Abstract
Purpose
To compare rebleeding rates following treatment of variceal hemorrhage with TIPS alone versus TIPS with variceal embolization in the covered stent-graft era.
Methods
In this retrospective study, 52 patients (M:F 29:23, median age 52 years) with hepatic cirrhosis and variceal hemorrhage underwent TIPS insertion between 2003 and 2008. Median Child–Pugh and MELD scores were 8.5 and 13.5. Generally, 10-mm diameter TIPS were created using covered stent-grafts (Viatorr; W.L. Gore and Associates, Flagstaff, AZ). A total of 37 patients underwent TIPS alone, while 15 patients underwent TIPS with variceal embolization. The rates of rebleeding and survival were compared.
Results
All TIPS were technically successful. Median portosystemic pressure gradient reductions were 13 versus 11 mmHg in the embolization and non-embolization groups. There were no statistically significant differences in Child–Pugh and MELD score, or portosystemic pressure gradients between each group. A trend toward increased rebleeding was present in the non-embolization group, where 8/37 (21.6%) patients rebled while 1/15 (6.7%) patients in the TIPS with embolization group rebled (P = 0.159) during median follow-up periods of 199 and 252 days (P = 0.374). Rebleeding approached statistical significance among patients with acute hemorrhage, where 8/32 (25%) versus 0/14 (0%) rebled in the non-embolization and embolization groups (P = 0.055). A trend toward increased bleeding-related mortality was seen in the non-embolization group (P = 0.120).
Conclusions
TIPS alone showed a high incidence of rebleeding in this series, whereas TIPS with variceal embolization resulted in reduced recurrent hemorrhage. The efficacy of embolization during TIPS performed for variceal hemorrhage versus TIPS alone should be further compared with larger prospective randomized trials.
Keywords: Transjugular intrahepatic portosystemic shunt (TIPS), Variceal hemorrhage, Embolization, Rebleeding
Introduction
Transjugular intrahepatic portosystemic shunts (TIPS) represent an established interventional therapy for complications of portal hypertension, including medically refractory or recurrent gastroesophageal variceal hemorrhage [1–3]. The efficacy of TIPS in treating bleeding complications of portal hypertension has been shown in several large studies [4, 5]. Early TIPS were performed using bare metal stents, which were associated with high rates of shunt stenosis and dysfunction [6, 7]. More recently, covered stent-grafts have been shown to improve TIPS patency [8–11], and the specialized expanded polytetrafluoroethylene (e-PTFE) covered Viatorr stent (W.L. Gore and Associates, Flagstaff AZ) is now widely used for TIPS creation. Direct prospective comparison of TIPS created using bare metal stents with TIPS made using covered stent-grafts has shown improved patency rates and reduced rates of recurrent variceal hemorrhage among patients receiving Viatorr TIPS [11].
Given the improved patency and reduced rebleeding rates provided by covered stent-grafts, the need to embolize gastroesophageal varices at the time of TIPS creation is controversial. Widespread variceal coil embolization provides a theoretical measure of protection against future bleeding and has been validated in TIPS performed using bare metal stents [12]. Despite this, some studies argue that TIPS creation alone is sufficient to manage variceal gastrointestinal hemorrhage in most instances [13, 14]. Patients with varices that continue to fill in spite of a post-TIPS reduction in the portosystemic gradient to less than 12 mmHg [15], patients who have large caliber portosystemic collateral vessels [13, 16], patients with submucosally coursing vessels [17], and patients who have gastric varices or gastric antral vascular ectasia [18] have been cited as specific populations with increased bleeding risk that may benefit from variceal embolization. Given the lack of consensus in literature regarding the role of variceal embolotherapy during TIPS procedures in the covered stent-graft age, this study was undertaken with the purpose of comparing rebleeding rates following TIPS with concurrent coil embolization of gastroesophageal varices with TIPS alone in the Viatorr era.
Materials and methods
This study was in compliance with the Health Insurance Portability and Accountability Act, and the institutional review board of our institution granted approval with waiver of consent for inclusion in the study. All patients provided written informed consent for procedures.
