Abstract
Objective
The aim of this study was to explore the role of farnesoid X receptor (FXR) in liver lipid metabolism of non-alcoholic fatty liver disease (NAFLD) patients.
Methods
In this study, pathology and clinical criteria confirmed NAFLD in patients. Fatty acid synthetase (FAS)-positive liver cells were visualized by laser scanning confocal microscopy. Levels of FXR, liver X receptor (LXR), sterol regulatory element binding protein 1C (SREBP-1C), and small heterodimer partner (SHP) proteins were detected by Western blot. FXR, LXR, and SHP mRNA levels were measured by real-time PCR.
Results
In patients with NAFLD, a significant positive relationship between the degree of hepatic steatosis and serum triglycerides and cholesterol (correlation coefficient > 0.5, P < 0.05) was seen. The NAFLD patients had more FAS protein in liver, which suggests that there could have been more of fatty acid synthesis in hepatic cells (P < 0.05). The levels of FXR protein and mRNA were decreased in patients with NAFLD (P < 0.05), while those of LXR and SREBP-1C were increased (P < 0.05). The levels of SREBP-1C positively correlated with the degree of hepatic steatosis. There were no differences between the levels of SHP protein and mRNA both in NAFLD patients and normal controls (P > 0.05).
Conclusion
Our data showed that the decreased expression of hepatic FXR is associated with an increased expression of LXR, SREBP-1C, and hepatic triglyceride synthesis; furthermore, increased SREBP-1C is associated with the degree of hepatic steatosis in the NAFLD patients.
Keywords: FXR, Human, NAFLD, Lipid metabolism
Introduction
Non-alcoholic fatty liver disease (NAFLD) encompasses a disease spectrum ranging from non-alcoholic fatty liver (NAFL) to non-alcoholic steatohepatitis (NASH), which can progress to cirrhosis and hepatocellular carcinoma. Given that it is closely associated with metabolic syndrome and insulin resistance, NAFLD is currently recognized as the most common form of chronic liver disease and the leading cause of elevated liver enzyme tests in many parts of the world. NAFLD can coexist with viral hepatitis, being closely related to type 2 diabetes, metabolic syndrome, and cardiovascular factors. Therefore, NAFLD is a current and future challenge for liver treatment units.
The pathogenesis of NAFLD is complex but often explained by a “two-hit” theory. The first hit is triglyceride (TG) deposition in liver cells mainly due to insulin resistance (IR). The second hit is liver inflammation and fibrosis, mainly oxidative stress. Fat (mainly TG) accumulation in liver cells is the basic initiator of NAFLD [1, 2], which does not only occur locally in the liver. Hepatic deposits are responsible for the hepatic manifestations of a disordered lipid metabolism [3]. Research on ligand-dependent nuclear receptors has provided new opportunities to elucidate the pathogenesis of these diseases and to find effective treatment.
Nuclear homologous steroid hormone receptors are a family of ligand-dependent transcription factors, including the classic steroid hormone receptors and thymic hormone receptors, as well as many orphan nuclear receptors whose physiological ligands or activators have not been determined. They bind DNA sequences (response elements) in transcription-factor gene promoters of target genes and activate them [4]. The metabolic nuclear receptor superfamily is closely involved with carbohydrate, lipid, and energy metabolism [5]. It plays a key role in many important biochemical processes, such as insulin-signal transduction, oxidative stress, inflammation, cell growth, and differentiation [5, 6]. As transcription factors, they strictly control the expression of their target genes, as well as the major intracellular antioxidant systems. Their malfunction is common and forms the pathophysiological basis of a variety of metabolic diseases. Recent research has shown that disorders of hepatic lipid metabolism that result in NAFLD are due to activation or inhibition of nuclear receptors or lipid metabolism-related genes [7–10]. Recently, in vitro experiments and studies in animal models have suggested that the farnesoid X receptor (FXR), a bile acid receptor (BAR), plays a central role in lipid metabolism in liver cells. It can affect two other nuclear receptors, liver X receptor (LXR) and sterol regulatory element binding protein 1C (SREBP-1C), which are closely related to fat metabolism and its regulation.
