Abstract
Background
Approximately one-third of patients undergoing interferon-α (IFN-α) therapy for treatment of the hepatitis C virus (HCV) develop major depression, which decreases functioning and may lead to the reduction or discontinuation of treatment.
Objective
The authors examined the efficacy of citalopram in preventing IFN-α-induced depression in HCV patients.
Method
This was a randomized, controlled trial comparing citalopram with placebo in 39 HCV patients.
Results
The rate of IFN-α-induced depression in the sample was 15.4% (6/39). Randomization to citalopram did not decrease the statistical likelihood of developing IFN-α-induced depression (10.5% for citalopram vs. 20.0% for placebo).
Conclusion
Citalopram does not prevent depression onset; however, an empirically-supported treatment recommendation for IFN-α-induced depression includes monitoring depressive symptoms throughout antiviral therapy and initiating psychiatric treatment at the initial signs of depression.
Approximately 3 million to 4 million individuals in the United States are estimated to be infected with the hepatitis C virus (HCV).1 Of individuals who test positive for HCV, approximately 25% to 30% have clinically significant disease (e.g., fibrosis, cirrhosis, hepatocellular carcinoma) and receive antiviral therapy.2 To-date, the most effective therapy for HCV is pegylated interferon α (IFN-α) used in combination with ribavirin.3,4 However, IFN-α-based therapies are associated with multiple neuropsychiatric side effects in many patients who receive them for the treatment of HCV, malignant melanoma, and other conditions.
The reported incidence rates for neuropsychiatric side effects among patients receiving IFN-α vary, given that many of the studies have not used objective and validated measures of depressive symptomatology or criterion-based instruments.5,6 Among individuals with HCV, IFN-α-induced depression generally occurs in 30%-or-more of patients on therapy.7 The onset of side effects usually occurs within the first 12-to-16 weeks of beginning IFN-α therapy. The symptoms of IFN-α-induced depression in HCV patients are similar to those seen in major depressive disorder; these include depressed mood, irritability, fatigue, apathy, anhedonia, psychomotor retardation, sleep disturbance, sexual dysfunction, memory impairment, and diminished ability to concentrate.7,8 The development of IFN-α-induced depression is also worrisome because symptoms may lead to the discontinuation of antiviral therapy.5 The depressive symptoms associated with antiviral therapy are dose-limiting and can compromise otherwise effective therapy, and they are a source of considerable distress to some patients.9
Although IFN-α-induced depression is common, earlier data suggest that symptoms respond to treatment. Initial studies with malignant-melanoma patients receiving antiviral therapy, administered at a much higher dose than treatment for HCV, suggest that prophylactic treatment with selective serotonin reuptake inhibitors (SSRIs) may prevent IFN-α-induced major depression.10,11 This treatment may allow malignant-melanoma patients to remain on IFN-α therapy. In a prospective study of 39 HCV patients treated with IFN-α, 33% of patients developed a major depressive episode during treatment.8 Furthermore, 85% of patients who developed IFN-α-induced major depression responded to antidepressant treatment, thus allowing patients to complete a course of IFN-α therapy with clinical benefits. These findings, combined with results from malignant-melanoma patients,10,11 raised the question of whether prophylactic treatment with HCV patients reduces the risk for or prevents IFN-α-induced depression.
Two open-label studies have indicated that prophylactic SSRIs may prevent development of IFN-α-induced depression in HCV patients.12,13 Conversely, in the first double-blind placebo-controlled study to evaluate the efficacy of preventing IFN-α-induced depression in 33 HCV patients, prophylactic treatment with paroxetine did not decrease the likelihood of developing depression.14 In a second double-blind, placebo-controlled study (N=61), paroxetine was not associated with a significant difference in the development of IFN-α-induced depression in HCV patients.15 However, this second trial demonstrated that prophylactic SSRI treatment may reduce the severity of depressive symptoms in patients with higher pretreatment depression-severity scores.
