Abstract
Purpose
Bioartificial liver assist devices (BLADs) are expected to bridge liver failure patients to liver transplantation, but porcine endogenous retroviruses (PERVs) still pose a potential risk in pig-to-human xenotransplantation and thereby limit the use of bioartificial liver therapy. In our lab, fluidized-bed BLADs based on microencapsulated primary porcine hepatocytes have been successfully used to treat liver failure pigs. We detected the risk of PERVs transmission of microencapsulated primary porcine hepatocytes—the key component of fluidized-bed BLADs, to evaluate the biosafety of this device for further clinical applications.
Methods
Microencapsulated primary porcine hepatocytes (cell diameter = 300 μm) were cultured in Dulbecco’s modified Eagles medium (DMEM). Microencapsulated cell culture supernatants were collected at 6, 12, 24 and 72 h. HEK-293 were cocultured with these supernatants, and the cocultured cells were harvested every 7 days. RT-PCR was used to detect PERVs transmission. RT-qPCR was used to get the number of virus copies. PK-15 was used as the positive control whereas HepG2 was used as the negative control.
Results
PERV was detected in all supernatants, and the viral load of the supernatants increased with time. Moreover, cocultured 293 cells were positive for PERV-specific sequences.
Conclusion
The kind of fluidized-bed BLADs based on microencapsulated primary porcine hepatocytes have risk of PERVs transmission. Further extensive pre-clinical study focused on biosafety is warranted.
Keywords: Microencapsulated primary porcine hepatocytes, Bioartificial liver assist devices, Porcine endogenous retroviruses, RT-PCR, RT-qPCR
Introduction
Bioartificial liver support system devices have been successfully used to treat the liver failure patients [1–3]. The most widely used types were hollow fiber reactors, which have been shown to have great effect on liver failure patients [4–7]. While the hollow fiber bioreactors may excel in mass transfer conditions, they fall short in keeping hepatocytes attachment and ensuring exchange efficiency. The fluidized-bed bioreactor based on microencapsulated primary porcine hepatocytes, however, provides both immunoprotection and a high surface area that facilitates maximal material transport. Microencapsulated hepatocytes have been successfully used in cell transplantation and bioartificial liver support system for liver function replacement [8–11]. Fluidized-bed bioreactor has been shown to have a significant effect on preventing the increase of serum ammonia and intracranial pressure in the acute liver failure model of pig [12–17]. In our lab, fluidized-bed BLADs (Fig. 1, CN patent 200710070279.0) based on microencapsulated primary porcine hepatocytes have also been used to treat the liver failure pigs, and the results were encouraged (data not shown). However, with the use of porcine cells, the risk of PERVs transmission has to be considered because PERVs are integrated in the pig genome and can infect human cells in vitro [18–22]. Currently, it is unclear whether the bioartificial liver support system may result in PERVs infection [23]. Recently, Fruhauf indicated that PERVs released from a bioartificial liver system infect primary cultured human cells [24]. Here, we explore the evidence of PERVs transmission in microencapsulated primary porcine hepatocytes in an effort to evaluate the biosafety of the fluidized-bed BLADs based on microencapsulated primary porcine hepatocytes, because the clinical use and the biosafety of them have not been extensively studied.
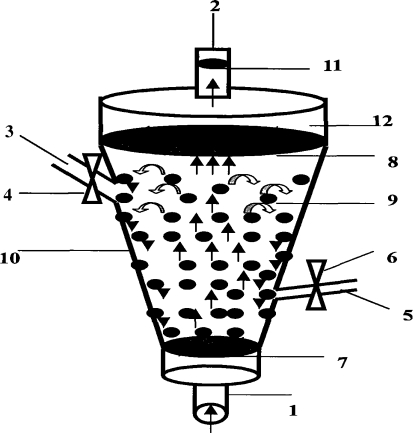
Fig. 1.