FXR was isolated from the cDNA library of rat liver originally, and was named farnesoate receptor because it was activated by superphysiological concentrations of farnesoate [11]. In 1999, some groups found that bile acids were the natural ligands of FXR, which is since known as the BAR [12, 13]. FXR is expressed abundantly in liver, intestines, kidneys, etc. Studies have shown that FXR is involved in the regulation of many metabolic pathways through regulation of its related target genes. Activation of FXR by small heterodimer partner (SHP) down-regulates LXR and its target genes, SREBP-1C, and fatty acid synthetase (FAS), which inhibit the synthesis of triglycerides, and promote degradation of triglycerides and fatty acid oxidation by activating lipoprotein lipase, PPARα. FXR, therefore, has an important regulatory function in triglyceride metabolism.
LXR, another key regulatory gene of the liver lipid metabolism, also belongs to the orphan nuclear receptor family, so named, because of its abundance in the liver. The promoters, SREBP-1C and FAS, both contain LXR-binding sites. On the one hand, LXR promotes fat synthesis by inducing SREBP-1C gene expression and regulating the activity of SREBP-1C; on the other hand, it also increases fat synthesis by directly affecting the promoter of the fatty acid synthetase gene to increase levels of FAS mRNA. Watanabe et al. reported that SREBP-1C and expression of its target gene in the liver in LXR gene knockout mice (LXRα/β−/−mice) were significantly lower than in wild-type mice. After giving bile acids, the expression of hepatic SHP significantly increased in both types of mice. Serum TG levels and hepatic expression of SREBP-1C in wild-type mice were much lower than prior administration. However, no effects on TG or SREBP-1C were reported in LXRα/β−/−mice, which suggest that LXR is necessary for bile acids to reduce triglyceride in vivo by effects on SHP [14]. SREBP-1C is a nuclear transcription factor, also known as an adipocyte determination and differentiation factor. It is involved in regulation of the fatty acids, TG synthesis-related enzymes, and fat synthesis by regulating the transcription of genes—encoding key enzymes—in adipogenesis [15]. Adipogenesis was found to be increased in transgenic mice with SREBP-1C gene over-expression. However, there is no FXR-binding site on the SREBP-1C promoter. FXR inhibition of SREBP-1C occurs via an SHP pathway enhancing SHP expression. As a nuclear inhibitory receptor, SHP inhibits SREBP-1C expression. In SHP gene knockout mice, endogenous bile acids and the FXR agonist GW4060 did not reduce the expression of SREBP-1C [14, 16, 17].
The atypical nuclear receptor, SHP, is an inhibitory nuclear receptor present mainly in the liver, heart, pancreas, skeletal muscle, and adipose tissue. It is a special orphan nuclear receptor, lacking conventional DNA-binding sites and only contains a putative ligand-binding domain. It does not directly bind target gene promoter sequences to regulate its transcription, but interacts with other nuclear factors to inhibit their transcriptional activity. SHP has broad regulatory function by interaction with many nuclear factors. Animal experiments showed that SHP is an important target gene of FXR in the regulation of lipid metabolism [18].
Fatty acid synthetase is a key enzyme of the fat synthesis pathway in vivo. It catalyzes reactions of acetyl-CoA and malonyl-CoA to synthesize long-chain fatty acids. Ruderman et al. showed that malonyl-CoA is a physiological fuel signal that controls and reduces appetite, acting as a switch in the hypothalamus. FAS inhibition blocks the pathway of fat synthesis, which reduces fat synthesis, increases malonyl-CoA, and thus reduces appetite. Therefore, FAS is thought to be an important site of regulation of energy consumption and storage [16]. It regulates FAS on a transcriptional level by controlling the quantity of the fatty acid synthetase mRNA. The FAS promoter simultaneously contains LXR and SREBP-1C binding sites, both of which up-regulate FAS mRNA. Compared with LXR, SREBP-1C is the main regulator of the FAS gene expression.
These results suggest that the decreased FXR increases the expression of LXR through SHP and subsequently its target gene SREBP-1C and FAS expression, thus promoting the synthesis of liver triglycerides. FXR may be an effective target in the treatment of NAFLD. However, very few human studies were done in this area. In the current clinical study, we wanted to explore the regulation pathways in human liver to further clarify the pathogenesis of NAFLD, as well as provide a basis for use of nuclear receptors as a target for the treatment of NAFLD.
