Abstract
Polycystic ovarian syndrome is a common disorder characterized by ovulatory dysfunction and hyperandrogenemia (HA). Its origins begin peripubertally, as adolescent HA commonly leads to adult HA and decreased fertility. HA reduces inhibition of gonadotropin releasing hormone pulse frequency by progesterone, causing rapid LH pulse secretion and further increasing ovarian androgen production. Obese girls are at risk for HA and develop increased LH pulse frequency with elevated mean LH by late puberty. Many girls with HA do not exhibit normal LH pulse sensitivity to progesterone inhibition. Thus, HA may adversely affect LH pulse regulation during pubertal maturation leading to persistent HA.
Keywords: polycystic ovarian syndrome, gonadotropin releasing hormone, GnRH pulse generator, obesity, puberty, hyperandrogenemia, adolescent
Introduction
Polycystic ovarian syndrome (PCOS) is the leading cause of infertility, affecting approximately six to eight percent of reproductive-age women and is associated with obesity in 60% of affected women in the United States, insulin resistance and hyperinsulinemia in 50–70%, diabetes mellitus in 4–10%, and markers of cardiovascular disease risk in 33%.1–3 Adolescents with PCOS have a 30–60% prevalence of metabolic syndrome, which is four to five times increased compared to age- and body mass index (BMI)-matched girls.4 Hallmarks of PCOS are clinical and/or biochemical evidence of hyperandrogenemia (HA) (i.e. excessive acne, hirsutism, or elevated free testosterone (T) levels) and evidence of anovulatory cycles (i.e. oligo/amenorrhea). Different definitions of PCOS currently contribute to heterogeneity of clinical and pathophysiological features within the diagnosis. The National Institutes of Health (NIH) criteria (1990) require hyperandrogenism and ovulatory dysfunction, but not polycystic ovarian morphology. The Rotterdam criteria (2003) identify women having two out of three features: androgen excess, ovulatory dysfunction, and polycystic morphology on ovarian ultrasound. This definition is more inclusive, as HA is not a required feature of PCOS. The inclusion of ovarian morphology in the definition of PCOS has been less validated and may not be useful in adolescent girls. Enlarged ovarian volume occurs in 50% of asymptomatic adolescent post-menarcheal girls, likely a developmental stage of maximal ovarian size and a normal variant in most girls.5 Therefore, the ability to discern abnormalities of morphological change in pubertal girls via ovarian ultrasound may be limited.
Evolution of PCOS
The origins of PCOS are possibly pre- or peri-pubertal, as clinical manifestations of the disorder frequently develop shortly after menarche. Both adolescents and adult women with PCOS have disruptions in the regulation of the gonadotropin releasing hormone (GnRH) pulse generator, characterized by rapid luteinizing hormone (LH) (and hence GnRH) pulsatility6, 7 with impaired inhibition by progesterone.8, 9 LH pulse sensitivity to slowing by progesterone can be restored in women with PCOS by antiandrogen therapy, suggesting that HA is responsible for the impaired feedback.10 HA during adolescence is recognized as a forerunner to adult PCOS, since it is associated with higher androgen levels in adulthood and lower fertility rates.11 Obese adolescents are at increased risk of HA12 and later development of PCOS.13
In-utero factors have been suggested by Barker and others to be an important contributor to insulin resistance and HA. Animal models support this idea, as intrauterine androgen exposure in primates14 and sheep15, 16 cause changes in LH secretion, LH pulse sensitivity to progesterone feedback, and insulin metabolism, especially when the animals are overfed postnatally.16 Low birth weight seems to be a factor in the development of PCOS in some girls in Spain and Italy.17, 18 However, studies in Finland, Amsterdam, and the United Kingdom have not supported this relationship in their populations.13, 19, 20
Role of hyperandrogenemia
In premenopausal women, approximately half of circulating T is derived from peripheral conversion of androstenedione, particularly in adipose tissue,21 with the remaining derived from ovarian and adrenal sources. Obesity alone increases androgen production, since adipose tissue, particularly from the abdominal region, has enzymes of steroidogenesis.22 LH is a major physiologic stimulus for ovarian androgen production from theca cells. Additionally, hyperinsulinemia related to obesity can contribute to HA through several mechanisms. Insulin can act as a co-gonadotropin with LH on ovarian theca cells to increase androgen production,23, 24 can increase adrenal responsiveness to ACTH for further androgen production,25 can potentiate hCG-mediated ovarian follicle arrest,26 and can cause pituitary hyperresponsiveness to GnRH for increased LH secretion in in vitro studies.27 Women with PCOS often have elevated LH and insulin levels and are obese, leading to compounded mechanisms for increased androgen production.