The purpose of the present study is to evaluate the efficacy of the antidepressant medication citalopram in preventing the development of IFN-α-induced depression in HCV patients. We built upon previous research by designing a trial that accounted for previous methodological limitations. Specifically, in earlier research, dose escalations of the study medication were not provided in a uniform manner when depressive symptoms worsened, and previous studies included patients receiving both pegylated IFN-α and nonpegylated IFN-α.14,15 In this study, all patients were randomized to citalopram or placebo 2 weeks before the initiation of pegylated IFN-α; the study design included dose escalations, and the study blind was not automatically broken when individuals displayed clinically significant depressive symptomatology. We hypothesized that 1) patients receiving citalopram would be less likely than those receiving placebo to develop IFN-α-induced depression; 2) patients receiving citalopram would be more likely to complete antiviral therapy; and 3) the study medication would not result in the development of significant side effects.
Method
Participants
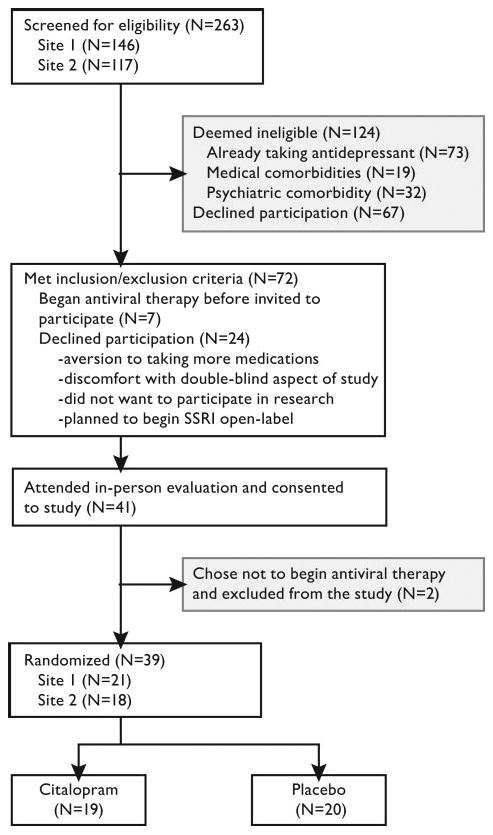
Participants were recruited from two Veterans Affairs Medical Centers: the Portland VA Medical Center (N=21) and the Seattle, WA, VA Medical Center of the Puget Sound Health Care System (N=18) between January 2003 and February 2006. Participants were included if they had HCV, were ≥18 years old, eligible for antiviral therapy, and agreed to undergo IFN-α/ribavirin treatment. Participants were excluded for ongoing depression or active psychotic symptoms during the previous 3 months, substance abuse in the previous 6 months, medical comorbidities that could interfere with treatment, or current antidepressant use. Figure 1 shows the participant-flow through the protocol. This study was approved by the Institutional Review Boards at the Portland VA Medical Center and Puget Sound Health Care System. All subjects completed informed consent before their participation in this study.
FIGURE 1. Flow of Participants Through Study Protocol.
Study Measures
A trained research assistant administered the Structured Clinical Interview for DSM–IV (SCID)16 in order to assess for current psychiatric disorders. The SCID was given 2 weeks before the initiation of antiviral therapy and again at study conclusion, or if patients entered the rescue arm of the trial. The SCID is a clinician-administered interview, and it was used as the primary outcome measure to assess development of IFN-α-induced depression.
Participants were administered other study measures 2 weeks before the initiation of antiviral therapy and at regularly scheduled follow-up appointments throughout treatment (at 0, 2, 4, 6, 8, 12, 16, 20, and 24 weeks), with the exception of the Beck Depression Inventory, 2nd Edition, which was obtained weekly. The Montgomery-Asberg Depression Rating Scale (MADRS), which is a clinician-administered measure of current depressive symptoms,17 was used as an outcome measure of depression severity. The MADRS provides a measure of depressive symptomatology that is less influenced by physical symptoms and may be more sensitive to changes in depressive symptoms in patients with chronic medical conditions. Participants also completed the Beck Depression Inventory, Version II (BDI–II), a 21-item, self-report inventory shown to have a high degree of reliability and validity.18 The BDI–II was used as an additional assessment of depression symptom severity. The Hamilton Rating Scale for Anxiety (Ham–A) is a 14-item measure of anxiety, with scores ranging from 0 to 56.19 The SSRI Side-Effect Checklist is a 22-item, self-report measure developed for this study to assess the potential side effects of the study medication. Questions are asked on a four-point Likert scale (a copy of this measure is available upon request). The Neurotoxicity Rating Scale (NRS) was used to monitor adverse events over the course of the study.20 The NRS comprises 39 self-report items; it is used in the evaluation of psychiatric and physical symptoms related to antiviral therapy. Participants received $10 or $20 for each study visit, depending on travel distance.
