Abstract
Whole cigarette smoke (WCS) is composed of approximately 5% particulates and 95% vapors by weight and is difficult to reproduce quantitatively in the laboratory, where typically, routine in vitro application of smoke normally only utilizes the particulate phase. In this study, we used a system for exposing epithelial cells cultured at an air-liquid interface to WCS. We hypothesized that the use of WSC in vitro was more relevant to what is seen in vivo than methods of cigarette smoke application that only use a small fraction of WCS [i.e., aqueous extract or cigarette smoke condensate (CSC)]. To test this hypothesis, we compared nicotine and cotinine concentrations (measured by mass spectrometry) in the airway surface liquid (ASL) of human primary bronchial epithelial cultures (HBECs) exposed to serial dilutions of WCS to the concentrations found in induced sputum of human subjects who had recently smoked a cigarette; this was also compared to the concentrations found after an exposure to a concentration of CSC commonly used in vitro. When measured by mass spectrometry, nicotine levels were not significantly different in induced sputum versus the ASL of HBECs exposed in vitro to a 1:30 exposure of WCS. However, HBECs that had been exposed to CSC returned significantly lower concentrations of ASL nicotine. These results suggest that nicotine is a good dosimetry marker of WCS exposure and provides direct evidence that the use of WCS is more relevant than the use of CSC for in vitro systems.
Introduction
Cigarette smoke-dependent chronic obstructive pulmonary disease and lung cancer are a major cause of worldwide morbidity, and as a consequence, they are highly researched. However, although the International Standards Organization (ISO) dictates the way a cigarette should be smoked (i.e., a standard ‘puff’ is a 35-mL draw over 2 s), no established protocols regarding how in vitro cell culture models should be exposed to cigarette smoke exist. This confusion arises in part from the fact that cigarette smoke is difficult to study because of the complexity of its constituent parts, with over 2000 different compounds being produced per puff (1). The majority (95%) of whole cigarette smoke (WCS) is gaseous phase by weight, with the remainder (5%) being particulates. The particulate phase is a mixture of lipid-soluble and water-soluble particulates. The particulate phase and the gaseous phase have been shown to have different properties. For example, a 1985 study by Church and Pryor (2) showed that the gaseous phase itself contains a strong oxidizing component that dominates, and the particulate phase is highly reductive. Because of the ease of collection, the most common methods of collecting cigarette smoke extract include passing WCS through Ringers solution to collect the aqueous phase or sonicating a WCS-exposed filter pad (a standard glass-fiber Cambridge filter pad that retains 99.9% of all particulate matter with a size greater than 0.1 μm) in dimethyl sulfoxide (DMSO) to collect the lipid soluble phase (CSC). These methods collect a small fraction (< 5%) of WCS, whilst smokers inhale all the components of WCS. However, these methods have the advantage of being relatively reproducible with dosing and are less complex than gas mixtures.
Measurements have previously been made of nicotine and its metabolites in urine and saliva by mass spectrometry (MS) (3–5). However, no measurements have been made of WCS metabolites in airway surface liquid (ASL). Recent studies demonstrating an effect of WCS on airway ion transport have indicated a precise need for dosing of cigarette smoke at the apical membrane of airway epithelia (6,7). Thus, we set out to measure WCS in ASL in vivo versus in vitro using nicotine and cotinine as markers. ASL may be sampled in vivo using the induced sputum technique. This method has previously been shown to selectively sample ASL from the surfaces of the bronchial airways and limits contamination from saliva [i.e., it is comparable to ASL in vivo (8)]. ASL may also be sampled in vitro by lavaging cultures with Ringers solution, which has previously been shown to accurately sample ASL (9).
To expose human primary bronchial epithelial cultures (HBECs) to smoke, cultures were placed in a specially designed Perspex smoke exposure chamber (Figure 1) that allows the cultures to be exposed to WCS whilst maintaining an air-liquid interface and exposing the serosal surface of the cultures to flowing media to maintain a physiologic environment (10). We then compared induced sputum collected immediately after subjects had smoked one cigarette to ASL sampled by lavage following WCS (from one cigarette). To achieve this, we used a high-performance liquid chromatography–triple-quadrupole MS method (HPLC–MS–MS) for the determination of low nanomolar concentrations of nicotine and its breakdown product, cotinine, as cotinine levels are known to be directly related to nicotine absorption (11). As a control, we also tested a commonly used dose of cigarette smoke condensate (CSC). Thus, the goal of this study was to directly test whether the delivery of WCS or the gaseous phase of cigarette smoke (GPCS) to airway epithelial cells were more comparable to the in vivo situation than previously used smoke preparations.
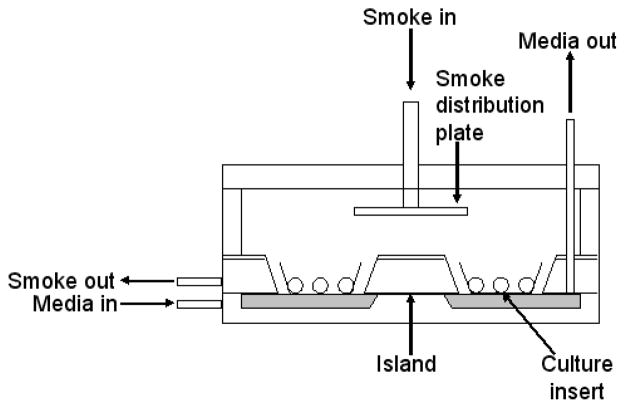
Figure 1.
