Abstract
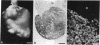
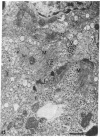
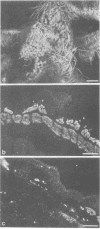
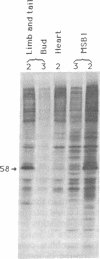
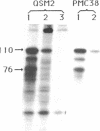
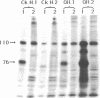
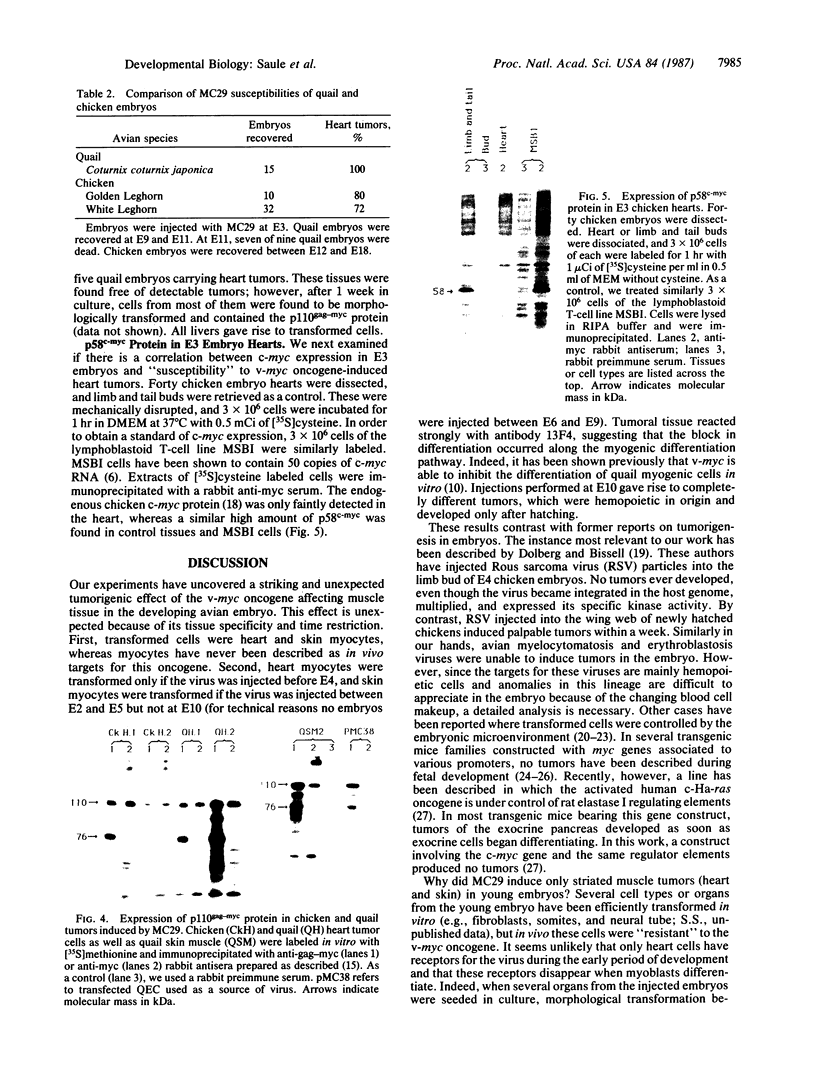
To determine if expression of the v-myc oncogene had any effect during ontogeny, we injected avian myelocytomatosis virus strain MC29 into avian embryos at various stages of development. The injection of MC29 at embryonic day 2 (E2) or 3 (E3) caused, about 10 days later, rhabdomyosarcomas of the heart and, in some cases, skin muscle hypertrophy. When the injection was performed at E4 or E5, the number of heart tumors declined, whereas the number of skin muscle tumors increased significantly. The p110gag-myc protein was found in all tumors analyzed. When the virus was injected intravenously into E10 embryos, no tumors appeared during embryonic life, in striking contrast to the results obtained from injections at earlier stages. The monoclonal antibody 13F4, which is specific for the myogenic lineage, bound strongly to tumoral heart tissue, whereas it bound weakly to normal cardiac cells. Comparison of the peaks of tumor incidence in relationship to the timing of injection suggests that the v-myc product could interfere in vivo with an early step of the muscle lineage differentiation program. In addition, we show that the p58c-myc protein, which is supposed to play an important role in the control of cell proliferation, is only faintly detected in the heart of normal E3 embryos, in contrast to limb and tail buds, which readily express detectable levels of p58c-myc.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Harris A. W., Pinkert C. A., Corcoran L. M., Alexander W. S., Cory S., Palmiter R. D., Brinster R. L. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985 Dec 12;318(6046):533–538. doi: 10.1038/318533a0. [DOI] [PubMed] [Google Scholar]
- Coll J., Righi M., Taisne C., Dissous C., Gegonne A., Stehelin D. Molecular cloning of the avian acute transforming retrovirus MH2 reveals a novel cell-derived sequence (v-mil) in addition to the myc oncogene. EMBO J. 1983;2(12):2189–2194. doi: 10.1002/j.1460-2075.1983.tb01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll J., Saule S., Martin P., Raes M. B., Lagrou C., Graf T., Beug H., Simon I. E., Stehelin D. The cellular oncogenes c-myc, c-myb and c-erb are transcribed in defined types of avian hematopoietic cells. Exp Cell Res. 1983 Nov;149(1):151–162. doi: 10.1016/0014-4827(83)90388-9. [DOI] [PubMed] [Google Scholar]
- Coppola J. A., Cole M. D. Constitutive c-myc oncogene expression blocks mouse erythroleukaemia cell differentiation but not commitment. Nature. 1986 Apr 24;320(6064):760–763. doi: 10.1038/320760a0. [DOI] [PubMed] [Google Scholar]
