Abstract
Purpose
Reported sustained virological response (SVR) rates in Asians with chronic hepatitis C (CHC) exceed those of other ethnic groups, but differences in body weight across races potentially confound this observed superior response. Our aim was to determine whether Asian race independently predicts SVR within a multicultural clinic setting.
Methods
Patients with genotype 1, 2 and 3 CHC prescribed peginterferon and weight-based ribavirin were included in this retrospective study. Logistic regression was performed to identify factors associated with SVR.
Results
Three-hundred ninety-two patients (BMI 26.9 ± 5.0 kg/m2, genotype 1 66%, viral load 5.9 ± 0.66 log10 IU/ml, advanced fibrosis 53%) were included in this study. Caucasians comprised 81%, South Asians 9% and Asians (Non-South) 10%. SVR was achieved by 54% overall, but was highest amongst Asians (Non-South) (79%) compared with South Asians (56%, P = 0.04) and Caucasians (50%, P < 0.001) despite a predominance of genotype 3 infection amongst the South Asians. Asians (Non-South) had the highest SVR rate even amongst those infected with genotype 1 (75%) and those with advanced fibrosis (77%). Independent of viral genotype, Asian (Non-South) race was a strong predictor of SVR (OR 5.10 vs. Caucasians, 95% CI 1.72–17.71, OR 7.84 vs. South Asians, 95% CI 1.62–37.84), as were treatment naïve status (OR 3.85, 95% CI 1.76–8.89), non-diabetic status (OR 3.70, 95% CI 1.30–11.11), non-obesity (OR 2.13, 95% CI 1.06–4.35), peginterferon α2a (2.08 vs. α2b, 95% CI 1.16–3.85), steatosis <10% (OR 2.0, 95% CI 1.05–3.85) and ribavirin exposure (mg/kg/day) (OR 1.13, 95% CI 1.01–1.28).
Conclusion
Asian (Non-South) race is a strong independent predictor of SVR.
Keywords: Asian, Sustained virological response, Body mass index, Weight, Obesity, Diabetes, Chronic hepatitis C
Introduction
The current standard of care in the treatment of chronic hepatitis C (CHC) is combination therapy with pegylated interferon and ribavirin [1, 2]. A number of viral and host factors impact the likelihood of achieving sustained virological response (SVR). Infection with genotype 1 HCV and high baseline viral load are strongly associated with diminished treatment response rates [1–3]. Host factors associated with reduced rates of SVR include obesity, insulin resistance (and overt diabetes), presence of advanced fibrosis or hepatic steatosis and both patterns of hepatic gene expression and polymorphisms of the interferon-λ gene [4–9].
The response to antiviral therapy also appears to be influenced by ethnicity. Reduced rates of SVR have been reported amongst African Americans, Hispanics and South Asians when compared to their Caucasian counterparts [10–12]. In contrast, studies arising from Eastern Asia report high rates of SVR, 79% in patients with genotype 1 and 95% with genotype 2 CHC [13, 14]. The rate of SVR in Asians, not differentiated into South or Asians (Non-South), was significantly higher when compared to Caucasians (65 vs. 45%), but in that study, patients had received only 800 mg/day ribavirin regardless of viral genotype [15]. Whilst the latter reports suggest that Asians have a higher SVR rate than non-Asians, it is possible that the differences were due to systematic differences in how HCV was treated at different sites, such as the policies regarding dose reduction and the use of weight-based ribavirin.
The response to antiviral therapy does not appear to be uniform amongst all Asians. A small study reports that South Asians infected with the “favourable” genotype 3 HCV have a lower rate of SVR than Caucasians and to other Asian historical controls [12]. The reasons for a superior response in Asians from regions other than South Asia, hereafter referred to as Asians (Non-South), are not evident. Low body weight and body mass index (BMI) are potential factors which confound the high observed rates of SVR in Asians (Non-South). We have previously reported that obesity (BMI ≥ 30 kg/m2) is a negative predictor of SVR, where weight-based dosing of ribavirin was not used [16]. Individuals with low body weight receive a higher dose of ribavirin per kilogram body weight which is shown to be associated with lower relapse and higher SVR rates [17]. Lower body weight is reported to be associated with rapid virological response (RVR), the single best predictor of SVR [18].
Aside from these potential confounders, race-specific host genetic variation may influence the efficacy of interferon-based therapy. Genetic polymorphisms of human leukocyte antigen, TNFα-308 promoter gene, suppressor of cytokine signalling (SOCS)-3 gene and interferon-λ 28B (IL28B) are examples of host-related differences that may influence response to interferon [7–9, 19–22]. Ethnicity may be a surrogate for such genetic polymorphisms; studies are yet to determine how the prevalence of such genetic markers differs between racial groups and specifically how they relate to treatment outcome.
To determine whether there is a true discrepancy in treatment response between Asians (Non-South) and other ethnic groups, we examined the treatment response controlling for confounders including BMI and ribavirin exposure (ribavirin dose per kilogram body weight). To our knowledge, this is the first report comparing the response to the current therapeutic standard of care in Asians (Non-South) to South Asians, to Caucasians treated at a single site.
Patients and methods
This retrospective study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Research Ethics Board of the University Heath Network. All patients infected with genotypes 1, 2 or 3 CHC treated with pegylated interferon-α and ribavirin (outside the context of a clinical trial) from June 2001 to March 2009 at the Toronto Western Hospital were eligible for inclusion. Patients with HCV genotype 1 infection received 48 weeks of either pegylated interferon-α2a 180 mcg weekly plus ribavirin 1,000 or 1,200 mg daily [for body weight (BW) <75 or ≥75 kg, respectively] or pegylated interferon-α2b 1.5 mcg/kg weekly plus daily ribavirin 800 mg (BW < 65 kg), 1,000 mg (BW = 65–85 kg) or 1,200 mg (BW > 85–105 kg). Patients with genotype 2/3 infection received pegylated interferon-α2a 180 mcg weekly or pegylated interferon-α2b 1.5 mcg/kg weekly plus ribavirin 800–1,200 mg daily according to body weight (aiming for a dose of 13–15 mg/kg). The duration of therapy for genotype 2/3 patients was 24 weeks (standard therapy).