1 liquid import, 2 liquid export, 3 pre-filled liquid import 4, 6 valve, 5 sampling ports, 7, 8 cell sieve, 9 microencapsulated cells, 10 funnel-type shell, 11 cell filter, 12 Buffer region
Materials and methods
Primary cells and cell lines
Primary porcine cells were isolated and encapsulated from the Chinese experimental mini-pigs using the methods described by Yu et al. [25]. Two other cell lines PK15 (ATCC-CCL-33) and HEK-293 (ATCC-CRL-1573) were used. All cells were cultured in Dulbecco’s modified Eagles medium (DMEM) containing 10% fetal bovine serum, 100 μg penicillin/ml and 100 μg streptomycin/ml. For coculture experiments, microencapsulated cell culture supernatants were collected at 6, 12, 24 and 72 h, and then filtered through 0.45 μm membrane (Pall, USA) and centrifuged for 10 min at 1,000g to ensure no porcine cells or porcine cell debris have been transferred to human cell culture during the infection experiment. HEK-293 cells were incubated with 2 ml of the treated supernatants; the cocultured cells were harvested on day 7, 14, 21 and 28 for the isolation of the RNA. PK-15 culture supernatants were used as the positive control and HepG2 as the negative control.
Porcine endogenous retroviruses-specific RT-PCR
RNA was extracted using the QIAmp viral RNA Mini kit (Qiagen), which was then reverse transcribed using RT-PCR kit (Promega). For the PCR assay, the specific primers for the gap gene: (forward CGGCAAGAGAAGAATTTGACTAAGATC, reverse CAGTTCCTTGCCCAGTGTCCTCTT) were used. The standard program of 95°C for 2 min, 30 cycles of 95°C for 55 s, 57°C for 55 s, 72°C for 30 s, and one cycle of 72°C for 5 min was applied [26].
Detection of virus copies by RT-qPCR
Sixteen samples of gap gene cDNA sequences were detected by fluorescence quantitative PCR, and one pair of primers was designed using online Primer 3 software (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi), gagF (CGGCAAGAGAAGAATTTGACTAAGA), gagR (ACATTGGTCCTTGTCGAGTGG). 16 cDNA samples and 5 samples of plasmid standardized criteria were carried out on three duplication of quantitative PCR experiments using 2X SYBR MASTER MIX(Qiagen) to get the copy numbers of target gene fragments, according to standardized plasmid concentration gradient.
Results
Porcine endogenous retroviruses expression
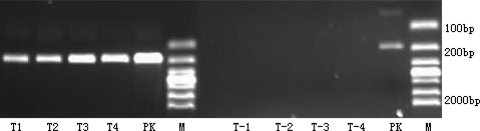
The RT-PCR product of the 6, 12, 24, 72 h microencapsulated cell culture supernatants was positive for the PERV gap gene (Fig. 2).
Fig. 2.
T1, T2, T3, T4, T-1, T-2, T-3, T-4 : 6, 12, 24, 72 h microencapsulated primary porcine hepatocytes and microencapsulated HepG2 cells’ culture supernatants; PK:PK-15 cell’ culture supernatants; M: 2000 bp Marker
Infection of HEK-293 cells with porcine endogenous retroviruses
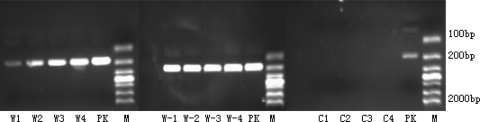
To investigate the transmission potency of the PERV, the infected HEK-293 cells by microencapsulated primary porcine hepatocytes culture supernatants were collected for extracting RNA and then for RT-PCR. The samples were found to be positive for PERV gene after 28 days of incubation. While the HEK-293 cells infected by PK15 were also positive, the HepG2 group was negative (Figs. 3, 4, 5).
Fig. 3.
W1, W2, W3, W4; W-1, W-2, W-3, W-4; C1, C2, C3, C4: 7, 14, 21 and 28 days infected HEK-293 cells by microencapsulated primary porcine hepatocytes, PK-15 cells, HepG2cells’ culture supernatants; PK: PK-15 cell’ culture supernatants; M: 2000 bp Marker
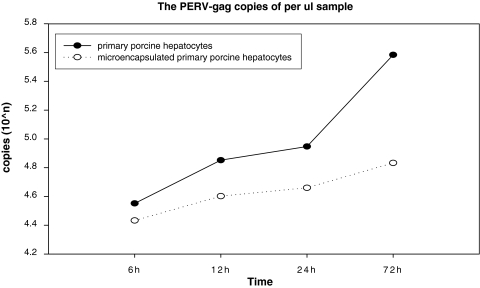
Fig. 4.