HA is associated with excess weight during puberty,12, 28, 29 with a prevalence between 60–94% in our cohort of obese girls (BMI-for-age ≥95%),12 and can be ameliorated with weight loss.28, 29 Circulating androgen levels normally rise slightly during puberty, with early morning elevations of serum T seen in prethelarchal girls.30 In normal weight pubertal girls, levels of total T increase and sex hormone binding globulin (SHBG) decrease, leading to higher circulating free T levels.12, 28 Obese girls have even higher levels of T, with diminished SHBG throughout all stages of puberty, leading to marked elevations of free T (Fig. 1).12 Dehydroepiandrosterone sulfate (DHEA-S), an adrenal androgen, is modestly increased in obese compared to normal weight girls in early puberty, but differences disappear by the end of puberty.
Fig. 1.
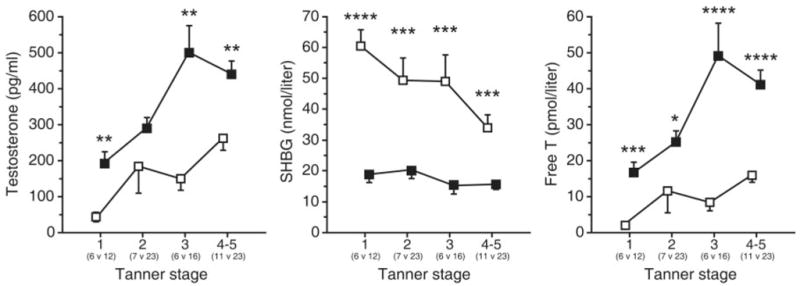
Early morning hormone levels in nonobese (open squares) and obese (solid squares) peripubertal girls by breast Tanner stage. Data are presented as mean ± SEM. Differences were assessed with Wilcoxon rank sum tests before Bonferroni correction: * p < 0.05, ** p ≤ 0.01, *** p ≤ 0.001, *** p ≤ 0.0001. Conversion from conventional to SI units: total T × 3.47 (nmol/L). (McCartney et al, 2007,12 adapted with permission from The Endocrine Society.)
Hyperandrogenemia and the GnRH pulse generator
GnRH pulse frequency designates preferential production of LH (via high frequency pulses) versus follicle stimulating hormone (FSH) (via low frequency pulses) in normal adult women.31 Pulse frequency is regulated by progesterone in the presence of estradiol8 such that increased progesterone production by the corpus luteum slows LH pulse frequency to favor FSH production, which aids in follicular development for the next menstrual cycle (Fig. 2). Women with PCOS have abnormally rapid LH pulses with reduced response to progesterone feedback,8 contributing to elevations in serum LH:FSH ratios. Since LH stimulates theca cell steroid production and FSH regulates follicular conversion of androgen to estrogen, this relative increase in LH leads to increased ovarian androgen synthesis with limited aromatization to estradiol. The resultant increase in serum T contributes to maintaining increased LH pulse frequency,10 creating a vicious loop leading to production of more ovarian androgens.
Fig. 2.
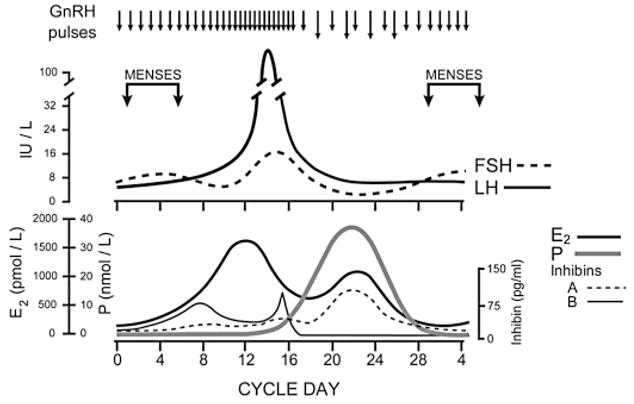
Schematic diagram of hormonal patterns during an ovulatory menstrual cycle. (Marshall JC and Eagleson CA, 1999,36 with permission from Elsevier.)