Clinical setting, patients and tumors
Between May 2003 and September 2008, 52 patients with liver cirrhosis who underwent TIPS for treatment of gastroesophageal variceal hemorrhage were identified among all patients undergoing TIPS (n = 132) at a single academic university affiliated hospital situated in a large metropolitan area and were selected for study. Inclusion criteria for TIPS consisted of (a) age greater than 18 years, (b) known hepatic cirrhosis based on histological or typical cross-sectional imaging (ultrasound, computed tomography or magnetic resonance imaging) findings, and (c) acute (within 24 h) or recent (more than 24 h prior) hemorrhage from gastroesophageal varices refractory to endoscopic therapy.
Patients, liver disease and bleeding data
A total of 52 patients (29 men, 23 women, median age 52 years, range 26–76 years) were included. Patient demographics, liver disease characteristics and bleeding data are summarized in Table 1, and showed no statistically significant differences between the study groups. Underlying liver disease included alcoholic (n = 21), hepatitis B and/or hepatitis C virus (n = 9), alcoholic and hepatitis C virus (n = 6), non-alcoholic steatohepatitis (NASH) (n = 7), primary biliary cirrhosis (n = 5), autoimmune (n = 2), alpha-one antitrypsin deficiency (n = 1) and unknown (n = 1) causes of cirrhosis. Liver disease included Child–Pugh class A (n = 6), B (n = 28) and C (n = 18) disease (median Child–Pugh score = 8.5, 95% CI 7.0–9.4, range 5–15). Median model for end-stage liver disease (MELD) score was 13.5 (95% CI 11.0–17.4, range 4–47). Bleeding sources included esophageal (n = 31) or gastric varices (n = 21). As much as 46 patients suffered acute hemorrhage requiring emergent TIPS insertion, while 6 patients had recent variceal hemorrhage occurring within 4 days to 2 months before TIPS placement.
Table 1.
Patient demographics, liver disease characteristics and bleeding data
| Group | All TIPS | TIPS without embolization | TIPS with embolization | P value |
|---|---|---|---|---|
| Patients | 52 | 37 | 15 | – |
| Age (years) | 52 (26–76) | 52 (26–76) | 52 (32–60) | 0.650 |
| Sex (M:F) | 29:23 | 20:17 | 9:6 | 0.765 |
| Child–Pugh score | 8.5 (5–15) | 9.0 (5–15) | 8.0 (6–14) | 0.920 |
| MELD score | 13.5 (4–47) | 14.0 (4–47) | 13.0 (4–34) | 0.327 |
| Varices (esophageal:gastric) | 31:21 | 24:13 | 7:8 | 0.350 |
| Bleeding (acute:recent) | 46:6 | 32:5 | 14:1 | 0.659 |
Values in parenthesis indicate range
TIPS transjugular intrahepatic portosystemic shunt, M male, F female, MELD model for end-stage liver disease, PSG portosystemic gradient
TIPS procedures
TIPS procedures were performed in the interventional radiology suite using general anesthesia administered by the anesthesiology service. TIPS were created from a right internal jugular vein approach. Patients were prepared and draped in standard sterile fashion while supine on the angiographic procedure table. Routine internal jugular venous access was gained using sonographic guidance. Percutaneous access was dilated to accept a ten French sheath. Typically, a five French multipurpose angled or reverse curve catheter was used to engage the right hepatic vein. After hepatic venography and pressure measurement, mapping wedged hepatic venography was performed using balloon occlusion technique and carbon dioxide. Next, a Rösch-Uchida transjugular liver access set (Cook Co., Bloomington, IN) was used to gain access to the right portal vein. After catheter passage into the portal vein and direct portal vein pressure measurement, dilatation of the hepatic parenchymal tract was performed using a 6 mm × 4 cm or 8 mm × 4 cm balloon. Next, direct portography was performed with measurement of the liver parenchymal tract length. Subsequently, Viatorr stents were deployed across the hepatic parenchymal tract in a standard manner. If the distal portion of the Viatorr stent fell short of the hepatic vein/inferior vena cava (IVC) junction, additional Viatorr stents were utilized to extend the stent at the discretion of the primary operator. Post-deployment balloon angioplasty was performed using an 8 mm × 4 cm or 10 mm × 4 cm balloon. After measurement of the final portal and right atrial pressures, completion shunt venography was performed.