The PERV-gag gen copies of primary porcine hepatocytes and microencapsulated primary porcine hepatocytes supernatants increased with time, and the viral load of microencapsulated cells supernatants are lower than the naked cells at each time point
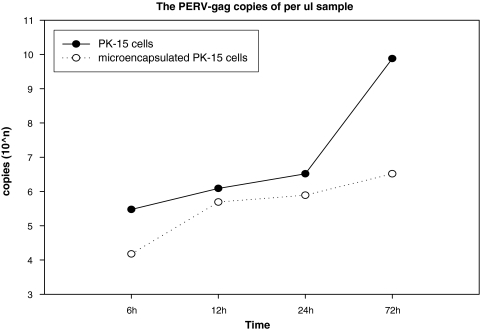
Fig. 5.
The PERV-gag gen copies of PK-15 cells and microencapsulated PK-15 cells supernatants show the same result with Fig. 3
Virus copies of the samples
Figures 4 and 5 show the growth trend of viral load of the microencapsulated cells and naked ones.
Discussion
Cell microencapsulation technology is mainly used in the field of islet transplantation and bioartificial liver support systems [27–29]. Due to the usage of porcine cells, the PERV transmission is always concerned. Although microencapsulation has immunoprotection effect, it remains unclear whether encapsulation has sufficiently reduced the risk of PERV transmission [30]. The key component of the fluidized-bed BLADs based on microencapsulated primary porcine hepatocytes is the microencapsulated cells, which perform the synthetic and regulatory functions. In this study, we demonstrated that in the RT-PCR products from 6, 12, 24 and 72 h microencapsulated porcine hepatocytes culture supernatants, the PERV-gag gene could be detected. As a positive control, the PERV-gag gene can also be detected in the microencapsulated PK-15 cell culture supernatants, and PERV titers of microencapsulation cells culture supernatant increased with time. This indicates that during the treatment of liver failure using the microencapsulated porcine hepatocytes, the PERV virus particles could be released into the reactor loop line and enter the blood circulation of the treatment models, bringing the risk of viral infection. The number of viruses entering the circulation, hence the risk of transmission increases as the duration of the treatment extends. Here, we chose 72 h as the experimental endpoint based on two reasons. First, we integrated the previous treatment time of all artificial liver support systems, including experimental and clinical ones. They last from minimal 4 h (AMC-BAL, Amsterdam) to maximal 168 h (ELAD, USA), and so we chose the middle time 72 h as the endpoint. Second, we found that the viral load of the supernatants increased with time, but after 72 h, the growth trend is not obvious (data was not shown there), so we think the 72 h is the ideal endpoint to assess the biosafety of this kind of device. In addition, we detected PERV-gag gene in the infected HEK-293 cells by microencapsulated primary porcine hepatocyte supernatants in samples from the 7, 14, 21, 28 days. This suggests that microencapsulated primary porcine hepatocytes can infect human cells in vitro, which means fluidized-bed BLADs based on microencapsulated primary porcine hepatocytes can result in the PERVs transmission.
This conclusion is consistent with the majority of the results from PERV in vitro infection experiments, and may indicate that there is no difference in the PERV transmission between the microencapsulated cells and naked cells. However, whether the microencapsulated porcine cells could cause the PERV infection in vivo requires further investigations.
Overall, these experimental results indicate that when using microencapsulated porcine cell-based fluidized-bed reactor to treat liver failure patients, there may be a risk of transmission of porcine retrovirus. Of course, factors such as the materials, pore size of encapsulation, the number of pig cells in the encapsulation, the contact time of the plasma and the microencapsulated cells may all have an impact on the result. This finding gives us some guides: we need to adjust the structure and pore size of our microcapsules to avoid the virus infection, and when we invent a new bioreactor, the pathogen transmission is still a problem that cannot be ignored. We therefore suggest that more extensive pre-clinical studies focused on biosafety to be conducted.
Conflict of interest
This material is based upon work funded by Zhejiang Provincial Natural Science Foundation of China under Grant No. Y206005 and Zhejiang Provincial Science Foundation of China under Grant No. 2007C23009.