Clues to the etiological factors related to the instigating events leading to PCOS might be learned from adolescents. In girls, LH is independently and positively correlated with free T when adjusting for age, pubertal stage, BMI, DHEA-S, and insulin.28 This might suggest that pre-existing neuroendocrine abnormalities affecting LH production during puberty lead to HA. Alternatively, HA might impair inhibition of the GnRH pulse generator, leading to high frequency pulses that favor LH production. Therefore, further study of normal and abnormal development of GnRH pulse generator regulation during puberty may help discern more precise etiological mechanisms for the HA in PCOS.
The maturation of the GnRH pulse generator undergoes a typical developmental progression during childhood. During infancy in girls, the GnRH pulse generator is active, producing FSH to high adult levels by about two months of age and LH to early pubertal levels by about four months of age, which both then decrease slowly over the first year to prepubertal levels finally by four years.32 As early as age five to six years (prethelarchal), small pulses of LH (and by inference GnRH) can be seen intermittently throughout the day with pulses of increased amplitude entrained to sleep,30, 33, 34 which are quite sensitive to progesterone inhibition.9 These augmented nighttime LH pulses are associated with overnight increases in sex steroid production (primarily progesterone and T), which diminish during the following daytime hours.30, 35 As puberty progresses, LH pulses gain increasing amplitude and frequency initially throughout the nighttime hours and then during the day.34, 35 By the end of puberty, daytime LH pulses have higher frequency than nighttime pulses with increased amplitude during sleep (Fig. 3).34, 35
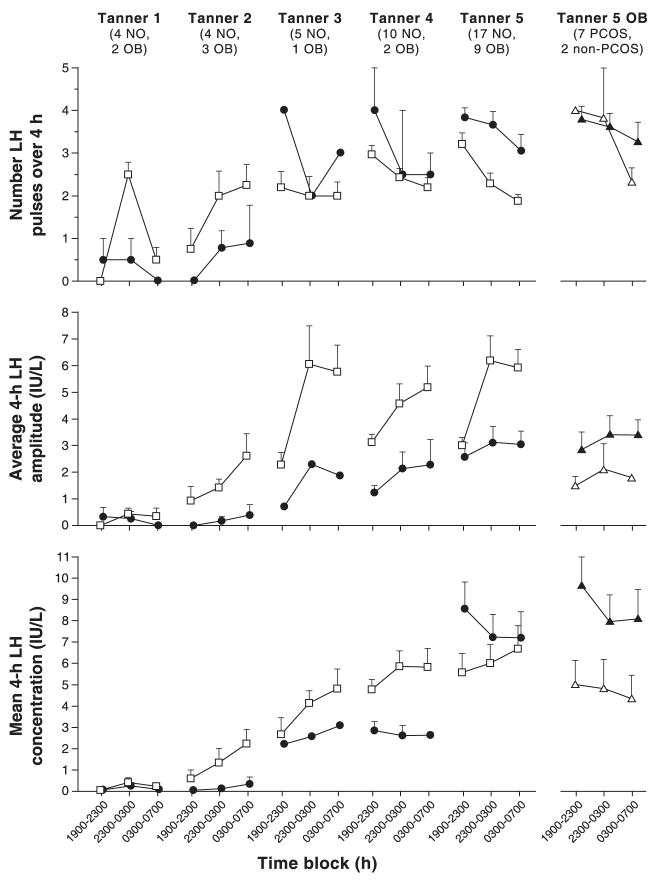
Fig. 3.
Late evening and overnight LH characteristics in nonobese (open squares) and obese (solid circles) peripubertal girls by breast Tanner stage. Data are presented as mean ± SEM. The numbers of subjects are below the Tanner stage labels: NO = nonobese, OB = obese. The last column shows obese Tanner 5 girls only with PCOS (solid triangles) and without PCOS (open triangles). Due to blood volume constraints, time point 1900–2300 (subject awake) is used as a surrogate for daytime hormone levels. (McCartney et al, 2009,35 with permission from The Endocrine Society.)