Coil embolization of gastroesophageal varices was performed following TIPS insertion at the discretion of the primary operator, based on the number and size of varices, as well as the presence and degree of angiographic filling following TIPS placement. Coil embolization was not necessarily dependent on reduction of the portosystemic pressure gradient to <12 mmHg. In general, selective catheterization of the coronary vein or gastric varix was performed using a five French angled catheter (Glidecath; Terumo Medical Corp., Somerset, NJ) or microcatheter (Renegade Hi-Flo; Boston Scientific, Natick MA). Embolization was then performed using 0.035 or 0.018 in. metallic coils (Nester or MicroNester; Cook Co., Bloomington, IN). Post-embolization completion venography was then performed. After embolization, all vascular devices were removed, and access site hemostasis was obtained using manual compression. Following the procedure, patients were monitored in an intensive care unit setting. Of note, no anticoagulant therapy or additional treatments were utilized to help maintain shunt patency after TIPS.
Clinical follow-up and measured outcomes
Immediate post-procedure, follow-up was performed while patients remained hospitalized following TIPS. Outpatient hepatology clinic follow-up was performed when possible, excluding patients who expired or did not return for scheduled follow-up. The primary outcome measure of this study was post-TIPS rebleeding, assessed by overall incidence and time to rebleeding events. Rebleeding was defined as recurrent gastroesophageal variceal hemorrhage requiring transfusion of two or more units of blood. Patient survival comprised a secondary outcome measure. Rebleeding and survival were analyzed from the date of TIPS insertion until death or date of most recent clinical follow-up. Data were censored in November 2008. TIPS technical success was defined as successful creation of a shunt between the hepatic vein and an intrahepatic portal venous branch. TIPS hemodynamic success was defined as reduction in the portosystemic pressure gradient to an absolute value <12 mmHg or relative reduction by more than 10 mmHg.
Statistical analysis
Quantitative measures were compared using a Mann–Whitney test for independent samples. Demographic data were compared using a Fisher’s exact test. Patient rebleeding rates and survival were assessed using the technique of Kaplan–Meier. Statistical analysis was performed utilizing a commercially available software package (MedCalc; MedCalc Software, Belgium). P values ≤0.05 were considered to be significant.
Results
TIPS procedures
TIPS insertion was technically successful in all 52 patients. Hemodynamic success was also achieved in all 52 patients. A single Viatorr stent-graft was utilized for TIPS creation in 47 patients, and two Viatorr stent-grafts were used in five patients. Fifty patients had 10 mm diameter stents inserted, while two patients had different diameter stents placed (8 mm stent in one patient in the embolization group and 12 mm stent in one patient in the non-embolization group). Median initial portosystemic gradient was 19.0 mmHg (95% CI 16.0–21.0, range 10–32 mmHg) and final portosystemic gradient was 6.0 mmHg (95% CI 5.0–8.0, range 1–16 mmHg) among all patients treated. There was no statistically significant difference in median initial (19.0 vs. 17.0 mmHg, P = 0.567) and final (6.0 vs. 6.0 mmHg, P = 0.524) portosystemic pressure gradient among the non-embolization and embolization treatment groups, respectively. No statistically significant differences were found when comparing pre- and post-TIPS portosystemic pressure gradient among patients partitioned by class of Child–Pugh liver disease or cause of cirrhosis (alcoholic, hepatitis B and/or hepatitis C virus, alcoholic and hepatitis C virus, and other causes). There were no immediate procedure-related complications.
Coil embolization
Among the 52 patients treated, gastroesophageal varices were angiographically evident in 43 patients during initial (pre-stent) direct portal venography (28/37 in the non-embolization group and 15/15 in the embolization group). Coil embolization was performed in 15 patients during TIPS insertion. Vessels embolized included the coronary vein (n = 14) and a gastrosplenic varix (n = 1). No contrast flow was seen through the coil pack on completion of TIPS venography following embolization in any of the cases.