Diagrammatic representation of the whole cigarette smoke exposure chamber. The chamber was attached to a Borgwaldt LM1 diluter smoke engine via the “smoke in” tube and smoke was evenly distributed over the apical surfaces of the cultures, by means of the distribution plate. During this time, warmed (37°C) culture medium continuously flowed across the serosal surfaces of the cultures. The horizontal medium inflow tube was below the level of the culture inserts, while the bottom of the vertical outflow tube was fractionally above the base of the inserts, to ensure the level of medium remained constant (10).
Experimental Methods
Collection of induced sputum
All subjects smoked a full cigarette (various commercial brands) immediately (within 5 min) prior to sputum induction. Sputum induction and processing were carried out as previously outlined (8). Briefly, the induction procedure involved three separate 7 min inhalation periods of 3%, 4%, and 5% hypertonic saline administered after baseline spirometry. At the end of each 7-min inhalation period, subjects performed a three-step cleansing procedure to remove saliva before a cough attempt as follows: 1. the mouth was rinsed and gargled with water, 2. the back of the throat was cleared (without coughing), and 3. the nose was blown. The subject was then instructed to perform a chesty-type cough without clearing the back of the throat. The sample was expectorated into a sterile specimen cup that was placed on ice throughout the procedure. The time between smoking and sample collection was approximately a total of 20 min. Samples were kept at −20°C until processed; they were then treated with 0.1% dithiothreitol to disperse mucus, washed with DPBS buffer (5 min, at room temperature), filtered (48–52-micron pore size mesh filter), then centrifuged at 15,000 × g for 5 min before 25 μL of supernatant was sent for MS analysis. Sputum cell viability was determined with the use of a Neubauer hemacytometer and Trypan Blue exclusion staining.
Human bronchial epithelial cultures
Human excess donor lungs and excised recipient lungs were obtained at the time of lung transplantation from portions of main stem or lobar bronchi, and cells were harvested by protease digestion (12). Cells were seeded directly as primary cultures on 12-mm Transwell Col membranes (T-Col, Costar, Cambridge, MA) in modified bronchial epithelial growth medium under airway liquid interface conditions and maintained at an air-liquid interface; cultures were typically used 2–5 weeks after seeding. At this time, the cultures had a differentiated morphology, including a distinct ASL/mucus layer indistinguishable from ASL in vivo.
2R4F cigarettes
The development of the 1R4F cigarette was a joint effort by the National Cancer Institute Agriculture Research Service-USDA and the Tobacco and Health Research Institute. The blend specifications for this cigarette [Flue-cured 32.51%; Burley 19.94%; Maryland 1.24%; Oriental 11.08%; Reconstituted (Schweitzer) 27.13%; Glycerin 2.80%; Invert sugar 5.30%] were based on achieving a design for a cigarette that would deliver approximately 9.2 mg of particulates and 0.8 mg of nicotine. These consistent levels were achieved through filtration, air dilution, and blend selection (Tobacco Research and Development Center, University of Kentucky, Lexington, KY). The 2R4F is the second run of the 1R4F.
Preparation and exposure of HBECs to whole and gaseous phase of cigarette smoke
WCS was prepared by smoking 2R4F cigarettes to ISO standard conditions [Routine analytical cigarette-smoking machine—Definitions and standard conditions (ISO 3308:2000)]. This consisted of a 35-mL puff taken over a 2 s duration, and one “puff” every 60 s was applied to the cultures using a Borgwaldt LM1 diluter smoke engine. To generate the GPCS, a Cambridge Filter pad, which retains 99.9% of all particulate matter with a size greater than 0.1 μm, was inserted into the smoke line. Both WCS and GPCS were transferred directly to the apical surface of cultures, which were contained in a specially designed Perspex smoking chamber, keeping the cultures at an air-liquid interface (Figure 1). Unless stated otherwise, WCS and GPCS were applied to the cultures for the time it took for one cigarette to burn, using ISO standard conditions (approximately 10 min and 11 “puffs”). WCS was diluted by a Borgwaldt smoke engine to various dilutions (1:3, 11: 0, 1:30, 1:100) using room air. After “smoking”, in order to measure nicotine and cotinine levels, 500 μL Ringers solution was lavaged over the culture’s apical surface and incubated at 37°C for 10 min (i.e., the time between start of smoking and sample collection was a total of 20 min). In some instances, the lavage was frozen at −20°C prior to MS.