Study Design
Randomized Assignment to Treatment Groups
We used a double-blind, randomized design to assign participants to an experimental condition: citalopram or placebo (Figure 1). We used block randomization by recruitment site, whereby patients within each geographical block were randomly assigned to receive either the citalopram or identically-appearing placebo. The first dose was administered 2 weeks before initiation of antiviral therapy.
Antiviral Therapy
Antiviral therapy included weekly injections of pegylated interferon-α (IFN-α) along with twice-daily oral doses of ribavirin. The doses of IFN-α and ribavirin were determined according to standard antiviral therapy guidelines and were monitored by a hepatology clinic provider. The intended duration of antiviral therapy was a minimum of 24 weeks (genotypes 2 and 3), although some patients continued with treatment for 48 weeks (genotype 1). Patients were given the option to discontinue treatment at any time.
Administration and Monitoring of Citalopram or Placebo
Upon entry into the study, 20-mg tablets of citalopram (Forest Laboratories, Inc., United States) or identical-appearing tablets of placebo were dispensed to participants blindly. Participants were asked to self-administer 1 tablet orally on a daily basis. Depressive symptoms were monitored by rating scales at each clinical visit, and the severity of depression, as measured by BDI–II scores, determined whether dose increases were necessary. Patients with a BDI–II score ≤14 were considered to be nondepressed and continued with their previous dose. BDI–II scores ≥15 prompted a dose increase of 20 mg/day. Up to two dose increases were allowed through the course of the study, on the basis of clinical need, with the maximum daily dose set at 3 tablets of citalopram (60 mg/day), or 3 tablets of placebo. Consecutive dose increases were separated by at least 2 weeks, in order to allow time for the dose increase to take effect.
Participants with moderate-to-severe depression, as defined by a weekly BDI–II ≥21 or MADRS ≥25, or suicidal thoughts, were placed in the rescue arm of the study. In the rescue arm, the blinded condition code for that participant was broken by contacting the research pharmacist. If the participant was in the placebo condition, he or she received open-label treatment with citalopram. Participants in the citalopram condition received an increased dose of open-label citalopram. If the depressive symptoms did not show a significant reduction, defined as a 50% reduction of maximum depression severity within 4 weeks, the participant was taken off IFN-α therapy. IFN-α was also discontinued at the request of participants.
Open-Label Antidepressant Treatment and Post-Therapy Dose Titration
After 24 weeks, the code for treatment group assignment was broken for all participants. Those who continued with antiviral therapy for 48 weeks were offered open-label citalopram for the duration of IFN-α therapy. Participants were monitored and evaluated for any side effects or depressive symptoms.
Statistical Analysis
Baseline characteristics were compared by chi-square analyses for categorical variables and analysis of variance for continuous variables. Odds ratios (ORs), derived from logistic-regression analyses, were conducted to evaluate the likelihood of developing major depressive disorder (MDD) during IFN-α treatment, by group assignment. In this analysis, group assignment was the independent variable, and depression status, assessed with the SCID, was the dependent variable. This study was originally designed to have power greater than 80% to detect significant differences in rates of IFN-α-induced depression. This assumes that 35% of patients assigned to placebo, and 10% assigned to citalopram, would develop IFN-α-induced depression.8,10 However, because of recruitment difficulties, there were not sufficient subjects enrolled in the recruitment period. The low rate of major depression in the entire sample (15.4%) and in the control group (20.0%) further limited the power to detect differences between groups.