Materials and methods
Patient inclusion criteria
This study was approved by the Ethics Committee at The Second Affiliated Hospital Chongqing Medical University. Forty patients who underwent cholecystectomy for gallstones and were generally well without fever or clinical jaundice or aminotransferase elevations (less than two times of normal upper limit) gave their informed consent to participate in this study. From this group, patients who also had NAFL confirmed by pathology and clinical methods were selected as a NAFLD group. The others without NAFLD comprised a normal control group (N). The diagnosis of NAFLD was made based on the clinical, pathology, and diagnostic criteria of NAFLD according to the histopathology criteria summarized by attendees of the AASLD Single Topic Conference 2002 [19]. Patients with viral, drug-induced, alcoholic or autoimmune liver disease, or liver cancer were excluded. Tuberculosis, cancer and other systemic diseases were ruled out. The pathology and clinical characteristics of the patients in the study are summarized in Table 1.
Table 1.
Clinical/pathology features of patients
| Variable | NAFLD group | N group |
|---|---|---|
| Mean age (range), years | 47.76 (24–60) | 51.80 (44–62) |
| Sex (male:female) | 6:14 | 8:12 |
| Mild hepatic steatosis | 10/20 | 0/20 |
| Moderate hepatic steatosis | 6/20 | 0/20 |
| Severe hepatic steatosis | 4/20 | 0/20 |
Liver tissue collection
Hepatic tissues of about 1 cm diameter, following biopsy of the left edge of the liver, were preserved immediately at −80°C. Some of the samples were fixed with 10% formalin, embedded in paraffin, and cut into sections for H and E staining and histochemistry. The rest were stored in liquid nitrogen for total protein and RNA isolation.
Serum lipids measurement
Fasting antecubital venous blood without anticoagulants—5.0 ml samples collected in the morning—was centrifuged for 15 min at 3,500 rpm. Then serum lipids were measured by colorimetric assay [20].
Liver FAS protein evaluation
Liver FAS protein was observed using immunofluorescence and laser confocal microscopy. Paraffin sections were deparaffinized, hydrated, and samples incubated overnight at 4°C with first antibody, FAS mouse monoclonal antibody (SC-56095A, SANTA, at a dilution of 1:50) after antigen heating and treatment with 0.4% Triton X-100 at 37°C for an hour. Antibody labeling using FITC as chromogen was performed and counterstained with 100 μg/ml propidium iodide (PI) at room temperature for 30 min and then covered with 50% glycerol. Images were acquired under identical exposure conditions, analyzed with an Image-Pro Plus 6.0 analysis system (purchased from Media Cybernetics, USA) and measured as areas of integrated optical density (AIOD).
Western blotting analysis of FXR, SHP, LXR, and SREBP-1C
The nuclear and cytoplasmic proteins of hepatic tissues were extracted according to the specifications of Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime, P0028). Protein concentrations were determined by using a Bradford assay kit (Bio-Rad, USA). FXR, SHP, and LXR protein was then separated by 12% SDS-PAGE, and SREBP-1C by 10% SDS-PAGE and transferred to polyvinylidene fluoride membranes (Dupont, USA). The membranes were washed with PBS containing 0.1% Tween 20 and blocked with 5% (w/v) non-fat milk powder in 0.1% Tween 20-PBS (pH 7.4) for 2 h. The membranes were then incubated with goat anti-rabbit FXR and SHP polyclonal antibody (diluted 1:100), or rabbit anti-mouse LXR and SREBP-1C monoclonal antibody (diluted 1:500 and 1:200), and horseradish peroxidase-conjugated goat anti-rabbit IgG (diluted 1:100,000, Santa Cruz, USA). Finally, the immune complexes were developed with DAB. The relative amount of protein was quantified from the optical density of the corresponding band by the Bio-Image analysis system (Bio-Rad Gel Doc 2000, USA).