Preparation of CSC
CSC was prepared by smoking 2R4F cigarettes using ISO standard conditions of a 35-mL puff taken over a 2 s duration and one “puff” every 60 s. The total particulate matter from each of the cigarettes was collected onto Cambridge filter pads. The condensate was extracted with DMSO to yield a concentration of 10 mg/mL and frozen immediately at −80°C. The apical surfaces of cultures were exposed to 20 μL 1 μg/mL CSC for either 10 min or 1 h, before 500 μL Ringers solution was lavaged over the culture’s apical surface and frozen at −20° prior to MS. Additionally, 500 μL of basolateral Ringers solution was collected at the end of the time points and frozen at −20°C. In the literature, concentrations of CSC used varies considerably (~0.1 μg/mL to ~20 μg/mL) (13,14); 0.4 μg/mL is a concentration that has been shown in airway epithelial cells (A549) to have > 90% survival and < 5% apoptosis (13); we chose a higher concentration (1 μg/mL) that has been shown to not result in significant apoptosis of the cells, but is representative of a concentration commonly used in in vitro studies (13).
Measurement of particulate matter
To measure the amount of particulate matter in WCS and a 1:30 dilution of WCS, a pre-weighed Teflo TM particle collection filter (47 mm, 2.0 μm, P/N R2PJ047, Pall Life Sciences, East Hills, NY) was placed in front of the “smoke-in” port of the tissue culture chamber and particulates were collected over the set-exposure time (10 min) of one cigarette. After collection, the filters were re-weighed and the amount of particulates per smoke volume calculated.
Measurement of nicotine in WCS and GPCS
Nicotine was measured using the method of Hammond and Leaderer (15). In brief, particulate nicotine was collected on a 37-mm Cambridge glass filter, while vapor phase nicotine was collected downstream on a similar filter that had been treated with sodium bisulfate. A watchglass was filled with an aqueous solution of 4% sodium bisulfate, the filter was placed on the surface of the solution for a few seconds, until it had absorbed the solution, and then the filter was placed on a clean glass plate to dry. This method coated the filter with 7–10 mg of sodium bisulfate. The two filters were contained within one cassette and were separated by a stainless steel support screen and a Teflon o-ring.
Desorption of nicotine from treated filters
Treated filters were place in centrifuge tubes containing 2 mL of water and 100 μL of ethanol and vortex mixed for 1 min. Two milliliters of 10 N sodium hydroxide was added to form the free base of nicotine, and again vortex mixed for 1 min. Nicotine was then concentrated by a liquid–liquid extraction into heptanes by adding 250 μL of ammoniated heptane (gaseous ammonia was bubbled though heptanes for 30 s) and vortex mixing for an additional min. An aliquot of the heptanes layer was removed immediately for analysis by gas chromatography.
Desorption of nicotine from particulate matter
Filters containing the particulate matter collected were desorbed ultrasonically in dichloromethane. Solutions were evaporated to 1 mL, and an aliquot of the solution was taken for analysis.
Measurement of HBEC viability and morphology after WCS exposure
For the viability assay, cells were stained with 5 μM propidium iodide for 10 min at 37°C. Cultures were imaged and then exposed to WCS for 10 min, followed by further 10 min incubation at 37°C before being imaged again. As a non-viable cell control, 70% ethanol was added to the WCS-exposed cultures before imaging. To label the morphology of live cells, cultures were incubated for 30 min with 3 μM Calcein-AM at 37°C and then rinsed (3×) with PBS. Cultures were imaged and then exposed to WCS for 10 min, followed by a further 10 min incubation at 37°C before being imaged again. To observe any subtle morphological changes, HBECs that had or had not been exposed to 10 min WCS were fixed for 1 h in osmium tetraoxide in perfluorcarbon before being processed by conventional methods for light microscopy and stained with Richardson’s stain. Cultures were imaged using 100× lens.
Standards and reagents
Nicotine, cotinine, cimetidine, and all salts were obtained from Sigma-Aldrich (St. Louis, MO). Nicotine and cotinine solutions (1 mM) were prepared in ethanol; dilutions required for the standard curve (50 μM, 10 μM, 5 μM, 1 μM, 500 nM, 250 nM, 100 nM, 50 nM, and 25 nM) were made using standard Ringers solution. The internal standard, cimetidine, was prepared at 10 nM in methanol.
MS analysis
Nicotine and cotinine concentrations were determined by HPLC–MS–MS. The system consisted of two Shimadzu Scientific (Columbia, MD) solvent delivery pumps, a Valco (Houston, TX) switching valve, a thermostated (6°C) LEAP HTC autosampler (Carrboro, NC), and an Applied Biosystems (Foster City, CA) API4000 triple-quadrupole MS. Reversed-phase gradient chromatography was used to elute the compounds from an Aquasil (C18, 5 μm, 50 × 2.1 mm) analytical column at a flow rate of 0.5 mL/min, following a 5-μL injection. Starting conditions for each injection were 100% water with 0.1% formic acid (v/v). This was held constant for 0.5 min while the column eluted to waste. After 0.5 min, the eluent was directed to the MS and the organic phase (methanol with 0.1% formic acid, v/v) increased linearly, reaching 90% organic 2 min post-injection. This was held for 1 min to wash the column. The column was re-equilibrated to starting conditions for the final 1 min. Total run time was 4 min. The MS was connected to the HPLC system by a TurboIonSpray interface. Nitrogen, from a Peak Scientific (Bedford, MA) nitrogen generator, was used as the curtain, nebulizer, and collision gases. User-controlled voltages, gas pressures, and source temperature were optimized via direct infusion for nicotine, cotinine, and cimetidine. All compounds were analyzed in positive ion mode using the following transitions preset in multiple reaction monitoring scans: nicotine 163.1 → 84.5, cotinine 177.2 → 80.45, and cimetidine 253.1 → 117.0. Automated sample acquisition and data analysis were performed using Analyst software (version 1.4.1, Applied Biosystems). Calibration curves were generated based on peak-area ratios (analyte: cimetidine) from 50 nM to 50 μM. Curves for both nicotine and cimetidine followed a quadratic fit with 1/x weighting. Typical r values were 0.997 or greater, and accuracy was between 90 and 110%. Automated sample acquisition and data analysis were performed using Analyst software (version 1.4.1, Applied Biosystems).