Post-TIPS rebleeding and patient survival
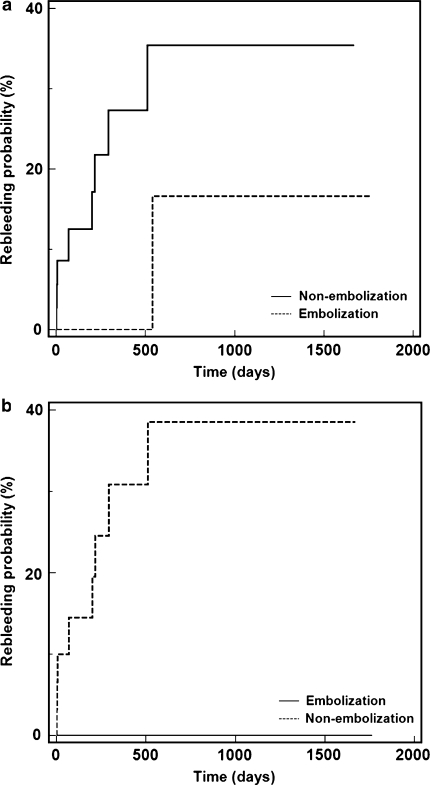
Following TIPS insertion, 9/52 (17.3%) patients suffered recurrent gastroesophageal variceal hemorrhage; 8/37 (21.6%) patients in the TIPS alone group rebled, while 1/15 (6.7%) patients in the TIPS with coil embolization group rebled (P = 0.159, Fig. 1) during median clinical follow-up time period of 199 days (95% CI 43–317, range 1–1,669 days) and 252 days (95% CI 33–12,16, range 1–1,763 days), respectively (P = 0.374). As such 36 patients underwent 0–12 months follow-up, 6 patients underwent 13–24 months follow-up, 4 patients underwent 25–36 months follow-up, 4 patients underwent 37–48 months follow-up and 2 patients underwent ≥49 months follow-up. Median time to recurrent hemorrhage was 199 days (95% CI 2–521, range 1–538 days) for all patients with rebleeding; 4/9 patients (all in the non-embolization group) rebled within 7 days (early rebleeding), whereas 5/9 patients rebled beyond 199 days (late rebleeding). Among the nine patients exhibiting rebleeding after TIPS, median portosystemic gradient after initial shunt insertion was 7 mmHg (range 4–16 mmHg), and only 1/9 patient had a portosystemic gradient greater than 12 mmHg after TIPS (this patient did not undergo coil embolization). While most of the rebleeding patients had Child–Pugh class C liver disease, no statistically significant differences in rebleeding rates were found among patients partitioned by Child–Pugh class using Kaplan–Meier analysis: Child–Pugh A 0/6 (0%), Child–Pugh B 4/28 (14%), Child–Pugh C 5/18 (28%), P = 0.184. Additionally, while most of the rebleeding patients had alcoholic cirrhosis, no statistically significant differences in rebleeding rates were found among patients partitioned by cause of cirrhosis (alcoholic, hepatitis B and/or hepatitis C virus, alcoholic and hepatitis C virus and other causes) using Kaplan–Meier analysis: alcoholic cirrhosis 7/21 (33%), hepatitis B and/or hepatitis C virus cirrhosis 0/9 (0%), alcoholic and hepatitis C virus cirrhosis 0/6 (0%) and other causes 1/16 (6%), P = 0.082.
Fig. 1.