- Dolberg D. S., Bissell M. J. Inability of Rous sarcoma virus to cause sarcomas in the avian embryo. Nature. 1984 Jun 7;309(5968):552–556. doi: 10.1038/309552a0. [DOI] [PubMed] [Google Scholar]
- Dony C., Kessel M., Gruss P. Post-transcriptional control of myc and p53 expression during differentiation of the embryonal carcinoma cell line F9. Nature. 1985 Oct 17;317(6038):636–639. doi: 10.1038/317636a0. [DOI] [PubMed] [Google Scholar]
- Enrietto P. J., Payne L. N., Hayman M. J. A recovered avian myelocytomatosis virus that induces lymphomas in chickens: pathogenic properties and their molecular basis. Cell. 1983 Dec;35(2 Pt 1):369–379. doi: 10.1016/0092-8674(83)90170-8. [DOI] [PubMed] [Google Scholar]
- Falcone G., Tatò F., Alemà S. Distinctive effects of the viral oncogenes myc, erb, fps, and src on the differentiation program of quail myogenic cells. Proc Natl Acad Sci U S A. 1985 Jan;82(2):426–430. doi: 10.1073/pnas.82.2.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré F., Martin P., Begue A., Ghysdael J., Saule S., Stéhelin D. Préparation et caractérisation d'antisera spécifiques dirigés contre différents domaines polypeptidiques codés par l'oncogène c-myc humain pour étudier l'expression de ce gène introduit dans des cellules de caille ou de rat. C R Acad Sci III. 1986;303(15):633–636. [PubMed] [Google Scholar]
- Gerschenson M., Graves K., Carson S. D., Wells R. S., Pierce G. B. Regulation of melanoma by the embryonic skin. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7307–7310. doi: 10.1073/pnas.83.19.7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Metcalf D. Expression of myb, myc and fos proto-oncogenes during the differentiation of a murine myeloid leukaemia. Nature. 1984 Jul 19;310(5974):249–251. doi: 10.1038/310249a0. [DOI] [PubMed] [Google Scholar]
- Gootwine E., Webb C. G., Sachs L. Participation of myeloid leukaemic cells injected into embryos in haematopoietic differentiation in adult mice. Nature. 1982 Sep 2;299(5878):63–65. doi: 10.1038/299063a0. [DOI] [PubMed] [Google Scholar]
- Graf T., Beug H. Avian leukemia viruses: interaction with their target cells in vivo and in vitro. Biochim Biophys Acta. 1978 Nov 17;516(3):269–299. doi: 10.1016/0304-419x(78)90011-2. [DOI] [PubMed] [Google Scholar]
- Hiruma T., Hirakow R. An ultrastructural topographical study on myofibrillogenesis in the heart of the chick embryo during pulsation onset period. Anat Embryol (Berl) 1985;172(3):325–329. doi: 10.1007/BF00318980. [DOI] [PubMed] [Google Scholar]