Statistical methods
All data is presented as mean ± standard error. Statistically, significance between groups was assessed using non-parametric Student’s unpaired t test and equivalence was used when appropriate. Data were inspected by analysis of variance to demonstrate whether or not the data was derived from a single population and normally distributed. Values of n refer to the number of experiments in each group.
Results
In vivo nicotine and cotinine concentrations
Concentrations of nicotine and cotinine in the ASL in vivo following exposure to WCS have not previously been reported. We sampled induced sputum that is not contaminated with saliva, as it has previously been shown to be comparable to ASL from bronchial surfaces (8). However, based on reported plasma and serum nicotine levels (16), it was predicted that a method with a limit of quantification of 5 nM to 5 μM would be sufficient to analyze this parameter. We collected induced sputum from subjects immediately (i.e., within 5 min) after they smoked one full cigarette, to determine the concentrations of nicotine and cotinine after WCS deposition in the ASL in vivo. Levels of nicotine found in induced sputum were 33.6 ± 5.5 μM (n = 12) (Figure 4A), whereas cotinine concentrations were found to be significantly lower (6.5 ± 1.1 μM; n = 12) (Figure 4B).
WCS does not affect HBEC viability, integrity, or morphology
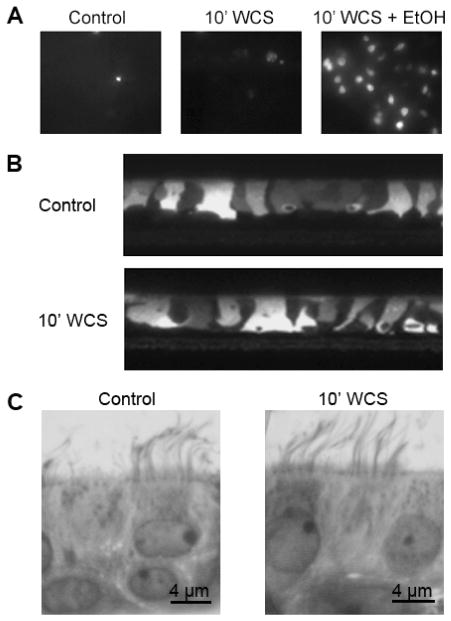
To test whether WCS affected the viability or morphology of live HBECs, we loaded cultures with either propidium iodide or Calcein-AM before exposing them to WCS. We then used fluorescence microscopy to measure changes in HBEC properties pre/post WCS exposure. To determine whether WCS exposure caused any subtle changes on HBEC morphology, we also fixed HBECs ± WCS in Osmium Tetraoxide in perfluorocarbon. The sections of these cultures were then stained with Richardsons stain and imaged using a 100× lens. Using these approaches, it appeared that exposure of HBECs to WCS did not affect either the viability or morphology of either the live or fixed cultures (Figure 2).
Figure 2.
WCS does not affect HBEC integrity. Viability (A). All cultures (control cultures, cultures 10 min after undiluted WCS exposure and WCS-exposed cultures after addition of 70% ethanol) were stained with 5 μM propidium iodide, which fluoresces upon binding to DNA of dead cells (all images are representative from n = 4 cultures per group). Integrity (B). Calcein-stained live HBECs imaged by confocal microscopy pre- (top) and post- (bottom) 10-min WCS exposure (all images are representative from n = 4 cultures per group). Morphology (C). Control HBECs and HBECs exposed to WCS for 10 min were fixed in 1% osmium tetraoxide suspended in perfluorocarbon and stained with Richardson’s stain before being imaged by light microscopy with 100× lens.
