>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 23.Santerre JP, Ten hove P, VanderKamp NH, Brash JL. J Biomed Mater Res. 1992;26:39–57. doi: 10.1002/jbm.820260105. [DOI] [PubMed] [Google Scholar]
- 24.Korematsu A, Li YJ, Murakami T, Sakurai I, Kodama M, Nakaya T. J Mater Chem. 1999;9:647–653. [Google Scholar]
- 25.Ishihara K, Tanaka S, Furukawa N, Kurita K, Nakabayashi N. J Biomed Mater Res. 1996;32:391–399. doi: 10.1002/(SICI)1097-4636(199611)32:3<391::AID-JBM12>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 26.Wu X, Tang X, Xian M, Wang PG. Tetrahedron Lett. 2001;42:3779–3782. [Google Scholar]
- 27.Saavedra JE, Dunams TM, Flippen-Anderson JL, Keefer LK. J Org Chem. 1992;57:6134–6138. [Google Scholar]
- 28.Keefer LK. Current Topics Med Chem. 2005;5:625–636. doi: 10.2174/1568026054679380. [DOI] [PubMed] [Google Scholar]
- 29.Saavedra JE, Booth MN, Hrabie JA, Davies KM, Keefer LK. J Org Chem. 1999;64:5124–5131. doi: 10.1021/jo9901539. [DOI] [PubMed] [Google Scholar]
- 30.Mowery KA, Schoenfisch MH, Saavedra JE, Keefer LK, Meyerhoff ME. Biomaterials. 2000;21:9–21. doi: 10.1016/s0142-9612(99)00127-1. [DOI] [PubMed] [Google Scholar]
- 31.Drago RS. Adv Chem Ser. 1962;36:143–149. [Google Scholar]
- 32.Hrabie JA, Saavedra JE, Davies KM, Keefer LK. Org Prep Proced Int. 1999;31:189–192. doi: 10.1080/00304940902801968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yun SY, Kittong Y, Oh BK, Cha GS, Nam H. Anal Chem. 1997;69:868–873. doi: 10.1021/ac9605455. [DOI] [PubMed] [Google Scholar]
- 34.Lee Y, Oh BK, Meyerhoff ME. Anal Chem. 2004;46:536–544. doi: 10.1021/ac035064h. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt HHHW, Kelm M. Determination of Nitrite and Nitrate by the Griess Reaction. In: Feelish M, Stamler JS, editors. Methods in Nitric Oxide Research. John Wiley; New York: 1996. p. 491. [Google Scholar]
- 36.Reynolds MM, Zhou ZR, Oh BK, Meyerhoff ME. Org Lett. 2005;7:2813–2816. doi: 10.1021/ol050541z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.