Girls with obesity, however, have altered maturation of the GnRH pulse generator (Fig. 3). Initially, their LH pulses have lower amplitude and frequency overnight compared to normal weight girls, consistent with lower mean LH concentrations.35 By mid-puberty, however, they surpass normal weight girls in LH pulse frequency during both day and night, although their pulse amplitude and mean LH concentrations remain lower. By the end of puberty, obese girls have significantly higher LH pulse frequency which diminishes only slightly with sleep. Although they still have lower LH pulse amplitude, their mean LH concentrations have now become elevated as in adults with PCOS, likely reflecting the increased LH pulse frequency. Obese girls with clinical evidence of PCOS have even less nighttime diminution of LH pulse frequency, greater pulse amplitude, and significantly higher mean LH concentrations compared to obese girls of similar pubertal stage without clinical evidence of PCOS. Perhaps this designates a continuum of developmental LH pulse abnormalities during puberty which leads to adolescent PCOS symptoms in more affected individuals.
Possible mechanisms for abnormal development of GnRH pulse regulation
During early puberty, the GnRH pulse generator is exquisitely sensitive to inhibition by progesterone. LH pulses are essentially abolished by administered progesterone during early pubertal stages.9 As puberty progresses, normal girls exhibit reduced sensitivity to progesterone inhibition, similar to normal adult women (Fig. 4). In adolescents with HA, however, approximately 60% do not appropriately suppress LH pulsatility after progesterone administration, similar to adult women with PCOS (Fig. 4).9 Interestingly, in girls with HA, the reduction in LH pulse frequency after progesterone decreases with increasing fasting insulin levels, suggesting that hyperinsulinemia may further contribute to impaired GnRH regulation.9
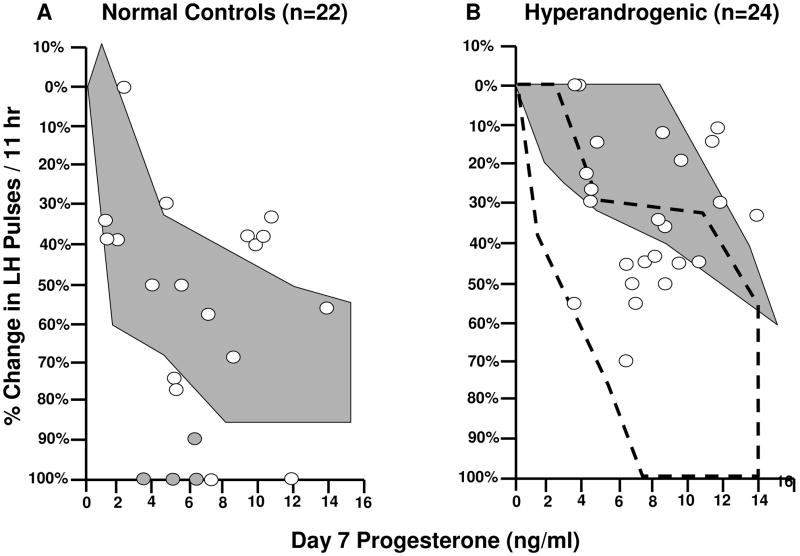
Fig. 4.
Percent change in LH pulse frequency during 11 hr following 7 days of oral E2 and P plotted as a function of mean plasma P on day 7 in control (A) and HA (B) adolescent girls. Shaded circles represent girls with breast Tanner stage 1–2; open circles represent girls with Tanner stage 3–5. The shaded background areas represent range of response to a similar protocol in adult control women (A) and women with PCOS (B). The outlined area in B represents the range of responses in control adolescent girls. Conversion from conventional to SI units: P × 3.18 (pmol/L). (Blank et al,9 2009, with permission from The Endocrine Society.)