Concentrations of nicotine and cotinine in airway surface liquid in vitro
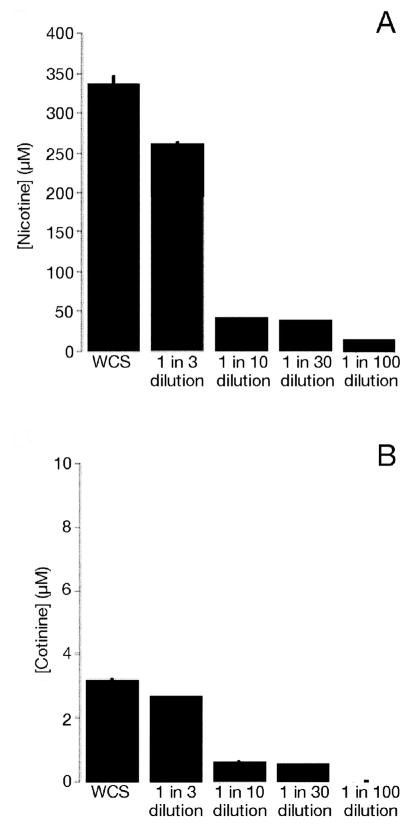
To determine the concentrations of nicotine and cotinine in the ASL after WCSe exposure, we exposed the apical surface of HBECs to varying dilutions of WCS (see Preparation and exposure of HBECs to whole and gaseous phase of cigarette smoke). The amount of nicotine detected in ASL decreased as the smoke was diluted by the smoke engine, but remained in the micromolar range (WCS, 336.8 ± 10.4 μM; 1:3 dilution, 261.3 ± 1.2 μM; 1:10 dilution, 40.9 ± 0.1 μM; 1:30 dilution, 37.1 ± 0.2 μM; 1:100 dilution, 11.8 ± 0.5 μM; n = 4). (Figure 3A). ASL cotinine concentrations in HBECs exposed to smoke were much lower than nicotine, and at 1:100 dilution of WCS, cotinine was not detected (WCS, 3.2 ± 0.006 μM; 1:3 dilution, 2.6 ± 0.02 μM; 1:10 dilution, 0.6 ± 0.008 μM; 1:30 dilution, 0.5 ± 0.01 μM; 1:100 dilution, no peak; n = 4) (Figure 3B).
Figure 3.
Concentrations of nicotine and cotinine in ASL (in vitro) after cigarette smoke exposure. All cultures were exposed to WCS under thin-film conditions then ASL obtained by lavage. Nicotine in WCS (336.8 ± 10.4 μM); 1:3 (261.3 ± 1.2 μM); 1:10 (40.9 ± 0.1 μM); 1:30 (37.1 ± 0.2 μM); 1:100 (11.8 ± 0.5 μM) (A). Cotinine in WCS (3.2 ± 0.006 μM); 1:3 (2.6 ± 0.02 μM); 1:10 (0.6 ± 0.008 μM); 1:30 (0.5 ± 0.01μM); 1:100 (no peak) (B). n = > 4 for all conditions.
Comparison of in vitro and in vivo nicotine and cotinine concentrations
To test whether our smoke exposure system provided comparable ASL nicotine and cotinine concentrations to what occurs in vivo, we compared the nicotine and cotinine concentrations found in induced sputum to ASL samples collected from HBECs exposed to various dilutions of cigarette smoke (all samples were collected approximately 10–20 min after smoke exposure). Levels of nicotine in induced sputum were not significantly different from those found after a 1:30 dilution of WCS (induced sputum, 33.6 ± 5.5 μM; 1:30 dilution, 37.1 ± 0.2 μM; n = 4) (Figure 3A). This was in contrast to cotinine concentrations, which were significantly lower in in vitro (1:30 dilution) samples compared to in vivo samples (induced sputum, 6.5 ± 1.1 μM; 1:30 dilution, 0.5 ± 0.02 μM; n = 4) (Figure 3B).
A comparison of nicotine dosing using different methods of smoke production
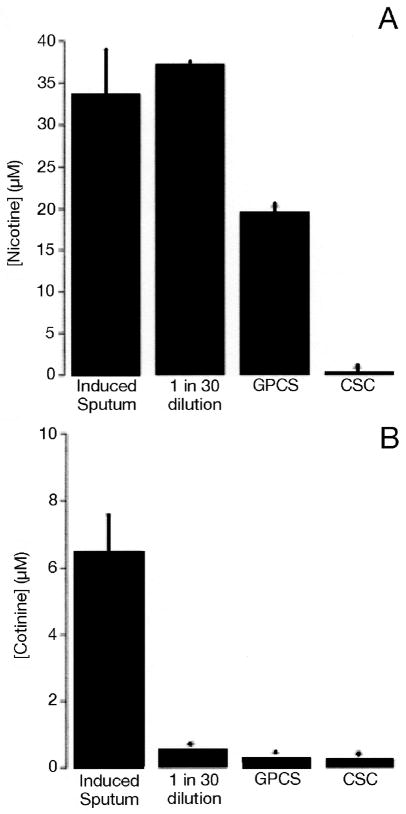
To evaluate different methods of smoke exposure, we measured nicotine exposure levels in ASL from HBECs and induced sputum samples exposed to WCS, GPCS, and CSC (at two different time points). The amount of nicotine found in GPCS and the 1 h CSC exposed cultures was significantly lower than the nicotine found in either the 1:30 dilution of WCS or the induced sputum from cigarette smokers (1:30 dilution, 37.1 ± 0.2 μM; GSC, 19.4 ± 0.1 μM, CSC, 0.1 ± 0.007 μM; induced sputum, 33.6 ± 5.5 μM; n = 4; P < 0.05), suggesting that the WCS exposure yields concentrations of nicotine that are more comparable to the in vivo measurements. In contrast, ASL concentrations of cotinine from WCS, GPCS, and 1 h CSC-exposed cultures were all significantly lower from that seen in vivo (1:30 dilution, 0.5 ± 0.02 μM; GSC, 0.3 ± 0.01 μM; CSC, 0.2 ± 0.01 μM; induced sputum, 6.5 ± 1.1 μM; n = 4) (Figure 4B), suggesting that nicotine is not metabolized to cotinine in vivo. Cotinine levels were found to be significantly lower than nicotine levels in all samples apart from those treated with CSC. Levels of both nicotine and cotinine in the samples collected from the cultures exposed to CSC for 10 min were both below the limit of detection of our system (i.e., < 5 nM).