To examine severity scores over time, a series of analysis of covariance (ANCOVA) statistics were conducted. A different analysis was conducted for depression severity, anxiety severity, antiviral treatment side effects, and side effects of the study medication. The factors included in these analyses were Treatment Group, Time (Weeks 4, 8, 12, 16, 20, and 24), and the interaction of the two. All repeated-measures analyses included baseline scores as a covariate. In instances of missing data, data were analyzed with the last observation carried forward. This study had power greater than 80% to detect a 10-point difference in scores on the BDI–II in 39 patients. All analyses were two-tailed and considered to be statistically significant at p<0.05.
Results
Table 1 presents a comparison of the baseline demographic characteristics of subjects assigned to the citalopram and placebo groups. No significant baseline differences existed between the two groups on any demographic or medical-related variable. The rate of IFN-α-induced major depression, as assessed with the SCID, in the entire sample, was 15.4% (6/39). Citalopram treatment was not significantly associated with prevention of IFN-α-induced depression (OR: 0.47; 95% confidence interval [CI]: 0.08–2.93). Major depression developed in 10.5% of subjects assigned to citalopram (2/19), versus 20.0% in the placebo group (4/20). Also, citalopram treatment was not associated with a significant reduction in the severity of depressive symptoms over the course of the study (Table 2). Neither depression severity scores, assessed with the MADRS or BDI–II, nor anxiety scores, as measured by the Ham–A, significantly differed between the two groups.
TABLE 1. Baseline Demographic and Treatment-Related Characteristics of Patients With Hepatitis C.
| Full Sample (N=39) | Citalopram Group (N=19) |
Placebo Group (N=20) |
Test Statistic [df] | |
|---|---|---|---|---|
| Age, years, mean (SD) | 53.0 (7.3) | 51.8 (5.1) | 54.2 (8.9) | F[1,38]=1.02 |
| Race/ethnicity, N (%) | χ2[1]=0.67 | |||
| White | 33 (84.6%) | 17 (89.5%) | 16 (80.0%) | |
| Other | 6 (15.4%) | 2 (10.5%) | 4 (20.0%) | |
| Employment, N (%) | χ2[2]=4.59 | |||
| Employed | 20 (51.3%) | 13 (68.4%) | 7 (35.0%) | |
| Retired | 8 (20.5%) | 2 (10.5%) | 6 (30.0%) | |
| Other | 11 (28.2%) | 4 (21.1%) | 7 (35.0%) | |
| Education, N (%) | χ2[1]=0.05 | |||
| High School or less | 13 (33.3%) | 6 (31.6%) | 7 (35.0%) | |
| Some college or more | 26 (66.7%) | 13 (68.4%) | 13 (65.0%) | |
| Gender, N (%) | χ2[1]=0.31 | |||
| Male | 36 (92.3%) | 18 (94.7%) | 18 (90.0%) | |
| Female | 3 (7.7%) | 1 (5.3%) | 2 (10.0%) | |
| HCV genotype, N (%) | χ2[1]=1.29 | |||
| 1 | 21 (53.8%) | 12 (63.2%) | 9 (45.0%) | |
| 2 or 3 | 18 (46.2%) | 7 (36.8%) | 11 (55.0%) | |
| History of major depression, N (%) | 5 (12.8%) | 2 (10.5%) | 3 (15.0%) | χ2[1]=0.17 |
| Current depression severity, mean (SD) | 4.3 (4.3) | 3.3 (3.9) | 5.3 (4.6) | F[1,38]=2.10 |
SD: standard deviation. All p values were nonsignificant. History of major depression was assessed with the Structured Clinical Interview for DSM–IV. Current depression severity was assessed with the Beck Depression Inventory, 2nd Edition.
TABLE 2. Psychiatric Severity Scores and Treatment-Related Side Effects During Antiviral Therapy.