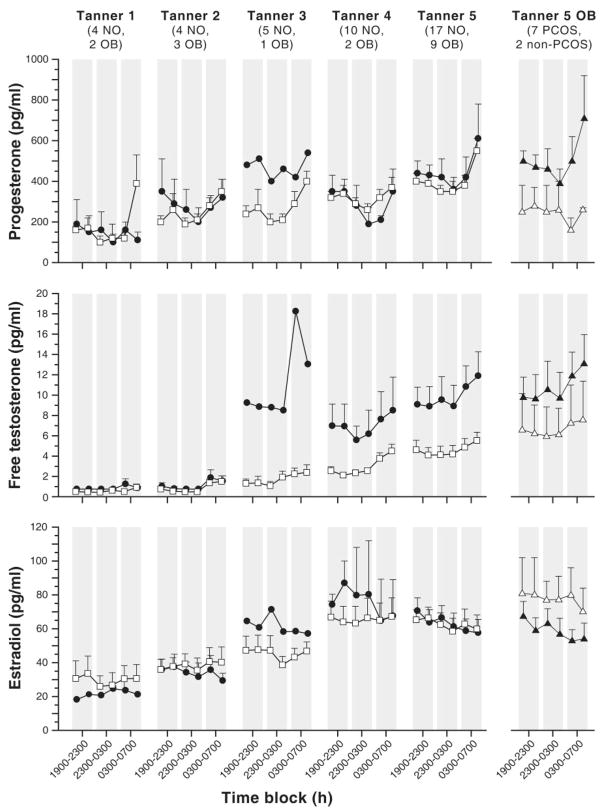
Progressively diminishing sensitivity to inhibition by progesterone may help determine the developmental patterns of the GnRH pulse generator during puberty. Progesterone levels increase overnight during early puberty in normal girls at the same time as the development of detectable sleep-associated LH pulses.35 The following day progesterone again falls to lower levels. In subsequent stages of puberty, daytime progesterone levels diminish to a lesser degree with a continued slight rise overnight.35 The overnight rise of progesterone may contribute to the subsequent suppression of daytime LH pulses during early puberty through exquisitely sensitive feedback inhibition. Free T has a similar overnight rise in plasma,30 whereas estradiol does not; however, levels of both hormones increase during daytime as puberty progresses (Fig. 5).35
Fig. 5.
Late evening and overnight sex steroid concentrations in nonobese (open squares) and obese (solid circles) peripubertal girls by breast Tanner stage. Data are presented as mean ± SEM. The numbers of subjects are below the Tanner stage labels: NO = nonobese, OB = obese. The last column shows obese Tanner 5 girls only with PCOS (solid triangles) and without PCOS (open triangles). Conversion from conventional to SI units: P × 3.18 (pmol/L); free T (pmol/L); E2 × 3.671 (pmol/L). (McCartney et al, 2009,35 with permission from The Endocrine Society.)
Given that androgens decrease feedback inhibition of LH pulsatility by progesterone in adult women with PCOS,10 we hypothesize that increasing androgen levels throughout puberty gradually impair the inhibitory effects of the overnight rise in progesterone. This would result in progressive daytime increases in LH pulsatility, as is seen in normal weight girls as they progress through puberty (Fig. 3). In obese girls with HA, increased androgen levels may further impair the normal patterns of progesterone inhibition during puberty, leading to earlier development of elevated daytime LH pulsatility.7 Over time, this change in the maturational pattern of LH pulses could enhance ovarian androgen production, especially in later puberty, leading to a symptomatic PCOS phenotype.
Further studies are needed to elucidate the precise mechanisms for the development of abnormal GnRH regulation in obese girls. The source of overnight increases in progesterone is unclear, since ovarian hormone production is thought to be minimal in early puberty. Alternatively, the adrenal gland and not the ovary may be responsible for this diurnal variation in progesterone levels, similar to diurnal cortisol variability. The role of androgens in decreasing progesterone sensitivity during pubertal maturation and the impact of progesterone inhibition also remain to be demonstrated. Studies to determine whether antiandrogen administration to obese girls could restore sensitivity to progesterone and normalize development of LH pulsatility are required to establish this concept.
Acknowledgments
No funding was received or given for publication, writing, or editorial assistance. We are grateful to L. Lockhart, M.P.H. for research coordination; M. DeBoer, M.D., W. Clarke, M.D., and S. Cluett, C.P.N.P. for subject recruitment for our studies; the GCRC staff and nurses; and the Ligand Core Lab of the Center for Research in Reproduction.
Funding: Our studies were supported by National Institutes of Health U54 Grant HD 28934-16 from the Eunice Kennedy Shriver Institute for Child Health and Human Development.
Footnotes
Disclosure of Interests: There are no relevant or significant financial, personal, political, intellectual, or religious conflicts of interest to disclose.
Contribution of Authorship: CM Burt Solorzano is the primary author of this manuscript, writing all drafts toward the final manuscript. JC Marshall contributed extensive manuscript intellectual, organizational, and editorial mentorship. CR McCartney, SK Blank, and KL Knudsen contributed intellectual and editorial advice to the preparation of the final manuscript.