Figure 4.
Concentrations of nicotine and cotinine in airway epithelial cells (in vitro) versus induced sputum (in vivo) after cigarette smoke exposure. ASL was obtained by lavage in vitro or following induction of sputum in vivo. Nicotine in induced sputum (33.6 ± 5.5 μM); 1:30 dilution of WCS (37.1 ± 0.2 μM); GPCS (19.4 ± 0.1 μM); CSC (0.1 ± 0.007 μM) (A). Cotinine in induced sputum (6.5 ± 1.1 μM); 1:30 dilution of WCS (0.5 ± 0.02 μM); GPCS (0.3 ± 0.01μM); CSC (0.2 ± 0.01 μM) (B). n = > 4 for all conditions.
Concentration of nicotine and particulate matter in WCS and 1:30 dilution of WCS
In order to make it easier for other researchers to replicate our exposure system, we measured the concentration of nicotine and particulate matter our system produces in both WCS and a 1:30 dilution of WCS. The amount of particulate matter found in WCS was 74.1 ± 16.6 mg/m3 compared to 26.5 ± 0.3 mg/m3 from the 1:30 dilution. The concentrations of nicotine and cotinine in both the particulate and gaseous phases of WCS and the 1:30 dilution of WCS at the ‘smoke-in’ port of our tissue culture chamber were also determined. Levels of nicotine were found to be higher in WCS than the 1:30 dilution for both the particulate (WCS, 315.3 ± 18.2 μM; 1:30 dilution, 82.5 ± 2.2 μM) and gaseous (WCS, 838.4 ± 62.4 μM; 1:30 dilution, 400.6 ± 91.4 μM) phases. The same pattern was seen for cotinine levels (particulate phase: WCS, 3.5 ± 0.3 μM; 1:30 dilution, 0.8 ± 0.04 μM; gaseous phase: WCS, 0.9 ± 0.2 μM; 1:30 dilution, 0.6 ± 0.2 μM). Levels of nicotine and cotinine measured in 1 μg/mL CSC were 4.98 ± 0.4 μM and 0.2 ± 0.003 μM, respectively, which were significantly lower than levels seen in either whole or 1:30 dilution of WCS.
Discussion
The superficial epithelium which lines the airways is the principle site of WCS deposition (17). Recent reports have shown that airway epithelia themselves can be directly altered by smoke exposure (6,7). However, methods of delivering cigarette smoke to cultured airway epithelial cells varies substantially between laboratories. Our study not only highlights the need for a standardized method for cigarette smoking in vitro, but suggests that WCS is the most appropriate method.
Cigarette smoke exposure is most often performed using lipid soluble (CSC) or aqueous extracts of cigarette smoke to treat cells in preference to WCS, because these methods have the advantage of being relatively easy to perform and reproduce. CSC is currently the most common vehicle for delivering components of cigarette smoke (7,19–21). However, these methodologies only utilize ~5% of the components of WCS. We reasoned that a system capable of accurately administering 100% of WCS in a reproductive fashion to apical surfaces of well-differentiated HBECs is required before being able to test the effects of cigarette smoke on airway epithelia in vitro. Accordingly, we compared the system developed by Phillips et al. (10) (Figure 1) to WCS deposition in vivo ASL (induced sputum) obtained from smokers directly after consumption of one cigarette. Nicotine appears to be present in substantial quantities in both WCS and CSC and is one of the more relatively stable constituents of cigarette smoke (18). Using nicotine as a marker of cigarette smoke exposure, we demonstrated that the amount of “smoke components” that come in contact with the ASL differs substantially depending on the preparation of smoke delivered. It should be noted, however, that while nicotine is a useful marker of cigarette smoke exposure, its levels may not reflect levels of other toxic chemicals in cigarette smoke, and toxicological effects of cigarette smoke preparations may not be proportional to nicotine levels in ASL. We were able to show that, in contrast to CSC where the amount of nicotine measured in the ASL of HBECs exposed to CSC is significantly different from concentrations measured in vivo, the nicotine concentrations found in the ASL of HBECs exposed to a 1:30 dilution of WCS were comparable to levels measured in vivo, confirming our hypothesis that the use of WCS has more toxicological relevance.