| Baseline | Week 4 | Week 8 | Week 12 | Week 16 | Week 20 | Week 24 | |
|---|---|---|---|---|---|---|---|
| MADRS | |||||||
| Citalopram group | 3.8 (4.2) | 7.5 (6.2) | 8.3 (6.0) | 9.5 (6.0) | 9.8 (8.8) | 9.3 (6.9) | 8.2 (6.6) |
| Placebo group | 3.0 (3.0) | 7.8 (7.3) | 7.8 (6.0) | 8.5 (8.9) | 8.2 (8.1) | 6.6 (7.6) | 5.6 (5.5) |
| BDI–II | |||||||
| Citalopram group | 3.3 (3.9) | 7.3 (5.8) | 6.4 (5.5) | 6.0 (4.1) | 7.6 (6.5) | 7.5 (5.1) | 6.3 (5.7) |
| Placebo group | 5.3 (4.6) | 6.8 (4.7) | 6.9 (5.1) | 7.8 (7.8) | 7.3 (6.7) | 6.4 (5.5) | 4.6 (3.6) |
| Ham–A | |||||||
| Citalopram group | 5.6 (5.3) | 9.1 (6.8) | 8.9 (6.1) | 9.2 (6.3) | 11.0 (8.7) | 9.7 (6.1) | 9.8 (6.1) |
| Placebo group | 5.7 (3.5) | 8.6 (5.7) | 9.1 (4.1) | 7.9 (4.9) | 9.3 (6.5) | 8.3 (4.3) | 7.4 (4.3) |
| SSRI Side-Effect Checklist | |||||||
| Citalopram group | 4.1 (4.1) | 9.0 (6.4) | 7.4 (4.5) | 8.8 (5.6) | 9.4 (6.7) | 8.8 (4.4) | 9.2 (6.7) |
| Placebo group | 5.4 (3.6) | 8.5 (6.3) | 9.9 (6.5) | 8.5 (4.9) | 9.2 (5.2) | 7.9 (4.8) | 6.8 (4.3) |
| Neurotoxicity Rating Scale | |||||||
| Citalopram group | 20.3 (16.1) | 24.1 (19.4) | 22.3 (20.7) | 24.8 (21.1) | 28.0 (24.5) | 26.7 (22.5) | 26.2 (21.3) |
| Placebo group | 20.7 (16.8) | 26.4 (20.0) | 28.2 (20.3) | 23.7 (16.1) | 25.7 (17.0) | 23.9 (17.8) | 19.5 (16.4) |
Values are mean (standard deviation). Based on repeated-measures ANCOVA, where the baseline score is included as a covariate. All p values were nonsignificant.
MADRS: Montgomery-Asberg Depression Rating Scale; BDI–II: Beck Depression Inventory, 2nd Edition; Ham–A: Hamilton Rating Scale for Anxiety; SSRI: selective serotonin reuptake inhibitor.
Assignment to citalopram or placebo was not associated with likelihood of completing the recommended course of treatment: 84.2% of participants in the citalopram group completed antiviral therapy, versus 75.0% of participants assigned to placebo (p=0.70). Reasons for dropping out of treatment did not differ between the two groups: these included medical factors (N=3), never starting IFN-α (N=2), noncompliance (N=1), and a combination of factors (N=2). Group assignment was not associated with likelihood of achieving early virologic response (genotype 1: 75.0% versus 44.4%, p=0.15; genotypes 2 and 3: 85.7% versus 81.8%, p=0.83) or sustained virologic response (genotype 1: 41.7% versus 33.3%, p=0.70; genotypes 2 and 3: 28.6% versus 63.6%, p=0.15).
Administration of citalopram was not associated with a significant change in medication- or treatment-related side effects. Repeated-measures analysis, using baseline scores as covariates, did not differ between the two groups on the Neurotoxicity Rating Scale: F[5,32]=2.17; p=0.08; partial η2=0.25, or the SSRI side-effect checklist, F[5,29]=1.71; p=0.16; partial η2=0.23.
A similar proportion of participants from both groups had dose changes in the study medication (26.3% versus 30.0%; p=0.80) and entered the rescue arm of the study (15.8% versus 20.0%; p=0.73); 18% of participants (N=7) entered the rescue arm of the study and received open-label antidepressant medication. Of these participants, only one person (14.3%) continued to report significant depressive symptomatology at the end of treatment.
Secondary analyses examined the impact of baseline depression severity scores during antiviral therapy. Only 1 of 23 participants with a MADRS score below the median (<3) developed IFN-α-induced depression. In participants with baseline scores above the median on the MADRS (>4), randomization to citalopram showed preliminary support for preventing IFN-α-induced depression; 1 of 7 patients assigned to citalopram (14%), and 4 of 9 assigned to placebo (44%) developed IFN-α-induced depression. However, the differences between groups did not achieve statistical significance (χ2=1.7; p=0.31).