References
- 1.Kerkhove MP, Hoekstra R, Chamuleau R, Gulik TM. Clinical application of bioartificial liver support systems. Ann Surg. 2004;240:216–230. doi: 10.1097/01.sla.0000132986.75257.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naruse K, Tang W, Makuuchi M. Artificial and bioartificial liver support: a review of perfusion treatment for hepatic failure patients. World J Gastroenterol. 2007;13:1516–1521. doi: 10.3748/wjg.v13.i10.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKenzie TJ, Lillegard JB, Nyberg SL. Artificial and bioartificlal liver support. Semin Liver Dis. 2008;28:210–217. doi: 10.1055/s-2008-1073120. [DOI] [PubMed] [Google Scholar]
- 4.Ellis AJ, Hughes RD, Wendon JA, Dunne J, Langley PG, Kelly JH, Gislason GT, Sussman NL, Williams R. Pilot-controlled trial of the extracorporeal liver assist device in acute liver failure. Hepatology. 1996;24:1446–1451. doi: 10.1002/hep.510240625. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe FD, Mullon CJP, Hewitt WR, Arkadopoulos N, Kahaku E, Eguchi S, Khalili T, Arnaout W, Shackleton CR, Rozga J, Solomon B, Demetriou AA. Clinical experience with a bioartificial liver in the treatment of severe liver failure—a phase I clinical trial. Ann Surg. 1997;225:484–491. doi: 10.1097/00000658-199705000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sauer IM, Zeilinger K, Obermayer N, Pless G, Grunwald A, Pascher A, Mieder T, Roth S, Goetz M, Kardassis D, Mas A, Neuhaus P, Gerlach JC. Primary human liver cells as source for modular extracorporeal liver support—a preliminary report. Int J Artif Organs. 2002;25:1001–1005. doi: 10.1177/039139880202501015. [DOI] [PubMed] [Google Scholar]
- 7.Mazariegos GV, Kramer DJ, Lopez RC, Shakil AO, Rosenbloom AJ, DeVera M, Giraldo M, Grogan TA, Zhu Y, Fulmer ML, Amiot BP, Patzer JF. Safety observations in Phase I clinical evaluation of the Excorp Medical Bioartificial Liver Support System after the first four patients. ASAIO J. 2001;47:471–475. doi: 10.1097/00002480-200109000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Gao Y, Xu J, Sun B, Jiang H-C. Microencapsulated hepatocytes and islets as in vivo bioartificial liver support system. World J Gastroenterol. 2004;10:2067–2071. doi: 10.3748/wjg.v10.i14.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diekmann S, Glockner P, Mittag A, Tarnok A, Bader A. Encapsulated hepatocytes for the application in a bioartificial liver support system. Cytometry A. 2007;71A:516–517. [Google Scholar]
- 10.Kinasiewicz A, Gautier A, Lewinska D, Bukowski J, Legallais C, Werynski A. Culture of C3A cells in alginate beads for fluidized bed bioartificial liver. Transplant Proc. 2007;39:2911–2913. doi: 10.1016/j.transproceed.2007.08.062. [DOI] [PubMed] [Google Scholar]
- 11.Kinasiewicz A, Gautier A, Smietanka A, Legallais C, Werynski A. Encapsulation of hepatic cells in alginate beads for fluidized bed bioartificial liver. Studies on chitosan membrane. Int J Artif Organs. 2008;31:655–657. doi: 10.1177/039139880803100411. [DOI] [PubMed] [Google Scholar]
- 12.Dore E, Legallais C. A new concept of bioartificial liver based on a fluidized bed bioreactor. Ther Apher. 1999;3:264–267. doi: 10.1046/j.1526-0968.1999.00152.x. [DOI] [PubMed] [Google Scholar]
- 13.Hwang YJ, Kim YI, Lee JG, Lee JW, Kim JW, Chung JM. Development of bioartificial liver system using a fluidized-bed bioreactor. Transplant Proc. 2000;32:2349–2351. doi: 10.1016/S0041-1345(00)01695-X. [DOI] [PubMed] [Google Scholar]
- 14.David B, Dore E, Jaffrin MY, Legallais C. Mass transfers in a fluidized bed bioreactor using alginate beads for a future bioartificial liver. Int J Artif Organs. 2004;27:284–293. doi: 10.1177/039139880402700404. [DOI] [PubMed] [Google Scholar]