A model to predict the percent deposition of cigarette smoke particles in the airways was developed by the Task Group on Lung Dynamics (1966), which predicted that approximately 30% of the particulates will deposit in the alveolar region and 5–10% in the tracheobronchial region (22). Thus, a 1:30 (or 3%) dilution of WCS is close to the amount of particulates/nicotine that would reach bronchial epithelial cells in vivo (i.e., ≥ 5%). Previously published reports have estimated that the concentration of nicotine in the alveolar lining fluid is 1–10 μg/mL (~6–60 μM) after smoking one cigarette (18,23,24), which is consistent with the values we have measured in vitro and in vivo in the ASL under similar conditions. Smokers chosen for this study were light (i.e., less than one pack/year). However, it is important to consider that the concentration of nicotine could be higher in chronic smokers because of its possible accumulation in the ASL at rates faster than it can be cleared by the airways.
Our study suggests that nicotine does not get converted to cotinine in freshly produced cigarette smoke in the ASL of HBECs after WCS exposure. This is not surprising, as nicotine is primarily metabolized in the liver by cytochrome P450 enzymes in vivo and this is likely the primary site of cotinine production, rather than the lungs. However, more cotinine than nicotine was detected in CSC-exposed ASL in vitro, suggesting that components of CSC may continue to react once CSC has been collected. This CSC may actually have a very different composition when applied to cells that are time-dependent. In support of this hypothesis, it is estimated that cigarette smoke contains over 1017 small oxygen- and carbon-centered free radicals in each puff of smoke (1,2) and although the half-life of most oxygen-free radicals is short (< 1 s), Church and Pryor (2) demonstrated the presence of cigarette smoke free radical activity in extracts, and postulated that continuous free radical regeneration occurs in the extract form. WCS also likely reacts with itself after production. However, in our system, it is delivered to culture surfaces within seconds after production in a similar fashion to in vivo and was never stored for subsequent usage.
Interestingly, although nicotine is thought to reside only in the particulate phase of cigarette smoke, we were still able to detect nicotine in samples exposed to GPCS, indicating that either nicotine is present in the gaseous phase or that the filters used to generate the gaseous phase do not remove 100% of all nicotine. Nicotine concentrations from GPCS-exposed cultures were significantly different to both in vivo and WCS-exposed cultures, but were significantly higher than those found in CSC-exposed cultures. This observation was confirmed by measuring nicotine directly from the “smoke-in” port of the tissue culture chamber in both the particulate and gaseous phases. Nicotine appeared to be present in higher concentrations in the vapor phase than in the particulate phase, suggesting that either the filters used to collect CSC are not sufficient in removing all particulates, or that nicotine is indeed present in both particulate and gaseous phases. These observations suggest the need for further investigation.
In conclusion, using nicotine as a marker of cigarette smoke exposure, we have demonstrated that we can reproducibly deliver WCS to the ASL of well-differentiated HBECs in a dose-dependent manner. Furthermore, we can adjust the dosing to match in vivo exposure levels. We conclude that the smoke exposure system designed by Phillips et al. (10) recapitulates normal smoking and should permit reliable/reproducible studies of WCS in vitro.
Acknowledgments
The authors wish to thank BAT for their gift of the Borgwaldt LM1 diluter smoke engine and smoke exposure chamber as well as for their expert advice. The help of the UNC CF Center Histology and Tissue Culture Cores are also gratefully acknowledged.
Supported by NIBR, UK, and SCCOR 1-P50-HL084934 Project II.
References
- 1.Li ZQ, Luo XM, Wei HJ, Feng YM, Zhang C. Inhibition of the transforming ability of cigarette smoking condensate-treated human fetal lung DNA induced by oltipraz. J Environ Pathol Toxicol Oncol. 1994;13:133–135. [PubMed] [Google Scholar]
- 2.Church DF, Pryor WA. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect. 1985;64:111–126. doi: 10.1289/ehp.8564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byrd GD, Chang KM, Greene JM, deBethizy JD. Evidence for urinary excretion of glucuronide conjugates of nicotine, cotinine, and trans-3′-hydroxycotinine in smokers. Drug Metab Dispos. 1992;20:192–197. [PubMed] [Google Scholar]
- 4.Jacob P, III, Yu L, Wilson M, Benowitz NL. Selected ion monitoring method for determination of nicotine, cotinine and deuterium-labeled analogs: absence of an isotope effect in the clearance of (S)-nicotine-3′,3′-d2 in humans. Biol Mass Spectrom. 1991;20:247–252. doi: 10.1002/bms.1200200503. [DOI] [PubMed] [Google Scholar]