Discussion
Our study examined the prophylactic use of citalopram to prevent the development of major depression in HCV patients undergoing antiviral therapy. This study was designed to assess whether prophylactic medication is helpful, and it attempted to address methodological limitations from previous research. Specifically, this is only the third randomized, double-blind, placebo-controlled study of the use of prophylactic medication among HCV patients undergoing antiviral therapy. This study built on methods of previous research14 by including dose escalations of the study medication and a mechanism for retaining the study blind in those who displayed clinically significant depressive symptoms. Contrary to study hypotheses, citalopram was not associated with decreased likelihood of developing IFN-α-induced depression. These data suggest that providing prophylactic citalopram to HCV patients undergoing antiviral therapy confers no significant advantage for reducing the likelihood of developing major depression.
Compared with previous research, the rate of IFN-α-induced depression in this study was low. We found a 15% rate of IFN-α-induced depression, whereas previous investigations suggest that approximately one-third of patients will develop clinically significant depression.7,8,15,21 However, in this study, a diagnosis of major depression was based on the results of a structured clinical interview with the SCID after thresholds for depression severity were crossed (BDI–II score ≥21 or MADRS score ≥25), whereas other studies12,13,22 relied on results from self-report measures, which may overestimate the occurrence of IFN-α-induced depression. In our sample, a reanalysis that used a cutoff score ≥17 on the BDI–II resulted in a rate of IFN-α-induced depression of 30.6%, which is consistent with previous research. However, when the results from this study were reanalyzed using this more liberal cutoff rate, the likelihood of developing IFN-α-induced depression was not significantly different between the two groups.
Previous research examining the efficacy of prophylactic antidepressant medication among patients undergoing antiviral therapy has been mixed. In 40 patients undergoing antiviral therapy for malignant melanoma, paroxetine prevented the development of IFN-α-induced depression.10 In an open-label trial (N=36), citalopram initiated 2 weeks before antiviral therapy was successful in preventing IFN-α-induced depression in HCV patients with comorbid psychiatric or substance-use disorder.13 A second open-label study with HCV patients found that SSRIs were successful in preventing IFN-α-induced depression in those with histories of depression from a previous course of antiviral therapy for HCV.12 In contrast, findings from two double-blind, placebo-controlled studies in HCV patients suggest that prophylactic treatment with paroxetine did not prevent development of IFN-α-induced depression.14,15 However, studies with HCV patients may not be applicable to malignant-melanoma patients, because malignant-melanoma patients receive significantly higher doses of IFN-α, which presumably would be associated with higher rates of IFN-α-induced depression.
In the present study, we accounted for methodological limitations of previous research with HCV patients by conducting a double-blind, placebo-controlled study of prophylactic treatment of depression where all participants received pegylated IFN-α, and the study blind was not automatically broken when depressive symptoms worsened. The results from the present study, combined with previous research, suggest that the prophylactic use of antidepressant medication for all HCV patients undergoing antiviral therapy is unnecessary. Recent data indicate that citalopram is efficacious in treating IFN-α-induced depression. In a double-blind, placebo-controlled study of 28 HCV patients who developed IFN-α-induced depression, citalopram was significantly more likely than placebo to reduce depression severity scores.23 Also, depressive symptoms responded to medication in only 1 to 2 weeks, thereby decreasing the likelihood that depressive symptoms would interfere with antiviral therapy.23 On the basis of available data, we recommend that all HCV patients undergoing antiviral therapy be closely monitored for exacerbation of depressive symptoms. If a patient develops clinically significant depression, treatment should be initiated promptly.24 Some HCV patients receiving antiviral therapy may be at increased risk of developing IFN-α-induced major depression because of a history of a psychiatric disorder, having developed IFN-α-induced depression during a previous unsuccessful treatment, a genetic predisposition,25 having higher baseline (pre-antiviral therapy) depression scores, or other factors. For these high-risk patients, prophylactic antidepressant medication may be helpful.12,13,15 Data from the present study suggest that prophylactic citalopram was not associated with an increase in treatment-related side effects or marked change in medication-related negative effects.