The HCV genotype testing prior to January 2007 was performed using INNO-LiPA HCV genotype assay (Innogenetics, Belgium), between January and November 2007 using Versant HCV Genotype 2.0 Assay (LiPA) (Seimens Healthcare Diagnostics, Tarrytown, NY, USA), for the latter part of the study period using HCVG Genotyping Assay (Third Wave Technologies, Madison, WI, USA). All three assays are able to distinguish HCV genotype 6 from genotype 1.
The clinic database was interrogated for patients’ age at initiation of treatment, gender, genotype, pretreatment viral load, diagnosis of diabetes mellitus (type 1 or 2), weight and height, and BMI. For those in whom liver biopsy had been performed as part of standard of care, fibrosis score (Laennec criteria) and steatosis (%) were recorded. The presence of cirrhosis by clinical criteria was also determined (splenomegaly, thrombocytopenia, radiological or clinical evidence of portal hypertension).
Self-reported ethnicity and country of origin were obtained from the database. Patients included were categorised as Caucasian, South Asian or Asian (Non-South). South Asians were patients originating from India, Pakistan, Sri Lanka or Bangladesh. Patients originating from Asian countries other than South Asia were categorised as Asian (Non-South). Because of their small numbers, patients of ethnicity other than those specified above were excluded from analysis.
Taking dose reductions or dose interruptions into account, the average daily ribavirin exposure (per kilogram body weight) was calculated for each patient using the equation:
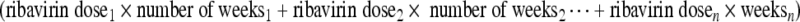 |
divided by total number of weeks of treatment, divided by body weight (kg).
Null responders (those achieving <2 log drop in viral load after 12 weeks of therapy) were included in the final analysis. Exclusion criteria were: extended duration treatment (>24 weeks in genotype 2/3 patients or >48 weeks in genotype 1); genotype 4, 5 and 6 infection; coexistence of other liver disease (HBV, HIV, autoimmune hepatitis, liver transplantation).
The primary outcome was SVR, defined as a negative HCV RNA PCR 6 months after cessation of antiviral therapy (Roche Amplicor assay, LLD 50 IU/ml, prior to 2007; Taqman assay LLD 15 IU/ml since 2007). Null responders, non-responders (positive HCV RNA at end of treatment) and relapsers (negative HCV RNA at end of treatment with subsequent positive HCV RNA after cessation of therapy) were grouped together and categorised “non-SVR”.
Data were analysed using SAS System v.9.1.3 (SAS Institute Inc., NC, USA). Student t tests, one way ANOVA, fisher exact test and χ2 test were performed (where applicable) in order to identify variables to enter the multivariate logistic regression model. BMI was analysed categorically based on World Health Organization definition of obesity (BMI ≥ 30) [23] and repeated after recategorising the Asian patients using the suggested race-specific BMI cut-off for obesity (BMI ≥ 27.5) [24]. Multiple logistic regression by intention-to-treat (ITT) was performed adjusting for cases with inadequate therapy, and treating those with missing treatment outcomes as non-SVR cases. As the primary aim of the study was to determine the influence of race (as a surrogate for host genetic variation) on treatment outcome, multiple logistic regression analysis was also performed to identify factors independently associated with SVR including only patients who received adequate dose and duration of therapy in line with the standard of care and also had a documented treatment outcome [per protocol (PP) analysis]. Inadequate treatment was defined as receiving less than 80% of the prescribed treatment dose for less than 80% of the duration of therapy (due to adverse events or lack of adherence).
Results
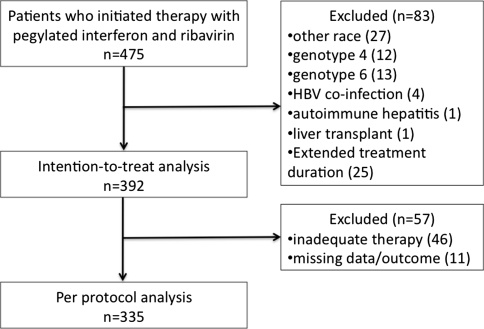
During the study period, 475 patients were prescribed pegylated interferon and ribavirin outside of a clinical trial. Eighty-three patients (17.5%) were excluded from the intention-to-treat analysis, and further 57 patients were excluded from the per protocol analysis (details in Fig. 1). Therefore, 392 patients were included in the intention-to-treat analysis and 335 included in the per protocol analysis. The vast majority of the patients (329/392, 84%) were interferon-naïve.
Fig. 1.
Patient flow illustrating the characteristics of the intention-to-treat and per-protocol cohorts
Patients’ characteristics
Table 1 lists the characteristics of the 392 patients included in the ITT analysis broken down into ethnic groups. The study cohort comprised Caucasians (81%), Asians (Non-South) (10%) and South Asians (9%). The Asian (Non-South) patients originated from Vietnam (19), China (7), Hong Kong (4), Taiwan (1), Singapore (1), Japan (1), Korea (1), Burma (1), Cambodia (1) and Philippines (3). There were clear differences in the characteristics of the racial groups that could be considered to potentially confound the contribution of race to treatment outcome. Genotype 1 was the most prevalent across the entire cohort (66%), except in South Asians where genotype 3 predominated (79%). Asians (Non-South) had the lowest body weight and the lowest prevalence of obesity (0%), but the highest prevalence of genotype 1 (82%) and highest prevalence of diabetes (18%). When race-specific BMI cut offs were used to redefine obesity in the Asians, the prevalence of obesity for Asians (Non-South) and South Asians were 9 and 58%, respectively. Gender, pretreatment viral load (and prevalence of high viral load >800,000 IU/ml) and type of pegylated interferon prescribed were not significantly different between the ethnic groups.