- 15.Carpentier B, Gautier A, Paullier R, Legallais C. Mass transfer studies in a model of fluidized bed bioartificial liver. Int J Artif Organs. 2006;29:509–512. [Google Scholar]
- 16.Kinasiewicz A, Gautier A, Lewinska D, Dufresne M, Paullier P, Bukowski J, Legallais C, Werynski A. Activity of cells within alginate beads produced for fluidized bed bioreactor (bioartificial liver) Int J Artif Organs. 2006;29:519–521. [Google Scholar]
- 17.Hwang YJ, Chang SK, Kim JY, Kim SG, Yun YG, Il Kim Y. Bioartificial liver system using a fluidized-bed bioreactor. Liver Transpl. 2008;14:S188–191. doi: 10.1002/lt.21440. [DOI] [PubMed] [Google Scholar]
- 18.Patience C, Takeuchi Y, Weiss RA. Infection of human cells by an endogenous retrovirus of pigs. Nat Med. 1997;3:282–286. doi: 10.1038/nm0397-282. [DOI] [PubMed] [Google Scholar]
- 19.Ritzhaupt A, Laan LJW, Salomon DR, Wilson CA. Porcine endogenous retrovirus infects but does not replicate in nonhuman primate primary cells and cell lines. J Virol. 2002;76:11312–11320. doi: 10.1128/JVI.76.22.11312-11320.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartosch B, Stefanidis D, Myers R, Weiss R, Patience C, Takeuchi Y. Evidence and consequence of porcine endogenous retrovirus recombination. J Virol. 2004;78:13880–13890. doi: 10.1128/JVI.78.24.13880-13890.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Machnik G, Sypniewski D, Mazurek U, Rozek-Kostorkiewicz J, Wilczok T. Hazards of porcine endogenous retroviruses (PERVs) in xenotransplantation. Med Weter. 2004;60:345–348. [Google Scholar]
- 22.Denner J. Recombinant porcine endogenous retroviruses (PERV-A/C): a new risk for xenotransplantation? Arch Virol. 2008;153:1421–1426. doi: 10.1007/s00705-008-0141-7. [DOI] [PubMed] [Google Scholar]
- 23.Denner J, Schuurman HJ, Patience C. Strategies to prevent transmission of porcine endogenous retroviruses, Chap. 5. In The International Xenotransplantation Association Consensus Statement On Conditions for Undertaking Clinical Trials of Porcine Islet Products in Type 1 Diabetes. Xenotransplantation 2009; 16:239–248 [DOI] [PubMed]
- 24.Fruhauf JH, Mertsching H, Giri S, Fruhauf NR, Bader A. Porcine endogenous retrovirus released by a bioartificial liver infects primary human cells. Liver Int. 2009;29:1553–1561. doi: 10.1111/j.1478-3231.2009.02087.x. [DOI] [PubMed] [Google Scholar]
- 25.Yu CB, Lv GL, Pan XP, Chen YS, Cao HC, Zhang YM, Du WB, Yang SG, Li LJ. In vitro large-scale cultivation and evaluation of microencapsulated immortalized human hepatocytes (HepLL) in roller bottles. Int J Artif Organs. 2009;32:272–281. doi: 10.1177/039139880903200504. [DOI] [PubMed] [Google Scholar]
- 26.Switzer WM, Shanmugam V, Heneine W. Polymerase chain reaction assays for the diagnosis of infection with the porcine endogenous retrovirus and the detection of pig cells in human and nonhuman recipients of pig xenografts. Transplantation. 1999;68:183–188. doi: 10.1097/00007890-199907270-00003. [DOI] [PubMed] [Google Scholar]
- 27.Lanza RP, Hayes JL, Chick WL. Encapsulated cell technology. Nat Biotechnol. 1996;14:1107–1111. doi: 10.1038/nbt0996-1107. [DOI] [PubMed] [Google Scholar]
- 28.Aoki K, Hakamada K, Seino K, Umehara Y, Itabashi Y, Sasaki M. Development of a bioartificial liver using microencapsulated xenohepatocytes. New Directions for Cellular and Organ Transplantation, vol 1210. 2000. p. 55–59
- 29.Kendall WF, Collins BH, Opara EC. Islet cell transplantation for the treatment of diabetes mellitus. Expert Opin Biol Ther. 2001;1:109–119. doi: 10.1517/14712598.1.1.109. [DOI] [PubMed] [Google Scholar]
- 30.Lim GJ, Zare S, Van Dyke M, Atala A. Cell Microencapsulation. In Therapeutic Applications of Cell Microencapsulation, vol 670. 2010. p. 126–136 [DOI] [PubMed]