A secondary study aim was to examine the effect of citalopram on antiviral treatment outcomes. Contrary to hypotheses, medication was not associated with decreased antiviral treatment side effects or likelihood of premature treatment discontinuation. Therefore, adding prophylactic citalopram during antiviral therapy may not improve treatment outcomes. However, citalopram was not associated with a significant difference in scores on the SSRI Side-Effect Checklist, suggesting that this medication is not toxic for HCV patients undergoing antiviral therapy.
There are several limitations regarding the study findings. First, the power to detect significant differences in rates of IFN-α-induced major depression was low, leaving the possibility of a Type II error. However, we also compared depression and anxiety severity scores between the two groups, and had adequate power to detect clinically significant differences, and the results for these analyses were also nonsignificant. Second, this study had specific inclusion/exclusion criteria, which limit the generalizability of the findings. We excluded patients who were already taking an antidepressant medication or who had current clinically-significant psychiatric symptoms. Third, this study took place at two VA Medical Centers, and VA patients may differ from the clinical presentation in other settings. Finally, all participants were followed closely by a research assistant. Patients were greeted at all appointments by the research assistant and received weekly follow-up phone calls. It is possible that the nonspecific support provided by the assistant may have moderated the severity of psychiatric symptoms in an unanticipated way.
In summary, results from this randomized, double-blind, placebo-controlled study, designed to evaluate the efficacy of citalopram in preventing IFN-α-induced depression in HCV patients, did not indicate that the use of SSRI medications was helpful. Although the use of citalopram was not associated with negative effects, a conservative and empirically supported approach to the treatment of IFN-α-induced depression suggests that prophylactic antidepressant use is not indicated. We recommend the careful monitoring of neuropsychiatric symptoms during antiviral therapy and initiating psychiatric treatment at the initial signs of depression.
Acknowledgments
This material is based on work supported in part by the Office of Research Development, Clinical Sciences Research and Development Service, from the Department of Veterans Affairs. Dr. Morasco receives support from the National Institutes of Health (K23DA023467-01A1). The authors also appreciate collaboration from members of the Northwest Hepatitis C Resource Center.
Footnotes
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Dr. Hauser has grant funding from Janssen Pharmaceutica and is currently on the speaker's bureau for AstraZeneca and Jazz Pharmaceutical. No other coauthor reports a potential conflict of interest.
References
- 1.Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999–2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 2.Yates WR, Gleason O. Hepatitis C and depression. Depress Anxiety. 1998;7:188–193. doi: 10.1002/(sici)1520-6394(1998)7:4<188::aid-da7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Dienstag JL, McHutchison JG. American Gastroenterological Association Technical Review on the Management of Hepatitis C. Gastroenterology. 2006;130:225–230. doi: 10.1053/j.gastro.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Deutsch M, Hadziyannis SJ. Old and emerging therapies in chronic hepatitis C: an update. J Viral Hepat. 2008;15:2–11. doi: 10.1111/j.1365-2893.2007.00887.x. [DOI] [PubMed] [Google Scholar]
- 5.Dieperink E, Willenbring M, Ho S. Neuropsychiatric symptoms associated with hepatitis C and interferon α: a review. Am J Psychiatry. 2000;157:867–876. doi: 10.1176/appi.ajp.157.6.867. [DOI] [PubMed] [Google Scholar]