Table 1.
Patient characteristics (ITT)
| Characteristic | Asian (Non-South) | South Asian | Caucasian | P value |
|---|---|---|---|---|
| Number of subjects | 39 (10%) | 34 (9%) | 319 (81%) | |
| Age (years) | 51.5 ± 11.0 | 46.7 ± 11.2 | 49.4 ± 9.2 | 0.105 |
| No. of males | 21 (54%) | 23 (68%) | 215 (67%) | 0.236 |
| Genotype | ||||
| 1 | 32 (82%) | 5 (15%) | 223 (70%) | <0.001 |
| 2 | 5 (13%) | 2 (6%) | 43 13%) | |
| 3 | 2 (5%) | 27 (79%) | 53 (17%) | |
| Pretreatment viral load (log10 IU/ml) | 6.0 ± 0.6 | 5.9 ± 0.8 | 5.8 ± 0.6 | 0.418 |
| Weight (kg) | 59.6 ± 12.0 | 74.9 ± 12.9 | 80.4 ± 15.6 | <0.001 |
| BMI (kg/m2) | 22.4 ± 3.5 | 27.5 ± 4.8 | 27.4 ± 4.9 | <0.001 |
| Obese (WHO definition) | 0 (0%) | 7 (27%) | 69 (24%) | <0.001 |
| Obese (Asian definition) | 3 (9%) | 15 (58%) | 69 (24%) | <0.001 |
| Diabetes (type 1 or 2) | 7 (18%) | 6 (18%) | 24 (8%) | 0.021 |
| Advanced fibrosis (F3–F4 or clinical features of cirrhosis) | 22 (59%) | 15 (48%) | 171 (58%) | 0.577 |
| Steatosis ≥10% | 5 (16%) | 15 (60%) | 73 (27%) | 0.001 |
| Treatment naïve status | 33 (85%) | 33 (97%) | 263 (82%) | 0.064 |
| PegIFN 2a | 18 (46%) | 15 (44%) | 200 (63%) | 0.023 |
| Calculated ribavirin dose received (mg/kg/day) | 15.0 ± 3.0 | 12.7 ± 2.3 | 12.6 ± 2.4 | <0.001 |
| Received “adequate” therapy (>80% dose, 80% duration) | 34 (87%) | 33 (97%) | 275 (86%) | 0.203 |
Data are expressed as n (%) or mean ± SD
Pretreatment liver biopsy had been performed in 338 of 392 patients. Fifty three percent of the entire cohort had either biopsy-proven advanced fibrosis (F3–F4) or clinical evidence of cirrhosis; there was no difference in prevalence across the racial groups. Steatosis ≥10% was observed in 28% of the liver biopsies. South Asians had the highest prevalence of steatosis ≥10% (60%) in keeping with the predominance of genotype 3 infection and prevalence of obesity amongst this sub-group.
Dose reduction of peginterferon was required in 8% of patients, and ribavirin dose reduction in 15% of patients, without difference in occurrence of dose reductions across the racial groups. Only two patients required ribavirin dose reduction within the first 4 weeks of therapy (1 Caucasian, 1 South Asian). Taking all dose reductions and interruptions into consideration, Asians (Non-South) received the highest mean calculated ribavirin dose/kg body weight across the racial groups (Table 1).
Sustained virological response
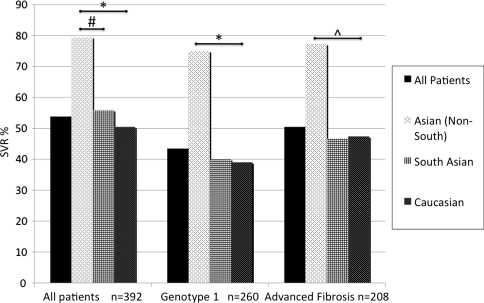
The SVR was achieved by 54% of the cohort; of the remaining 46% who did not achieve SVR, 55 (14%) were null-responders (genotype 1: 52, genotype 2: 1, genotype 3: 2). Figure 2 illustrates the unadjusted rates of SVR broken down into racial groups. Asians (Non-South) had the highest rate of SVR overall (31/39, 79%), higher than in South Asians (19/34, 56%, P = 0.04) and Caucasians (161/319, 50%, P < 0.001). The higher SVR in Asians (Non-South) was observed despite “traditional” negative predictors of response: SVR was achieved by 24/32 (75%) with genotype 1 CHC and 17/22 (77%) with advanced fibrosis.
Fig. 2.
Unadjusted SVR rates stratified into racial groups. SVR rates observed in Asian (Non-South) significantly higher than Caucasians across all patients, amongst genotype 1 patients and amongst those with advanced fibrosis. *P < 0.001, ^P = 0.03, #P = 0.04
The overall rates of SVR in genotype 2 and 3 were 82% (41/50) and 70% (57/82), respectively, without significant difference across the racial groups.
Factors associated with SVR
Univariate analysis
The factors associated with SVR by univariate analysis were: Asian (Non-South) race, younger age, treatment naïve status, genotype 2/3 infection, lower body weight, lower BMI and the absence of obesity. Pretreatment viral load, ribavirin dose/kg body weight received, and the type of peginterferon prescribed did not differ significantly between those who achieved SVR and those who did not (Table 2).