Details of Ethics Approval: This is a review article, which did not include previously unpublished human study data. All information relayed in this manuscript has been collected from previously published manuscripts, which have therefore undergone the ethical scrutiny of each corresponding journal. Studies previously reported from our research group have all received Institutional Review Board (IRB) approval from the University of Virginia and our General Clinical Research Center.
References
- 1.Azziz R, Sanchez LA, Knochenhauer ES, Moran C, Lazenby J, Stephens KC, et al. Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrinol Metab. 2004 Feb;89(2):453–62. doi: 10.1210/jc.2003-031122. [DOI] [PubMed] [Google Scholar]
- 2.Azziz R, Marin C, Hoq L, Badamgarav E, Song P. Health care-related economic burden of the polycystic ovary syndrome during the reproductive life span. J Clin Endocrinol Metab. 2005 Aug;90(8):4650–8. doi: 10.1210/jc.2005-0628. [DOI] [PubMed] [Google Scholar]
- 3.Shroff R, Kerchner A, Maifeld M, Van Beek EJ, Jagasia D, Dokras A. Young obese women with polycystic ovary syndrome have evidence of early coronary atherosclerosis. J Clin Endocrinol Metab. 2007 Dec;92(12):4609–14. doi: 10.1210/jc.2007-1343. [DOI] [PubMed] [Google Scholar]
- 4.Coviello AD, Legro RS, Dunaif A. Adolescent girls with polycystic ovary syndrome have an increased risk of the metabolic syndrome associated with increasing androgen levels independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2006 Feb;91(2):492–7. doi: 10.1210/jc.2005-1666. [DOI] [PubMed] [Google Scholar]
- 5.Mortensen M, Rosenfield RL, Littlejohn E. Functional significance of polycystic-size ovaries in healthy adolescents. J Clin Endocrinol Metab. 2006 Oct;91(10):3786–90. doi: 10.1210/jc.2006-0835. [DOI] [PubMed] [Google Scholar]
- 6.Waldstreicher J, Santoro NF, Hall JE, Filicori M, Crowley WF., Jr Hyperfunction of the hypothalamic-pituitary axis in women with polycystic ovarian disease: indirect evidence for partial gonadotroph desensitization. J Clin Endocrinol Metab. 1988 Jan;66(1):165–72. doi: 10.1210/jcem-66-1-165. [DOI] [PubMed] [Google Scholar]
- 7.Apter D, Butzow T, Laughlin GA, Yen SS. Accelerated 24-hour luteinizing hormone pulsatile activity in adolescent girls with ovarian hyperandrogenism: relevance to the developmental phase of polycystic ovarian syndrome. J Clin Endocrinol Metab. 1994 Jul;79(1):119–25. doi: 10.1210/jcem.79.1.8027216. [DOI] [PubMed] [Google Scholar]
- 8.Pastor CL, Griffin-Korf ML, Aloi JA, Evans WS, Marshall JC. Polycystic ovary syndrome: evidence for reduced sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 1998 Feb;83(2):582–90. doi: 10.1210/jcem.83.2.4604. [DOI] [PubMed] [Google Scholar]
- 9.Blank SK, McCartney CR, Chhabra S, Helm KD, Eagleson CA, Chang RJ, et al. Modulation of GnRH pulse generator sensitivity to progesterone inhibition in hyperandrogenic adolescent girls - Implications for regulation of pubertal maturation. J Clin Endocrinol Metab. 2009 Apr 7; doi: 10.1210/jc.2008-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eagleson CA, Gingrich MB, Pastor CL, Arora TK, Burt CM, Evans WS, et al. Polycystic ovarian syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 2000 Nov;85(11):4047–52. doi: 10.1210/jcem.85.11.6992. [DOI] [PubMed] [Google Scholar]
- 11.Apter D, Vihko R. Endocrine determinants of fertility: serum androgen concentrations during follow-up of adolescents into the third decade of life. J Clin Endocrinol Metab. 1990 Oct;71(4):970–4. doi: 10.1210/jcem-71-4-970. [DOI] [PubMed] [Google Scholar]