- 6.Schafer A, Wittchen HU, Seufert J, et al. Methodological approaches in the assessment of interferon-alfa-induced depression in patients with chronic hepatitis C: a critical review. Int J Meth Psychiatr Res. 2007;16:186–201. doi: 10.1002/mpr.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loftis JM, Hauser P. The phenomenology and treatment of interferon-induced depression. J Affect Disord. 2004;82:175–190. doi: 10.1016/j.jad.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Hauser P, Khosla J, Aurora H, et al. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol Psychiatry. 2002;7:942–947. doi: 10.1038/sj.mp.4001119. [DOI] [PubMed] [Google Scholar]
- 9.Valentine AD, Meyers CA. Neurobehavioral effects of interferon therapy. Curr Psychiatry Rep. 2005;7:391–395. doi: 10.1007/s11920-005-0042-3. [DOI] [PubMed] [Google Scholar]
- 10.Musselman DL, Lawson DH, Gumnick JF, et al. Paroxetine for the prevention of depression induced by high-dose interferon alpha. N Engl J Med. 2001;344:961–966. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- 11.Hauser P, Soler R, Reed S, et al. Prophylactic treatment of depression induced by interferon-α. Psychosomatics. 2000;41:439–441. doi: 10.1176/appi.psy.41.5.439. [DOI] [PubMed] [Google Scholar]
- 12.Kraus MR, Schafer A, Al-Taie O, et al. Prophylactic SSRIs during interferon α re-therapy in patients with chronic hepatitis C and a history of interferon-induced depression. J Viral Hepat. 2005;12:96–100. doi: 10.1111/j.1365-2893.2005.00554.x. [DOI] [PubMed] [Google Scholar]
- 13.Schaefer M, Schwaiger M, Garkisch AS, et al. Prevention of interferon-α associated depression in psychiatric risk patients with chronic hepatitis C. J Hepatol. 2005;42:793–798. doi: 10.1016/j.jhep.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 14.Morasco BJ, Rifai MA, Loftis JM, et al. A randomized trial of paroxetine to prevent interferon-α-induced depression in patients with hepatitis C. J Affect Disord. 2007;103:83–90. doi: 10.1016/j.jad.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Raison CL, Woolwine BJ, Demetrashvili MF, et al. Paroxetine for prevention of depressive symptoms induced by interferon-α and ribavirin for hepatitis C. Aliment Pharmacol Ther. 2007;25:1163–1174. doi: 10.1111/j.1365-2036.2007.03316.x. [DOI] [PubMed] [Google Scholar]
- 16.First MB, Gibbons M, Williams JB. Structured Clinical Interview of DSM–IV Axis I Disorders, Clinician Version (SCID–CV) Washington, DC: American Psychiatric Press, Inc.; 1997. [Google Scholar]
- 17.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 18.Beck AT, Steer RA, Brown GK. Beck Depression Inventory Manual. 2nd. New York: Psychological Corp.; 1996. [Google Scholar]
- 19.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 20.Valentine AD, Meyers CA, Talpaz M. Treatment of neurotoxic side effects of interferon-α with naltrexone. Cancer Invest. 1995;13:561–566. doi: 10.3109/07357909509024923. [DOI] [PubMed] [Google Scholar]
- 21.Constant A, Castera L, Dantzer R, et al. Mood alterations during interferon-alfa therapy in patients with chronic hepatitis C: evidence for an overlap between manic/hypomanic and depressive symptoms. J Clin Psychiatry. 2005;66:1050–1057. doi: 10.4088/jcp.v66n0814. [DOI] [PubMed] [Google Scholar]
- 22.Dieperink E, Ho SB, Thuras P, et al. A prospective study of neuropsychiatric symptoms associated with interferon-α-2b and ribavirin therapy for patients with chronic hepatitis C. Psychosomatics. 2003;44:104–112. doi: 10.1176/appi.psy.44.2.104. [DOI] [PubMed] [Google Scholar]
- 23.Kraus MR, Schafer A, Schottker K, et al. Therapy of interferon-induced depression in chronic hepatitis C with citalopram: a randomized, double-blind, placebo-controlled study. Gut. 2008;57:531–536. doi: 10.1136/gut.2007.131607. [DOI] [PubMed] [Google Scholar]
- 24.Loftis JM, Matthews AM, Hauser P. Psychiatric and substance use disorders in individuals with hepatitis C: epidemiology and management. Drugs. 2006;66:155–174. doi: 10.2165/00003495-200666020-00003. [DOI] [PubMed] [Google Scholar]
- 25.Kraus MR, Al-Taie O, Schafer A, et al. Serotonin-1A receptor gene HTR1A variation predicts interferon-induced depression in chronic hepatitis C. Gastroenterology. 2007;132:1279–1286. doi: 10.1053/j.gastro.2007.02.053. [DOI] [PubMed] [Google Scholar]