Table 2.
Univariate analysis: factors associated with SVR–ITT analysis
| Characteristic | SVR | No SVR | P value |
|---|---|---|---|
| Number of subjects | 211 (54%) | 181 (46%) | |
| Age (years) | 48.2 ± 10.1 | 50.9 ± 8.8 | 0.004 |
| No. of males | 134 (64%) | 125 (69%) | 0.293 |
| Race | |||
| Asian (Non-South) | 31 (79%) | 8 (21%) | 0.003 |
| South Asian | 19 (56%) | 15 (44%) | |
| Caucasian | 161 (51%) | 158 (49%) | |
| Genotype | |||
| 1 | 113 (54%) | 147 (81%) | <0.001 |
| 2 | 41 (19%) | 9 (5%) | |
| 3 | 57 (27%) | 25 (14%) | |
| Pretreatment viral load (log10 IU/ml) | 5.8 ± 0.8 | 5.9 ± 0.5 | 0.081 |
| Weight (kg) | 75.2 ± 15.7 | 81.1 ± 16.7 | <0.001 |
| BMI (kg/m2) | 26.1 ± 5.0 | 27.9 ± 4.9 | <0.001 |
| Obese (WHO definition) | 33 (17%) | 43 (28%) | 0.029 |
| Obese (Asian definition) | 38 (20%) | 49 (32%) | 0.019 |
| Diabetes (Type 1 or 2) | 15 (7%) | 22 (12%) | 0.109 |
| Advanced Fibrosis (F3–F4 or clinical features of cirrhosis) | 105 (54%) | 103 (61%) | 0.255 |
| Steatosis ≥10% | 43 (24%) | 50 (33%) | 0.107 |
| Treatment naïve status | 194 (92%) | 135 (75%) | <0.001 |
| PegIFN 2a | 134 (64%) | 99 (55%) | 0.095 |
| Calculated ribavirin dose received (mg/kg/day) | 13.0 ± 2.7 | 12.8 ± 2.5 | 0.359 |
| Received “adequate” therapy (>80% dose, 80% duration) | 189 (90%) | 153 (85%) | 0.130 |
Data are expressed as n (%) or mean ± SD
Multiple logistic regression by intention-to-treat
The final model included the factors associated with SVR on univariate analysis (including all variables with P < 0.1; Table 2) plus the factors identified to be confounders of race (Table 1). The final model therefore included race, viral genotype (2/3 vs. 1), age, baseline viral load, steatosis (<10 vs. ≥10%), pretreatment status (naïve vs. previous IFN exposure), ribavirin exposure (mg/kg/day), BMI category (non-obese vs. obese) and diabetic status (non-diabetic vs. diabetic) and type of peginterferon (α2a vs. α2b) (Table 3). Fibrosis stage (F0–F2 vs. F3–F4 or clinical evidence of cirrhosis) and “adequacy of therapy” were also retained as covariates because of their previously reported influence on SVR. The final model was also adjusted for gender as it was observed in post-hoc univariate analysis that a significantly higher proportion of males received peginterferon α2a than peginterferon α2b (70.4 vs. 59.7%, P = 0.038).
Table 3.
Multiple logistic regression of factors influencing SVR
| Predictor | Intention-to-treat | Per protocol | ||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P value | Odds ratio | 95% CI | P value | |
| Race | ||||||
| Asian (Non-South) versus Caucasian | 5.10 | 1.72–17.71 | 0.005* | 4.56 | 1.52–15.89 | 0.010* |
| South Asian versus Caucasian | 0.65 | 0.21–2.08 | 0.459 | 0.64 | 0.20–2.07 | 0.446 |
| Contrast | ||||||
| Asian (Non-South) versus South Asian | 7.84 | 1.62–37.84 | 0.009* | 7.13 | 1.46–34.84 | 0.014* |
| Genotype 2/3 versus 1 | 8.32 | 4.06–18.22 | <0.001* | 8.38 | 3.89–19.52 | <0.001* |
| Age (years) | 0.98 | 0.95–1.01 | 0.263 | 0.98 | 0.95–1.02 | 0.366 |
| Baseline viral load (log10 IU/ml): | 0.75 | 0.49–1.15 | 0.193 | 0.80 | 0.51–1.25 | 0.328 |
| Fibrosis: Advanced versus Early | 1.58 | 0.87–2.94 | 0.140 | 1.42 | 0.87–2.94 | 0.277 |
| Steatosis <10% versus ≥10% | 2.00 | 1.05–3.85 | 0.036* | 1.75 | 0.91–3.57 | 0.098 |
| Pretreatment status: naïve versus Previous IFN exposure | 3.85 | 1.76–8.89 | 0.001* | 2.95 | 1.29–7.11 | 0.012* |
| Ribavirin dose received (mg/kg/day) | 1.13 | 1.01–1.28 | 0.041* | 1.11 | 0.98–1.27 | 0.099 |
| BMI <30 versus ≥30 kg/m2 | 2.13 | 1.06–4.35 | 0.034* | 2.38 | 1.15–5.00 | 0.022* |
| Non-diabetic versus Diabetic | 3.70 | 1.30–11.11 | 0.018* | 3.70 | 1.23–12.50 | 0.025* |
| Pegylated interferon type (2a vs. 2b) | 2.08 | 1.16–3.85 | 0.014* | 2.04 | 1.11–3.85 | 0.022* |
| Gender (male vs. female) | 0.84 | 0.44–1.59 | 0.593 | 0.77 | 0.39–1.50 | 0.440 |
| Received “adequate” versus “inadequate” therapy | 1.06 | 0.42–2.67 | 0.899 | – | – | – |
* Variables reaching statistical significance (P < 0.05)
Asian (Non-South) race was clearly identified as a strong independent predictor of SVR (OR 5.10, 95% CI 1.72–17.71 vs. Caucasians; contrast OR 7.84, 95% I 1.62–37.84 vs. South Asians). The additional independent predictors of SVR were genotype 2/3 infection, treatment naïve status, non-diabetic status, BMI < 30, peginterferon α2a, steatosis <10% and ribavirin dose received (mg/kg/day). When the analysis was repeated after recategorising the Asian patients using the race-specific definition of obesity, obesity remained a negative predictor of SVR (OR 0.36, 95% CI 0.18–0.72, P = 0.004).