Real-time PCR quantification of FXR, SHP, and LXR mRNA levels
Total RNA was isolated using TRIzol reagent and digested with DNAse I. RNA concentrations were determined by spectrophotometry at 260 nm. The purity and condition of the RNA was determined by 260/280 nm absorption ratios, and visual examination of agarose gels stained with ethidium bromide. Reverse transcription was performed on each RNA sample (2 μg) using the cDNA high-capacity archive kit (TaKaRa Code DDR037S) from TaKaRa Biotechnology (Dalian) Co. Ltd. in a final reaction volume of 10 μl. One microliter total cDNA of each sample was diluted in 500 μl dH2O and prepared for real-time quantitative RT-PCR analysis. Two microliters of template cDNA sample was processed using an SYBR PrimeScript-PCR Kit (TaKaRa Code DDR041S) in a final reaction volume of 25 μl. Real-time RT-PCR measurements were performed on an ABI Prism 7300 sequence detector system. The relative mRNA levels were estimated by the standard method using GAPDH as the reference gene l. Primer sequences are shown in Table 2.
Table 2.
Primer sequences for real-time RT-PCR
| DNA amplified | Primer sequence 5′ → 3′ | Size (bp) |
|---|---|---|
| FXR | ||
| Sense | CCTGGATTTCTTCTGGACATT | 210 |
| Antisense | GGACCTGCCACTTGTTCTGT | |
| SHP | ||
| Sense | CACTGGGTGCTGTGTGAAGT | 136 |
| Antisense | TGATAGGGCGAAAGAAGAGG | |
| LXR | ||
| Sense | CTTGCTCATTGCTATCAGCATCTT | 86 |
| Antisense | ACATATGTGTGCAGCCTCT | |
| GAPDH | ||
| Sense | CGGGAAACTGTGGCGTGAT | 311 |
| Antisense | CAAAGGTGGAGGAGTGGGT | |
Statistical analysis
Data reported were mean ± SD and analyzed by using analysis of variance and the least significant difference t test, using SPSS software 13.0 (SPSS, Chicago, IL, USA). Survival studies were analyzed by Fisher’s exact P. Significance was accepted at P < 0.05.
Results
Correlation of the degree of hepatic steatosis in NAFLD patients and concentrations of serum triglyceride and cholesterol
The degree of hepatic steatosis and lipid profiles of patients are summarized in Table 3. The degree of lipid deposits in the liver and levels of triglyceride and cholesterol were positively correlated (correlation coefficient > 0.5, P < 0.05). However, the levels of blood lipid in NAFLD patients were found to be not significantly higher than normal.
Table 3.
Results of Spearman correlation coefficients
| Serum lipid (mmol/l) | Mild steatosis | Moderate steatosis | Severe steatosis | CR | P |
|---|---|---|---|---|---|
| Triglyceride | 1.16 ± 0.27 | 1.73 ± 0.23 | 3.07 ± 0.56 | 0.71059* | 0.0213 |
| Cholesterol | 3.08 ± 0.55 | 4.16 ± 0.26 | 4.87 ± 0.37 | 0.69955* | 0.0243 |
| High-density lipoprotein | 0.82 ± 0.19 | 0.84 ± 0.20 | 1.10 ± 0.35 | 0.34871* | 0.3234 |
| Low-density lipoprotein | 1.72 ± 0.32 | 2.44 ± 0.55 | 2.37 ± 0.65 | 0.40793* | 0.2419 |
| Free fatty acids | 1.13 ± 0.22 | 0.65 ± 0.41 | 1.32 ± 0.38 | 0.11916* | 0.7430 |
CR correlation coefficient
* Spearman correlation analysis
FAS protein content in hepatocytes of NAFLD patients was higher than in normal patients
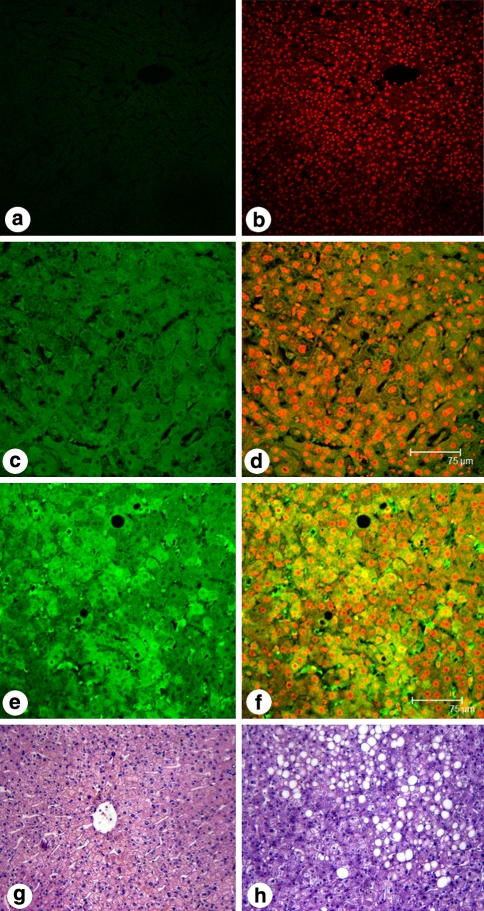
Hepatic FAS protein was evaluated by immunofluorescence and laser confocal microscopy. Strong green FITC signals were present in cytoplasm, and red PI signals in nuclei. In PBS, and alternative FAS antibody negative controls there was no green fluorescence in cytoplasm, only red fluorescence in nuclei (A and B). The presence of green fluorescence in hepatic cytoplasm supports the existence of FAS protein in normal control liver (C and D) (AIOD: 0.89 ± 0.21). The green fluorescence was obviously stronger in NAFLD liver indicating that FAS protein was higher in NAFLD patients (E and F) (AIOD: 1.46 ± 0.08) (P < 0.05) (Fig. 1).