- 12.McCartney CR, Blank SK, Prendergast KA, Chhabra S, Eagleson CA, Helm KD, et al. Obesity and sex steroid changes across puberty: evidence for marked hyperandrogenemia in pre- and early pubertal obese girls. J Clin Endocrinol Metab. 2007 Feb;92(2):430–6. doi: 10.1210/jc.2006-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laitinen J, Taponen S, Martikainen H, Pouta A, Millwood I, Hartikainen AL, et al. Body size from birth to adulthood as a predictor of self-reported polycystic ovary syndrome symptoms. Int J Obes Relat Metab Disord. 2003 Jun;27(6):710–5. doi: 10.1038/sj.ijo.0802301. [DOI] [PubMed] [Google Scholar]
- 14.Abbott DH, Tarantal AF, Dumesic DA. Fetal, infant, adolescent and adult phenotypes of polycystic ovary syndrome in prenatally androgenized female rhesus monkeys. Am J Primatol. 2009 Apr 14; doi: 10.1002/ajp.20679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forsdike RA, Hardy K, Bull L, Stark J, Webber LJ, Stubbs S, et al. Disordered follicle development in ovaries of prenatally androgenized ewes. J Endocrinol. 2007 Feb;192(2):421–8. doi: 10.1677/joe.1.07097. [DOI] [PubMed] [Google Scholar]
- 16.Steckler TL, Herkimer C, Dumesic DA, Padmanabhan V. Developmental programming: excess weight gain amplifies the effects of prenatal testosterone excess on reproductive cyclicity--implication for polycystic ovary syndrome. Endocrinology. 2009 Mar;150(3):1456–65. doi: 10.1210/en.2008-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ibanez L, de Zegher F, Potau N. Premature pubarche, ovarian hyperandrogenism, hyperinsulinism and the polycystic ovary syndrome: from a complex constellation to a simple sequence of prenatal onset. J Endocrinol Invest. 1998 Oct;21(9):558–66. doi: 10.1007/BF03350781. [DOI] [PubMed] [Google Scholar]
- 18.Pandolfi C, Zugaro A, Lattanzio F, Necozione S, Barbonetti A, Colangeli MS, et al. Low birth weight and later development of insulin resistance and biochemical/clinical features of polycystic ovary syndrome. Metabolism. 2008 Jul;57(7):999–1004. doi: 10.1016/j.metabol.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 19.Sadrzadeh S, Klip WA, Broekmans FJ, Schats R, Willemsen WN, Burger CW, et al. Birth weight and age at menarche in patients with polycystic ovary syndrome or diminished ovarian reserve, in a retrospective cohort. Hum Reprod. 2003 Oct;18(10):2225–30. doi: 10.1093/humrep/deg409. [DOI] [PubMed] [Google Scholar]
- 20.Cresswell JL, Barker DJ, Osmond C, Egger P, Phillips DI, Fraser RB. Fetal growth, length of gestation, and polycystic ovaries in adult life. Lancet. 1997 Oct 18;350(9085):1131–5. doi: 10.1016/s0140-6736(97)06062-5. [DOI] [PubMed] [Google Scholar]
- 21.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004 Jun;89(6):2548–56. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 22.Dieudonne MN, Pecquery R, Boumediene A, Leneveu MC, Giudicelli Y. Androgen receptors in human preadipocytes and adipocytes: regional specificities and regulation by sex steroids. Am J Physiol. 1998 Jun;274(6 Pt 1):C1645–52. doi: 10.1152/ajpcell.1998.274.6.C1645. [DOI] [PubMed] [Google Scholar]
- 23.Cara JF, Rosenfield RL. Insulin-like growth factor I and insulin potentiate luteinizing hormone-induced androgen synthesis by rat ovarian thecal-interstitial cells. Endocrinology. 1988 Aug;123(2):733–9. doi: 10.1210/endo-123-2-733. [DOI] [PubMed] [Google Scholar]
- 24.Nestler JE, Jakubowicz DJ, de Vargas AF, Brik C, Quintero N, Medina F. Insulin stimulates testosterone biosynthesis by human thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. J Clin Endocrinol Metab. 1998 Jun;83(6):2001–5. doi: 10.1210/jcem.83.6.4886. [DOI] [PubMed] [Google Scholar]