As peginterferon type (α2a vs. α2b) was observed to be a statistically significant predictor of SVR in the final model, a post-hoc analysis was performed which demonstrated that this finding translated to absolute (albeit unadjusted) SVR rates in our non-randomised cohort of 78 versus 81% in Asians (Non-South) (P = 1.0), 80 versus 58% in South Asians (P = 0.02), and 54 versus 45% in Caucasians (P = 0.11) for peginterferon α2a vs α2b, respectively.
Per protocol analysis
To ensure that the observed influence of race on treatment outcome was not confounded by the inclusion of patients that received inadequate therapy or follow-up, per protocol analysis was performed excluding these 57 cases (5 Asians (Non-South), 4 South Asians and 48 Caucasians). SVR was achieved by 79% of Asians (Non-South), 63% of South Asians and 52% of Caucasians. Asian (Non-South) race remained a strong independent predictor for SVR (OR 4.56, 95% CI 1.52–15.89 compared with Caucasians; contrast OR 7.13, 95% CI 1.46–34.84 vs. South Asians) in addition to genotype 2/3 infection, treatment naïve status, non-diabetic status, the absence of obesity and peginterferon α2a (Table 3). Intuitively, ribavirin dose (mg/kg/day) was not found to significantly influence SVR in the PP analysis as, by definition, all cases in the PP analysis had received “adequate” therapy. Steatosis was also no longer found to be significantly associated with SVR in the PP analysis, demonstrating its marginal influence on treatment outcomes, and emphasising the more important role of the host metabolic risk factors, namely obesity and diabetes, in influencing SVR.
Discussion
Within our multicultural outpatient clinic, we have demonstrated that Asian (Non-South) race is a strong independent predictor of sustained virological response to antiviral therapy. An overall SVR of 79% was observed in this group despite a higher mean age, 82% prevalence of genotype 1 infection, 59% prevalence of advanced liver fibrosis and a higher prevalence of diabetes in this cohort.
There are a number of strengths to our study that have allowed thorough testing of our hypothesis. Our uni-centric multicultural clinic population allowed the unique opportunity to compare treatment responses in multiple racial groups receiving the same standard of care. We identified differences in characteristics between the racial groups which potentially confound the contribution of race/ethnicity to the observed response to antiviral therapy; as anticipated Asians (Non-South) had lower body weight and thus received the highest dose of ribavirin per kg/body weight. Adjustment was made for both the confounders of body weight and the occurrence of ribavirin dose reductions by calculating ribavirin dose exposure per kilogram body weight. Having considered all these confounders, we have clearly demonstrated that Asian (Non-South) race is a strong and independent predictor of treatment response.
The distinction made between South Asians and Asians (Non-South) was decided upon in the light of small study which showed that South Asians with genotype 3 CHC had an inferior response to antiviral therapy when compared to Caucasians [12]. However, many subjects in the study by Freshwater et al. [25] had advanced fibrosis which is known to be associated with poor response to therapy. Having adjusted for confounders including fibrosis, we found no such difference in SVR rate between South Asians and Caucasians. Furthermore, distinguishing South Asians from Asians (Non-South) allowed us to demonstrate the superior response to antiviral therapy in Asians (Non-South) when compared with both South Asians and Caucasians.
Our findings strongly support the hypothesis that a race-specific genetic component has an effect on the response to pegylated interferon and ribavirin that overrides previously described factors shown to influence response to antiviral therapy. Recent work demonstrates that host genetic variation in IL28B predicts treatment-induced viral clearance in genotype 1-infected populations [7–9]. The prevalence and influence of this genetic variation differ in Japanese [9] when compared to Caucasians and African Americans [7, 8]. Although these recent findings were reported in separate studies differing in design and population base (and therefore subject to confounding), they appear to account for the variation in SVR rates observed across ethnic groups. Host genetics may also account for the difference in prevalence of metabolic disease (namely obesity and diabetes) across the races, thereby indirectly influencing the response to antiviral therapy.
We cannot exclude the influence of a viral (rather than host) factor since infections were likely to be acquired in different parts of the world where the distributions of viral geno-subtypes are different. HCV-1b is the dominant genotype in Asia–Pacific [26], whereas in Ontario (Canada), approximately 50% of genotype 1 is 1a and 50% is 1b (personal communication, Public Health Lab, Ontario, Canada). Nevertheless, it is interesting to note that in Japan, 59.4% SVR in genotype 1b [27] is not greatly superior to SVR reports for genotype 1 around the world.