Fig. 1.
Visualization of FAS protein in liver tissue by immunofluorescence staining using confocal laser microscopy. FAS protein was labeled with fluorescent antibody (green fluorescence), and nuclei were stained with PI (red fluorescence). a Negative control group with PBS incubation, b negative control group stained with PI after PBS incubation, c normal group labeled with fluorescent antibody (AIOD: 0.89 ± 0.21), d normal group labeled with fluorescent antibody and stained with PI, e NAFLD group labeled with fluorescent antibody (AIOD: 1.46 ± 0.08), f NAFLD group, labeled with fluorescent antibody and stained with PI, g an example of histomorphology of liver tissue from the normal group (H&E staining ×200), h an example of histomorphology of liver tissue from the NAFLD group (H&E staining ×200)
Hepatic FXR, LXR, and SREBP-1C protein content in NAFLD patients
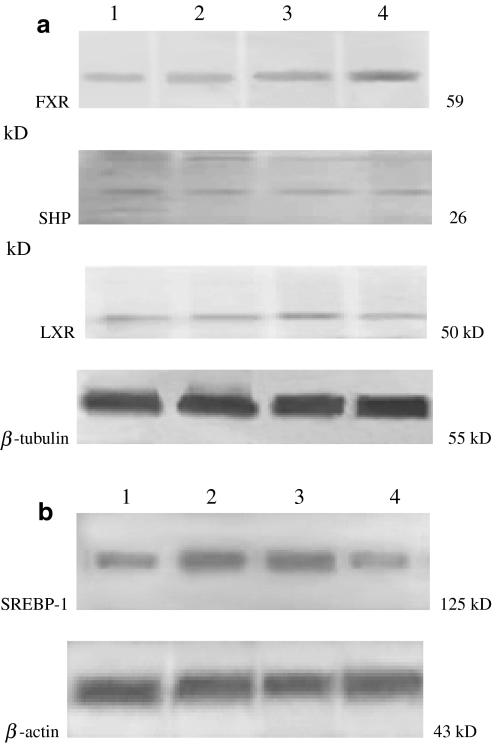
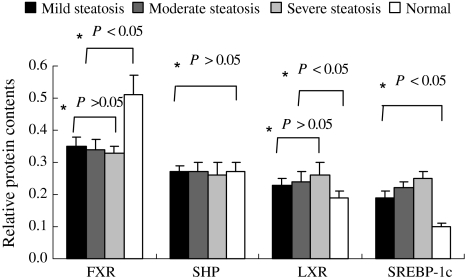
Hepatic FXR protein content was decreased in mild, moderate and severe steatosis (31.4, 33.3 and 35.3%, respectively) in NAFLD patients compared to normal, while LXR protein content was increased in patients with steatosis (mild 21.1%, moderate 26.3%, and severe 33.6%) compared to the normal (P < 0.05). However, there was no significant difference in SHP protein content between the two groups (P > 0.05). The SREBP-1C protein content was also increased in patients with steatosis (mild 90%, moderate 120%, and severe 150%), and its increase was significantly correlated with the degree of hepatic steatosis (P < 0.05), (Figs. 2, 3).
Fig. 2.