- 25.Moghetti P, Castello R, Negri C, Tosi F, Spiazzi GG, Brun E, et al. Insulin infusion amplifies 17 alpha-hydroxycorticosteroid intermediates response to adrenocorticotropin in hyperandrogenic women: apparent relative impairment of 17,20-lyase activity. J Clin Endocrinol Metab. 1996 Mar;81(3):881–6. doi: 10.1210/jcem.81.3.8772544. [DOI] [PubMed] [Google Scholar]
- 26.Poretsky L, Clemons J, Bogovich K. Hyperinsulinemia and human chorionic gonadotropin synergistically promote the growth of ovarian follicular cysts in rats. Metabolism. 1992 Aug;41(8):903–10. doi: 10.1016/0026-0495(92)90175-a. [DOI] [PubMed] [Google Scholar]
- 27.Soldani R, Cagnacci A, Yen SS. Insulin, insulin-like growth factor I (IGF-I) and IGF-II enhance basal and gonadotrophin-releasing hormone-stimulated luteinizing hormone release from rat anterior pituitary cells in vitro. Eur J Endocrinol. 1994 Dec;131(6):641–5. doi: 10.1530/eje.0.1310641. [DOI] [PubMed] [Google Scholar]
- 28.McCartney CR, Prendergast KA, Chhabra S, Eagleson CA, Yoo R, Chang RJ, et al. The association of obesity and hyperandrogenemia during the pubertal transition in girls: obesity as a potential factor in the genesis of postpubertal hyperandrogenism. J Clin Endocrinol Metab. 2006 May;91(5):1714–22. doi: 10.1210/jc.2005-1852. [DOI] [PubMed] [Google Scholar]
- 29.Reinehr T, de Sousa G, Roth CL, Andler W. Androgens before and after weight loss in obese children. J Clin Endocrinol Metab. 2005 Oct;90(10):5588–95. doi: 10.1210/jc.2005-0438. [DOI] [PubMed] [Google Scholar]
- 30.Mitamura R, Yano K, Suzuki N, Ito Y, Makita Y, Okuno A. Diurnal rhythms of luteinizing hormone, follicle-stimulating hormone, testosterone, and estradiol secretion before the onset of female puberty in short children. J Clin Endocrinol Metab. 2000 Mar;85(3):1074–80. doi: 10.1210/jcem.85.3.6445. [DOI] [PubMed] [Google Scholar]
- 31.Burger LL, Haisenleder DJ, Dalkin AC, Marshall JC. Regulation of gonadotropin subunit gene transcription. J Mol Endocrinol. 2004 Dec;33(3):559–84. doi: 10.1677/jme.1.01600. [DOI] [PubMed] [Google Scholar]
- 32.Winter JS, Faiman C, Hobson WC, Prasad AV, Reyes FI. Pituitary-gonadal relations in infancy. I. Patterns of serum gonadotropin concentrations from birth to four years of age in man and chimpanzee. J Clin Endocrinol Metab. 1975 Apr;40(4):545–51. doi: 10.1210/jcem-40-4-545. [DOI] [PubMed] [Google Scholar]
- 33.Boyar R, Finkelstein J, Roffwarg H, Kapen S, Weitzman E, Hellman L. Synchronization of augmented luteinizing hormone secretion with sleep during puberty. N Engl J Med. 1972 Sep 21;287(12):582–6. doi: 10.1056/NEJM197209212871203. [DOI] [PubMed] [Google Scholar]
- 34.Apter D, Butzow TL, Laughlin GA, Yen SS. Gonadotropin-releasing hormone pulse generator activity during pubertal transition in girls: pulsatile and diurnal patterns of circulating gonadotropins. J Clin Endocrinol Metab. 1993 Apr;76(4):940–9. doi: 10.1210/jcem.76.4.8473410. [DOI] [PubMed] [Google Scholar]
- 35.McCartney CR, Prendergast KA, Blank SK, Helm KD, Chhabra S, Marshall JC. Maturation of luteinizing hormone (gonadotropin-releasing hormone) secretion across puberty: evidence for altered regulation in obese peripubertal girls. J Clin Endocrinol Metab. 2009 Jan;94(1):56–66. doi: 10.1210/jc.2008-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marshall JC, Eagleson CA. Neuroendocrine aspects of polycystic ovary syndrome. Endocrinol Metab Clin North Am. 1999 Jun;28(2):295–324. doi: 10.1016/s0889-8529(05)70071-2. [DOI] [PubMed] [Google Scholar]