We did account for one important viral factor by using viral genotype assays that are able to distinguish genotype 6 (almost exclusively originating from South-Eastern Asia) from genotype 1. As the reported SVR rate in genotype 6 patients is 86% (significantly higher than that of genotype 1, 52%) [28], it was essential that this distinction be made. The INNO-Lipa and original Versant HCV genotype line probe assay have been reported to mischaracterise genotype 6 (subtypes 6c-6n) for genotype 1 [29]. All 13 genotype 6 cases identified and excluded from the present study were diagnosed using the INNO-LiPA, indicating that the assay was able to distinguish genotype 6 from 1 in our population. A recent study found no difference in SVR rate between Asian Americans and Caucasians with genotype 1 CHC when cases genotyped by the INNO-LiPA method were excluded [30]; however, they did not account for important host factors such as ribavirin dose reductions (which occurred significantly more often amongst their Asian patients) and the presence of type 2 diabetes (60% more prevalent in Asian Americans than non-Hispanic American Caucasians [31]); these factors may have biased their findings. In addition to distinguishing Asian (Non-South) from South Asian race, we demonstrated the independent influences of total ribavirin exposure (mg/kg/day), obesity and diabetes on SVR which were not addressed by the latter study [30].
Although a large, prospective trial has demonstrated similar SVR rates for peginterferon α2a compared with α2b [32], adjustment for ribavirin exposure in the present study (which was not performed in the aforementioned study) uncovered a statistically significant association between peginterferon α2a and SVR over peginterferon α2b in keeping with other recent reports [33–35]. However, we interpret this observation with caution since the primary aim of this non-randomised study was not to address the issue of peginterferon type on SVR, but rather the influence of ethnicity; the inclusion of “peginterferon type” in the final analysis merely served to adjust for any variation in peginterferon type administered between ethnic groups. Furthermore, the lower limit of the 95% confidence interval for the odds ratio for SVR of peginterferon 2a vs 2b approaches 1. Therefore, although the observation was statistically significant, we cannot conclude from our study that a clinically significant difference in influence of peginterferon type on SVR exists.
The present study was not accounted for to determine whether the difference in SVR observed was owing to a difference in early viral kinetics or the rate of null response, non-response or relapse. In a study from Taiwan early viral kinetics at week 4 (but not SVR) are reported to be influenced by BMI [36], but we have not observed this to be the case in Caucasians [37]. Evaluation of these secondary outcomes within a large sample size of patients of differing ethnic groups may give further insight into effects of race on treatment outcomes that we have identified. Additionally, advanced fibrosis and baseline viral load were not demonstrated to be independently associated with SVR in our cohort; we do not dispute their likely influence on SVR as reported by numerous much larger trials of antiviral therapy for CHC, but our findings suggest that race is a very strong predictor of SVR, superceding even these “classical” predictors of treatment response.
In summary, after considering all potential confounders, we report that individuals of Asian (Non-South) race have a superior response to pegylated interferon and ribavirin when compared to Caucasians and South Asians. This finding may influence the way Asian (Non-South) patients contemplating antiviral therapy are counselled and also supports the recent findings of a host genetic basis for response to antiviral therapy.
Acknowledgements
The authors wish to acknowledge Tamara Arenovich and Chris Meaney for performing the statistical analysis.
Conflict of interest VP received scholarship funding from the National Health and Medical Research Council of Australia and the University Health Network, Toronto, Canada. EJH has received grant support from the following: Axcan Pharma, Boehringer Ingelheim, Bristol-Myers Squib, Debio Pharma, Gilead Sciences, GlaxoSmithKline, Hoffman-LaRoche, Human Genome Sciences, Intercept Pharm, Merck, Tibotec, Vertex. EJH has acted as consultant for Axcan Pharma, Gilead Sciences, Hoffman-LaRoche, Merck, Tibotec. JH has acted as speaker for Axcan Pharma, Gilead Sciences, Hoffman-LaRoche, Merck, Tibotec. DKHW has no disclosures.
Abbreviations
- BMI
Body mass index
- CHC
Chronic hepatitis C
- CI
Confidence interval
- HCV
Hepatitis C virus
- IFN
Interferon
- OR
Odds ratio
- SVR
Sustained virological response
References
- 1.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/S0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 2.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr, Haussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 3.Hadziyannis SJ, Sette H, Jr, Morgan TR, Balan V, Diago M, Marcellin P, Ramadori G, Bodenheimer H, Jr, Bernstein D, Rizzetto M, Zeuzem S, Pockros PJ, Lin A, Ackrill AM. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346–355. doi: 10.7326/0003-4819-140-5-200403020-00010. [DOI] [PubMed] [Google Scholar]