Western blot analysis of FXR, SHP, LXR and SREBP-1C protein content. a FXR, SHP and LXR with β-tubulin as internal control. b SREBP-1C with β-actin as internal control. Lane 1 NAFLD group, mild steatosis; lane 2 NAFLD group, moderate steatosis; lane 3 NAFLD group, severe steatosis; lane 4 normal group
Fig. 3.
Relative protein contents in different subgroups of patients with NAFLD. Gray-scale analyses were performed on Western blots of FXR, SHP and LXR for mild, moderate, severe steatosis and normal groups. *One-way ANOVA test
mRNA expression levels of hepatic FXR
Hepatic FXR mRNA levels in NAFLD patients were lower than that of the normal group, but the opposite effect was seen in LXR mRNA levels. There was no significant difference in levels of SHP mRNA between the two groups.
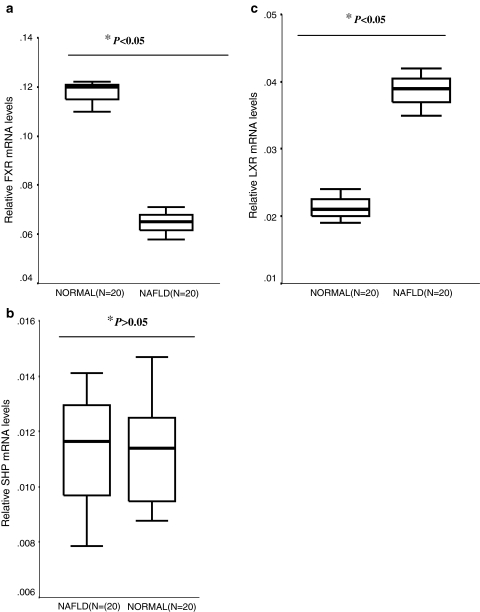
The relative FXR mRNA levels in the NAFLD group were significantly less than that of normal group (P < 0.05), and the average relative FXR mRNA levels in the NAFLD group were about 55.1% of the normal group. In contrast, the relative LXR mRNA levels in the NAFLD group were 1.8-fold higher compared to the normal group (P < 0.05). There was no significant difference between relative SHP mRNA levels in the two groups (P > 0.05) (Fig. 4).
Fig. 4.
Boxplot graphs of relative mRNA levels of FXR (a), SHP (b), and LXR (c) in NAFLD, and normal groups. *Independent t test
Discussion
It is widely known that an increase of triglyceride synthesis in liver plays an important role in NAFLD models [14, 21–23]. In rodents, an increase in the amount of synthesized lipids was observed in liver and adipose tissue cells, while in humans, it was primarily in the liver. Isotopic quantitative analysis showed that triglyceride production by FAS after taking food was only about 2–5% of the VLDL triglyceride output in normal liver cells. However, in NAFL patients, the ratio increased to about 20–30%. Furthermore, the high level of synthesis was maintained under the conditions of fasting, which suggests that the increase in fatty acid synthesis is one of the essential factors for triglyceride accumulation in liver cells of humans [24, 25]. Is the cause of the high synthesis in humans the same as in rodents? In obese, insulin resistance in ob/ob mice, serum triglycerides and cholesterol levels have been found to be increased, along with liver steatosis, and hepatic LXR, SREBP-1C mRNA levels were found to be significantly higher than normal [26].
In the current study, we compared levels of serum lipid and nuclear receptor expression in the liver in NAFLD patients. Peripheral blood triglycerides and total cholesterol in NAFL patients were found to be higher than that in non-fatty liver patients, consistent with the degree of liver lipid deposition. The LXR, SREBP-1C protein and mRNA levels, as well as FAS-positive staining by confocal laser scanning microscopy, were consistent with the lipid deposition in the liver of NAFLD patients. This finding is the same as that in animal studies suggesting that up-regulated expression of LXR, SREBP-1C and its target gene FAS is associated with an increase in synthesis of fatty acids and triglycerides. This may also be an important regulatory mechanism of liver steatosis in humans.