- 5.Benowitz NL, Jacob P, III, Fong I, Gupta S. Nicotine metabolic profile in man: comparison of cigarette smoking and transdermal nicotine. J Pharmacol Exp Ther. 1994;268:296–303. [PubMed] [Google Scholar]
- 6.Cantin AM, Hanrahan JW, Bilodeau G, Ellis L, Dupuis A, Liao J, Zielenski J, Durie P. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am J Respir Crit Care Med. 2006;173:1139–1144. doi: 10.1164/rccm.200508-1330OC. [DOI] [PubMed] [Google Scholar]
- 7.Kreindler JL, Jackson AD, Kemp PA, Bridges RJ, Danahay H. Inhibition of chloride secretion in human bronchial epithelial cells by cigarette smoke extract. Am J Physiol Lung Cell Mol Physiol. 2005;288:L894–L902. doi: 10.1152/ajplung.00376.2004. [DOI] [PubMed] [Google Scholar]
- 8.Alexis NE, Hu SC, Zeman K, Alter T, Bennett WD. Induced sputum derives from the central airways: confirmation using a radiolabeled aerosol bolus delivery technique. Am J Respir Crit Care Med. 2001;164:1964–1970. doi: 10.1164/ajrccm.164.10.2104051. [DOI] [PubMed] [Google Scholar]
- 9.Coakley RD, Boucher RC. Regulation and functional significance of airway surface liquid pH. JOP. 2001;2:294–300. [PubMed] [Google Scholar]
- 10.Phillips J, Kluss B, Richter A, Massey E. Exposure of bronchial epithelial cells to whole cigarette smoke: assessment of cellular responses. Altern Lab Anim. 2005;33:239–248. doi: 10.1177/026119290503300310. [DOI] [PubMed] [Google Scholar]
- 11.Galeazzi RL, Daenens P, Gugger M. Steady-state concentration of cotinine as a measure of nicotine-intake by smokers. Eur J Clin Pharmacol. 1985;28:301–304. doi: 10.1007/BF00543327. [DOI] [PubMed] [Google Scholar]
- 12.Matsui H, Randell SH, Peretti SW, Davis CW, Boucher RC. Coordinated clearance of periciliary liquid and mucus from airway surfaces. J Clin Invest. 1998;102:1125–1131. doi: 10.1172/JCI2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hellermann GR, Nagy SB, Kong X, Lockey RF, Mohapatra SS. Mechanism of cigarette smoke condensate-induced acute inflammatory response in human bronchial epithelial cells. Respir Res. 2002;3:22. doi: 10.1186/rr172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koethe SM, Kuhnmuench JR, Becker CG. Neutrophil priming by cigarette smoke condensate and a tobacco anti-idio-typic antibody. Am J Pathol. 2000;157:1735–1743. doi: 10.1016/S0002-9440(10)64810-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammond SK, Leaderer BP, Roche AC, Schenker M. Collection and analysis of nicotine as a marker for environmental tobacco smoke. Atmos Environ. 1987;21:457–462. [Google Scholar]
- 16.Jacob P, III, Wu S, Yu L, Benowitz NL. Simultaneous determination of mecamylamine, nicotine, and cotinine in plasma by gas chromatography–mass spectrometry. J Pharm Biomed Anal. 2000;23:653–661. doi: 10.1016/s0731-7085(00)00343-5. [DOI] [PubMed] [Google Scholar]
- 17.Laan M, Bozinovski S, Anderson GP. Cigarette smoke inhibits lipopolysaccharide-induced production of inflammatory cytokines by suppressing the activation of activator protein-1 in bronchial epithelial cells. J Immunol. 2004;173:4164–4170. doi: 10.4049/jimmunol.173.6.4164. [DOI] [PubMed] [Google Scholar]
- 18.Hoshino Y, Mio T, Nagai S, Miki H, Ito I, Izumi T. Cytotoxic effects of cigarette smoke extract on an alveolar type II cell-derived cell line. Am J Physiol Lung Cell Mol Physiol. 2001;281:L509–L516. doi: 10.1152/ajplung.2001.281.2.L509. [DOI] [PubMed] [Google Scholar]
- 19.Kode A, Yang SR, Rahman I. Differential effects of cigarette smoke on oxidative stress and proinflammatory cytokine release in primary human airway epithelial cells and in a variety of transformed alveolar epithelial cells. Respir Res. 2006;7:132. doi: 10.1186/1465-9921-7-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lavigne MC, Eppihimer MJ. Cigarette smoke condensate induces MMP-12 gene expression in airway-like epithelia. Biochem Biophys Res Commun. 2005;330:194–203. doi: 10.1016/j.bbrc.2005.02.144. [DOI] [PubMed] [Google Scholar]
- 21.Luppi F, Aarbiou J, van Wetering S, Rahman I, de Boer WI, Rabe KF, Hiemstra PS. Effects of cigarette smoke condensate on proliferation and wound closure of bronchial epithelial cells in vitro: role of glutathione. Respir Res. 2005;6:140. doi: 10.1186/1465-9921-6-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.N.R.C. (U.S.) Environmental Tobacco Smoke: Measuring Exposures and Assessing Health Effects. Commission on Life Sciences (CLS) National Academy Press; Washington, D.C: 1986. pp. 120–160. [PubMed] [Google Scholar]
- 23.Eiserich JP, van der Vliet A, Handelman GJ, Halliwell B, Cross CE. Dietary antioxidants and cigarette smoke-induced biomolecular damage: a complex interaction. Am J Clin Nutr. 1995;62:1490S–1500S. doi: 10.1093/ajcn/62.6.1490S. [DOI] [PubMed] [Google Scholar]
- 24.Holden WE, Maier JM, Malinow MR. Cigarette smoke extract increases albumin flux across pulmonary endothelium in vitro. J Appl Physiol. 1989;66:443–449. doi: 10.1152/jappl.1989.66.1.443. [DOI] [PubMed] [Google Scholar]