- Illmensee K., Mintz B. Totipotency and normal differentiation of single teratocarcinoma cells cloned by injection into blastocysts. Proc Natl Acad Sci U S A. 1976 Feb;73(2):549–553. doi: 10.1073/pnas.73.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek L., Hyland J. K., Watt R., Rosenberg M., Baserga R. Microinjected c-myc as a competence factor. Science. 1985 Jun 14;228(4705):1313–1315. doi: 10.1126/science.4001943. [DOI] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Lachman H. M., Skoultchi A. I. Expression of c-myc changes during differentiation of mouse erythroleukaemia cells. Nature. 1984 Aug 16;310(5978):592–594. doi: 10.1038/310592a0. [DOI] [PubMed] [Google Scholar]
- Leder A., Pattengale P. K., Kuo A., Stewart T. A., Leder P. Consequences of widespread deregulation of the c-myc gene in transgenic mice: multiple neoplasms and normal development. Cell. 1986 May 23;45(4):485–495. doi: 10.1016/0092-8674(86)90280-1. [DOI] [PubMed] [Google Scholar]
- Martin P., Henry C., Ferre F., Duterque-Coquillaud M., Lagrou C., Ghysdael J., Debuire B., Stehelin D., Saule S. Transformation of quail embryo fibroblasts by a retrovirus carrying a normal human c-myc gene. EMBO J. 1986 Jul;5(7):1529–1533. doi: 10.1002/j.1460-2075.1986.tb04393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J., Cheifetz S., Endo T., Nadal-Ginard B. Type beta transforming growth factor is an inhibitor of myogenic differentiation. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8206–8210. doi: 10.1073/pnas.83.21.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardanaud L., Altmann C., Kitos P., Dieterlen-Lievre F., Buck C. A. Vasculogenesis in the early quail blastodisc as studied with a monoclonal antibody recognizing endothelial cells. Development. 1987 Jun;100(2):339–349. doi: 10.1242/dev.100.2.339. [DOI] [PubMed] [Google Scholar]
- Quaife C. J., Pinkert C. A., Ornitz D. M., Palmiter R. D., Brinster R. L. Pancreatic neoplasia induced by ras expression in acinar cells of transgenic mice. Cell. 1987 Mar 27;48(6):1023–1034. doi: 10.1016/0092-8674(87)90710-0. [DOI] [PubMed] [Google Scholar]
- Ramsay G., Evan G. I., Bishop J. M. The protein encoded by the human proto-oncogene c-myc. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7742–7746. doi: 10.1073/pnas.81.24.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong P. M., Ziller C., Pena-Melian A., Le Douarin N. M. A monoclonal antibody specific for avian early myogenic cells and differentiated muscle. Dev Biol. 1987 Aug;122(2):338–353. doi: 10.1016/0012-1606(87)90299-5. [DOI] [PubMed] [Google Scholar]
- Stewart T. A., Pattengale P. K., Leder P. Spontaneous mammary adenocarcinomas in transgenic mice that carry and express MTV/myc fusion genes. Cell. 1984 Oct;38(3):627–637. doi: 10.1016/0092-8674(84)90257-5. [DOI] [PubMed] [Google Scholar]
- Vennström B., Moscovici C., Goodman H. M., Bishop J. M. Molecular cloning of the avian myelocytomatosis virus genome and recovery of infectious virus by transfection of chicken cells. J Virol. 1981 Aug;39(2):625–631. doi: 10.1128/jvi.39.2.625-631.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]