The current study also found that with increased lipid deposition in liver, the levels of FXR protein and mRNA were significantly reduced. It was negatively correlated with LXR and SREBP-1C gene expression, which suggests that FXR nuclear receptor inhibits LXR, SREBP-1C gene expression, thereby down-regulating FAS expression and lipid synthesis. In human liver, FXR negatively regulates lipid synthesis associated nuclear receptor, nuclear transcription factor, and adipogenic genes, which are closely involved in the pathophysiology of fatty liver. This result is also consistent with animal experiments which have shown that FXR inhibits expression of SREBP-1C and its target gene FAS through the SHP pathway, improves the lipid metabolism, and maintains lipid homeostasis [5, 14, 23]. However, we failed to detect differences in levels of SHP between patients with fatty liver and healthy people by Western blot and real-time PCR. It has been reported that SHP has wide regulatory function by interaction with many nuclear factors. However, the role of SHP is controversial in liver lipid metabolism. A study on FXR showed low levels of hepatic SHP in FXR−/−mice, and an increase in SREBP-1C with fat accumulation. Blood TG, bile acids or FXR agonists enhanced SHP expression and ameliorated hepatic steatosis and hyperlipidemia in obese rats [14]. This shows that the high expression of SHP is a protective factor for hepatosteatosis. However, recently Huang et al. provided data to the contrary. Their studies on SHP−/−/OB−/−and SHP transgenic mice showed minor hepatosteatosis, overtly decreased in fatty acids and triglycerides (56 and 85%, respectively), increased VLDL-TG secretion and serum TG, higher expression of liver SREBP-1C and FAS, and more lipid synthesis in SHP−/−/OB−/−mice compared with OB−/−mice. Furthermore, more liver steatosis and TG accumulation, high-expression SREBP-1C and PPAR-γ, elevated hepatic lipid synthesis were detected in transgenic mice [27]. Up to now, research on the SHP promoter sequence has shown that nuclear receptors (including FXR, RXR, LXRα, SREBP-1C, etc.) combine directly with their promoters to facilitate transcription, and SHP has different effects on different areas of metabolism by binding with different receptors. Some researchers have put forward the hypothesis that intrahepatic TG levels affect SHP expression, but this has not been supported by experiments [28]. It is not clear whether the lack of agreement of animal experiments with the current findings in humans is due to small sample sizes or inter-species differences. At present, no research has been reported on SHP expression in human lipid metabolism.
Our study is the first to show FXR, LXR, SREBP-1C, FAS regulation of lipid metabolism in human liver, suggesting that decreased FXR activity in the liver is an important factor in the pathogenesis of fatty liver. FXR probably down-regulates LXR, SREBP-1C expression, thereby inhibiting FAS expression to reduce fatty acid and triglyceride synthesis in liver. Therefore, FXR ligand agonists may be reasonable targets for the prevention of fatty liver and treatment of the related metabolic disorders.
References
- 1.Goldberg IJ, Ginsberg HN. Ins and outs modulating hepatic triglyceride and development of nonalcoholic fatty liver disease. Gastroenterology. 2006;130:1343–1346. doi: 10.1053/j.gastro.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 2.Saltiel AR. Holding the line on hepatic fat. Cell Metab. 2005;2:1–2. doi: 10.1016/j.cmet.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Hsiao P-J, Kuo K-K, Shin S-J, Yang Y-H, Lin W-Y, Yang J-F, et al. Significant correlations between severe fatty liver and risk factors for metabolic syndrome. J Gastroenterol Hepatol. 2007;22:2118–2123. doi: 10.1111/j.1440-1746.2006.04698.x. [DOI] [PubMed] [Google Scholar]
- 4.Lavery DN, McEwan IJ. Structure and function of steroid receptor AF1 transactivation domains: induction of active conformations. Biochem J. 2005;391:449–464. doi: 10.1042/BJ20050872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugden MC, Holness MJ. Role of nuclear receptors in the modulation of insulin secretion in lipid-induced insulin resistance. Biochem Soc Trans. 2008;36:891–900. doi: 10.1042/BST0360891. [DOI] [PubMed] [Google Scholar]