- 4.Romero-Gomez M, Del Mar Viloria M, Andrade RJ, Salmeron J, Diago M, Fernandez-Rodriguez CM, Corpas R, Cruz M, Grande L, Vazquez L, Munoz-De-Rueda P, Lopez-Serrano P, Gila A, Gutierrez ML, Perez C, Ruiz-Extremera A, Suarez E, Castillo J. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology. 2005;128:636–641. doi: 10.1053/j.gastro.2004.12.049. [DOI] [PubMed] [Google Scholar]
- 5.Conjeevaram HS, Kleiner DE, Everhart JE, Hoofnagle JH, Zacks S, Afdhal NH, Wahed AS. Race, insulin resistance and hepatic steatosis in chronic hepatitis C. Hepatology. 2007;45:80–87. doi: 10.1002/hep.21455. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Borozan I, Feld J, Sun J, Tannis LL, Coltescu C, Heathcote J, Edwards AM, McGilvray ID. Hepatic gene expression discriminates responders and nonresponders in treatment of chronic hepatitis C viral infection. Gastroenterology. 2005;128:1437–1444. doi: 10.1053/j.gastro.2005.01.059. [DOI] [PubMed] [Google Scholar]
- 7.Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ, Sulkowski M, McHutchison JG, Goldstein DB. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 8.Suppiah V, Moldovan M, Ahlenstiel G, Berg T, Weltman M, Abate ML, Bassendine M, Spengler U, Dore GJ, Powell E, Riordan S, Sheridan D, Smedile A, Fragomeli V, Muller T, Bahlo M, Stewart GJ, Booth DR, George J. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet. 2009;41:1100–1104. doi: 10.1038/ng.447. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, Nakagawa M, Korenaga M, Hino K, Hige S, Ito Y, Mita E, Tanaka E, Mochida S, Murawaki Y, Honda M, Sakai A, Hiasa Y, Nishiguchi S, Koike A, Sakaida I, Imamura M, Ito K, Yano K, Masaki N, Sugauchi F, Izumi N, Tokunaga K, Mizokami M. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105–1109. doi: 10.1038/ng.449. [DOI] [PubMed] [Google Scholar]
- 10.Jacobson IM, Brown RS, Jr, McCone J, Black M, Albert C, Dragutsky MS, Siddiqui FA, Hargrave T, Kwo PY, Lambiase L, Galler GW, Araya V, Freilich B, Harvey J, Griffel LH, Brass CA. Impact of weight-based ribavirin with peginterferon alfa-2b in African Americans with hepatitis C virus genotype 1. Hepatology. 2007;46:982–990. doi: 10.1002/hep.21670. [DOI] [PubMed] [Google Scholar]
- 11.Hepburn MJ, Hepburn LM, Cantu NS, Lapeer MG, Lawitz EJ. Differences in treatment outcome for hepatitis C among ethnic groups. Am J Med. 2004;117:163–168. doi: 10.1016/j.amjmed.2004.02.043. [DOI] [PubMed] [Google Scholar]
- 12.Freshwater DA, O’Donnell K, Mutimer DJ. Inferior response of Asian vs non-Asian hepatitis C genotype 3 infection to combination antiviral therapy. J Viral Hepat. 2008;15:115–119. doi: 10.1111/j.1365-2893.2007.00899.x. [DOI] [PubMed] [Google Scholar]
- 13.Yu ML, Dai CY, Huang JF, Chiu CF, Yang YH, Hou NJ, Lee LP, Hsieh MY, Lin ZY, Chen SC, Wang LY, Chang WY, Chuang WL. Rapid virological response and treatment duration for chronic hepatitis C genotype 1 patients: a randomized trial. Hepatology. 2008;47:1884–1893. doi: 10.1002/hep.22319. [DOI] [PubMed] [Google Scholar]
- 14.Yu ML, Dai CY, Huang JF, Hou NJ, Lee LP, Hsieh MY, Chiu CF, Lin ZY, Chen SC, Wang LY, Chang WY, Chuang WL. A randomised study of peginterferon and ribavirin for 16 versus 24 weeks in patients with genotype 2 chronic hepatitis C. Gut. 2007;56:553–559. doi: 10.1136/gut.2006.102558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Missiha S, Heathcote J, Arenovich T, Khan K. Impact of Asian race on response to combination therapy with peginterferon alfa-2a and ribavirin in chronic hepatitis C. Am J Gastroenterol. 2007;102:2181–2188. doi: 10.1111/j.1572-0241.2007.01431.x. [DOI] [PubMed] [Google Scholar]
- 16.Bressler BL, Guindi M, Tomlinson G, Heathcote J. High body mass index is an independent risk factor for nonresponse to antiviral treatment in chronic hepatitis C. Hepatology. 2003;38:639–644. doi: 10.1053/jhep.2003.50350. [DOI] [PubMed] [Google Scholar]
- 17.Reddy KR, Shiffman ML, Morgan TR, Zeuzem S, Hadziyannis S, Hamzeh FM, Wright TL, Fried M. Impact of ribavirin dose reductions in hepatitis C virus genotype 1 patients completing peginterferon alfa-2a/ribavirin treatment. Clin Gastroenterol Hepatol. 2007;5:124–129. doi: 10.1016/j.cgh.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Ferenci P, Laferl H, Scherzer TM, Gschwantler M, Maieron A, Brunner H, Stauber R, Bischof M, Bauer B, Datz C, Loschenberger K, Formann E, Staufer K, Steindl-Munda P. Peginterferon alfa-2a and ribavirin for 24 weeks in hepatitis C type 1 and 4 patients with rapid virological response. Gastroenterology. 2008;135:451–458. doi: 10.1053/j.gastro.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 19.Yu ML, Dai CY, Chen SC, Chiu CC, Lee LP, Lin ZY, Hsieh MY, Wang LY, Chuang WL, Chang WY. Human leukocyte antigen class I and II alleles and response to interferon-alpha treatment, in Taiwanese patients with chronic hepatitis C virus infection. J Infect Dis. 2003;188:62–65. doi: 10.1086/375554. [DOI] [PubMed] [Google Scholar]
- 20.Dai CY, Chuang WL, Chang WY, Chen SC, Lee LP, Hsieh MY, Hou NJ, Lin ZY, Huang JF, Wang LY, Yu ML. Tumor necrosis factor- alpha promoter polymorphism at position -308 predicts response to combination therapy in hepatitis C virus infection. J Infect Dis. 2006;193:98–101. doi: 10.1086/498244. [DOI] [PubMed] [Google Scholar]