- 6.Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454:470–477. doi: 10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- 7.Aranda A, Pascual A. Nuclear hormone receptors and gene expression. Physiol Rev. 2001;81:1269–1304. doi: 10.1152/physrev.2001.81.3.1269. [DOI] [PubMed] [Google Scholar]
- 8.Beaven SW, Tontonoz P. Nuclear receptors in lipid metabolism: targeting the heart of dyslipidemia. Annu Rev Med. 2006;57:313–329. doi: 10.1146/annurev.med.57.121304.131428. [DOI] [PubMed] [Google Scholar]
- 9.Portincasa P, Grattagliano I, Palmieri VO, Palasciano G. Current pharmacological treatment of nonalcoholic fatty liver. Curr Med Chem. 2006;13:2889–2900. doi: 10.2174/092986706778521878. [DOI] [PubMed] [Google Scholar]
- 10.Gaemers IC, Groen AK. New insights in the pathogenesis of non-alcoholic fatty liver disease. Curr Opin Lipidol. 2006;17:268–273. doi: 10.1097/01.mol.0000226118.43178.98. [DOI] [PubMed] [Google Scholar]
- 11.Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T, et al. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81:687–693. doi: 10.1016/0092-8674(95)90530-8. [DOI] [PubMed] [Google Scholar]
- 12.Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, et al. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 13.Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe M, Houten SM, Wang L, Moschetta A, Mangelsdorf DJ, Heyman RA, et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Investig. 2004;113:1408–1418. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferre P, Foufelle F. SREBP-1c transcription factor and lipid homeostasis: clinical perspective. Horm Res. 2007;68:72–82. doi: 10.1159/000100426. [DOI] [PubMed] [Google Scholar]
- 16.Ruderman N, Prentki M. AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nat Rev Drug Discov. 2004;3:340–351. doi: 10.1038/nrd1344. [DOI] [PubMed] [Google Scholar]
- 17.Ji C, Chan C, Kaplowitz N. Predominant role of sterol response element binding proteins (SREBP) lipogenic pathways in hepatic steatosis in the murine intragastric ethanol feeding model. J Hepatol. 2006;45:717–724. doi: 10.1016/j.jhep.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Lee YS, Chanda D, Sim J, Park YY, Choi HS. Structure and function of the atypical orphan nuclear receptor small heterodimer partner. Int Rev Cytol. 2007;261:117–158. doi: 10.1016/S0074-7696(07)61003-1. [DOI] [PubMed] [Google Scholar]
- 19.Association FLaALDSGotCLD Guidelines for diagnosis and treatment of nonalcoholic fatty liver disease. Chin J Hepatol. 2006;14:161–163. [PubMed] [Google Scholar]
- 20.Cooper GR, Myers GL, Smith SJ, Sampson EJ. Standardization of lipid, lipoprotein, and apolipoprotein measurements. Clin Chem. 1988;34:B95–B105. [PubMed] [Google Scholar]
- 21.Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/S0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 22.Pineda Torra I, Claudel T, Duval C, Kosykh V, Fruchart JC, Staels B. Bile acids induce the expression of the human peroxisome proliferator-activated receptor alpha gene via activation of the farnesoid X receptor. Mol Endocrinol. 2003;17:259–272. doi: 10.1210/me.2002-0120. [DOI] [PubMed] [Google Scholar]
- 23.Claudel T, Staels B, Kuipers F. The Farnesoid X receptor: a molecular link between bile acid and lipid and glucose metabolism. Arterioscler Thromb Vasc Biol. 2005;25:2020–2030. doi: 10.1161/01.ATV.0000178994.21828.a7. [DOI] [PubMed] [Google Scholar]
- 24.Tamura S, Shimomura I. Contribution of adipose tissue and de novo lipogenesis to nonalcoholic fatty liver disease. J Clin Investig. 2005;115:1139–1142. doi: 10.1172/JCI200524930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Investig. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimomura I, Matsuda M, Hammer RE, Bashmakov Y, Brown MS, Goldstein JL. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell. 2000;6:77–86. doi: 10.1016/S1097-2765(00)00009-5. [DOI] [PubMed] [Google Scholar]
- 27.Huang J, Iqbal J, Saha PK, Liu J, Chan L, Hussain MM, et al. Molecular characterization of the role of orphan receptor small heterodimer partner in development of fatty liver. Hepatology. 2007;46:147–157. doi: 10.1002/hep.21632. [DOI] [PubMed] [Google Scholar]
- 28.Trauner M. A little orphan runs to fat: the orphan receptor small heterodimer partner as a key player in the regulation of hepatic lipid metabolism. Hepatology. 2007;46:1–5. doi: 10.1002/hep.21801. [DOI] [PubMed] [Google Scholar]