- 21.Persico M, Capasso M, Russo R, Persico E, Croce L, Tiribelli C, Iolascon A. Elevated expression and polymorphisms of SOCS3 influence patient response to antiviral therapy in chronic hepatitis C. Gut. 2008;57:507–515. doi: 10.1136/gut.2007.129478. [DOI] [PubMed] [Google Scholar]
- 22.Walsh MJ, Jonsson JR, Richardson MM, Lipka GM, Purdie DM, Clouston AD, Powell EE. Non-response to antiviral therapy is associated with obesity and increased hepatic expression of suppressor of cytokine signalling 3 (SOCS-3) in patients with chronic hepatitis C, viral genotype 1. Gut. 2006;55:529–535. doi: 10.1136/gut.2005.069674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO. Obesity: preventing and managing a global epidemic. Report on a WHO Consultation in Obesity, Geneva 3–5 June, 1997. WHO/NUT/NCD/98.1. Technical Report Series Number 894. Volume WHO/NUT/NCD/98.1. Technical Report Series Number 894. Geneva: World Health Organization; 2000 [PubMed]
- 24.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–163 [DOI] [PubMed]
- 25.Powis J, Peltekian KM, Lee SS, Sherman M, Bain VG, Cooper C, Krajden M, Deschenes M, Balshaw RF, Heathcote EJ, Yoshida EM. Exploring differences in response to treatment with peginterferon alpha 2a (40kD) and ribavirin in chronic hepatitis C between genotypes 2 and 3. J Viral Hepat. 2008;15:52–57. doi: 10.1111/j.1365-2893.2007.00889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu ML, Chuang WL, Chen SC, Dai CY, Hou C, Wang JH, Lu SN, Huang JF, Lin ZY, Hsieh MY, Tsai JF, Wang LY, Chang WY. Changing prevalence of hepatitis C virus genotypes: molecular epidemiology and clinical implications in the hepatitis C virus hyperendemic areas and a tertiary referral center in Taiwan. J Med Virol. 2001;65:58–65. doi: 10.1002/jmv.2001. [DOI] [PubMed] [Google Scholar]
- 27.Yamada G, Iino S, Okuno T, Omata M, Kiyosawa K, Kumada H, Hayashi N, Sakai T. Virological response in patients with hepatitis C virus genotype 1b and a high viral load: impact of peginterferon-alpha-2a plus ribavirin dose reductions and host-related factors. Clin Drug Investig. 2008;28:9–16. doi: 10.2165/00044011-200828010-00002. [DOI] [PubMed] [Google Scholar]
- 28.Fung J, Lai CL, Hung I, Young J, Cheng C, Wong D, Yuen MF. Chronic hepatitis C virus genotype 6 infection: response to pegylated interferon and ribavirin. J Infect Dis. 2008;198:808–812. doi: 10.1086/591252. [DOI] [PubMed] [Google Scholar]
- 29.Noppornpanth S, Sablon E, Nys K, Truong XL, Brouwer J, Brussel M, Smits SL, Poovorawan Y, Osterhaus AD, Haagmans BL. Genotyping hepatitis C viruses from Southeast Asia by a novel line probe assay that simultaneously detects core and 5′ untranslated regions. J Clin Microbiol. 2006;44:3969–3974. doi: 10.1128/JCM.01122-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vutien P, Nguyen NH, Trinh HN, Li J, Garcia RT, Garcia G, Nguyen KK, Nguyen HA, Levitt BS, Keeffe EB, Nguyen MH. Similar treatment response to peginterferon and ribavirin in Asian and Caucasian patients with chronic hepatitis C. Am J Gastroenterol 2010;105:1110–1115 [DOI] [PubMed]
- 31.McNeely MJ, Boyko EJ. Type 2 diabetes prevalence in Asian Americans: results of a national health survey. Diabetes Care. 2004;27:66–69. doi: 10.2337/diacare.27.1.66. [DOI] [PubMed] [Google Scholar]
- 32.McHutchison JG, Lawitz EJ, Shiffman ML, Muir AJ, Galler GW, McCone J, Nyberg LM, Lee WM, Ghalib RH, Schiff ER, Galati JS, Bacon BR, Davis MN, Mukhopadhyay P, Koury K, Noviello S, Pedicone LD, Brass CA, Albrecht JK, Sulkowski MS. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009;361:580–593. doi: 10.1056/NEJMoa0808010. [DOI] [PubMed] [Google Scholar]
- 33.Rumi MG, Aghemo A, Prati GM, D’Ambrosio R, Donato MF, Soffredini R, Del Ninno E, Russo A, Colombo M. Randomized study of peginterferon-alpha2a plus ribavirin vs peginterferon-alpha2b plus ribavirin in chronic hepatitis C. Gastroenterology 2010;138:108–115 [DOI] [PubMed]
- 34.Ascione A, De Luca M, Tartaglione MT, Lampasi F, Di Costanzo GG, Lanza AG, Picciotto FP, Marino-Marsilia G, Fontanella L, Leandro G. Peginterferon alfa-2a plus ribavirin is more effective than peginterferon alfa-2b plus ribavirin for treating chronic hepatitis C virus infection. Gastroenterology 2010;138:116–122 [DOI] [PubMed]
- 35.Awad T, Thorlund K, Hauser G, Stimac D, Mabrouk M, Gluud C. Peginterferon alpha-2a is associated with higher sustained virological response than peginterferon alfa-2b in chronic hepatitis C: systematic review of randomized trials. Hepatology 2010;51:1176–1184 [DOI] [PubMed]
- 36.Hsu CS, Liu CH, Liu CJ, Chen CL, Lai MY, Chen PJ, Chen DS, Kao JH. Factors affecting early viral load decline of Asian chronic hepatitis C patients receiving pegylated interferon plus ribavirin therapy. Antivir Ther. 2009;14:45–54. [PubMed] [Google Scholar]
- 37.Pattullo V, Ravindran NC, Mazzulli T, Wong DK, Heathcote EJ. Pegylated interferon plus optimized weight-based ribavirin dosing negate the influence of weight and body mass index on early viral kinetics and sustained virological response in chronic hepatitis C. J Viral Hepat 2010. doi:10.1111/j.1365-2893.2010.01248.x [DOI] [PubMed]