Graphs of Kaplan–Meier estimation of a rebleeding probability among all patients undergoing TIPS with variceal coil embolization versus TIPS without embolization (P = 0.159). b Rebleeding probability among patients with acute hemorrhage undergoing TIPS with variceal coil embolization versus TIPS without embolization (P = 0.055)
A subgroup analysis of patients who underwent TIPS for acute (within 24 h) bleeding demonstrated recurrent hemorrhage in 8/46 patients, including 8/32 (25%) patients in the TIPS alone group and 0/14 (0%) patients in the TIPS with coil embolization group (P = 0.055, Fig. 1) during median clinical follow-up time periods of 208 days (95% CI 41–401, range 1–1,669 days) and 197 days (95% CI 32–1,277, range 1–1,763 days), respectively (P = 0.504). A subgroup analysis of rebleeding comparing patients by type of varices showed recurrent hemorrhage in 3/21 (14%) patients with gastric varices (2/13 in the non-embolization group and 1/8 in the embolization group, P = 0.770) and 6/31 (19%) patients with esophageal varices (6/24 in the non-embolization group and 0/7 in the embolization group, P = 0.166). As such 3/9 patients who suffered rebleeding had no angiographically evident gastroesophageal varices during initial direct portal venography at the time of TIPS placement (final post-TIPS portosystemic gradients in these patients were 9, 5, and 5 mmHg). It is unknown if these patients had elevation of portal pressure at the time of rebleeding. None of these patients underwent TIPS venography or pressure measurement after the rebleeding event. Furthermore, there were no corroborative clinical changes, such as new onset of ascites, to suggest development of new portal hypertension.
Endoscopic confirmation of rebleeding source was available in 5/9 patients with recurrent hemorrhage. Rebleeding occurred from the same varices as the patient’s original gastrointestinal hemorrhage in 5/8 patients (1 early, 4 late rebleeders) in the non-embolization group. In the remaining 3/8 patients in the non-embolization group, endoscopy was not performed due to very early rebleeding and active fluid and blood product resuscitation.
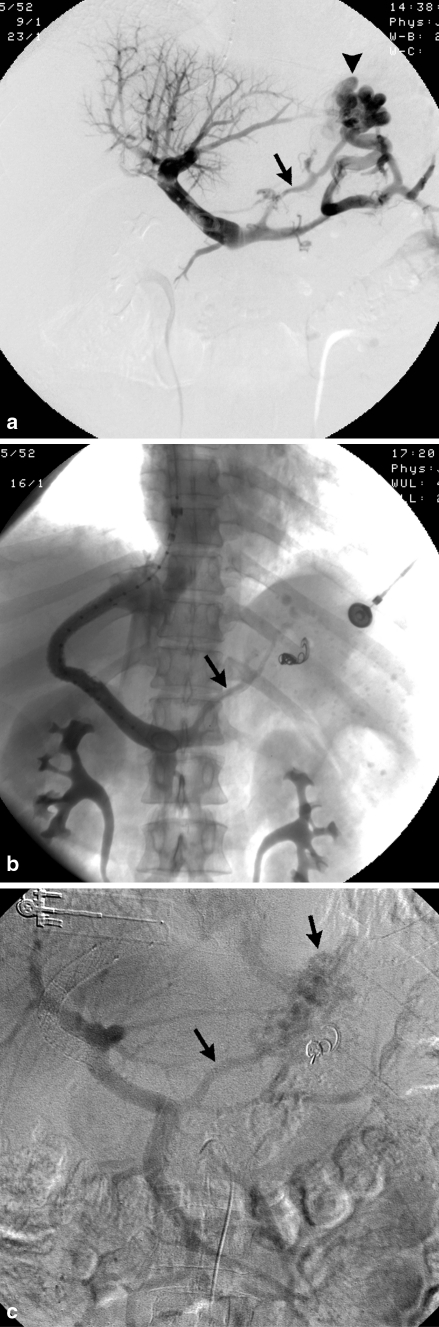
Of the nine patients with rebleeding, eight were in the TIPS alone group and one was in the TIPS with embolization group; 2/8 patients in the non-embolization group underwent coronary vein embolization after their rebleeding episode. In these two patients, the measured portosystemic pressure gradients were 7 and 15 mmHg at the time of embolization as compared to 4 and 16 mmHg immediately following TIPS, and no evidence of shunt dysfunction was identified venographically. In the non-embolization group, 2/8 patients underwent endoscopy with intervention (banding or electrocautery), and both had resolution of bleeding. In the non-embolization group, 3/8 patients underwent endoscopy without intervention and only one of these three patients survived. In the non-embolization group, 1/9 patients underwent no treatment and did not survive. The single patient in the embolization group who rebled demonstrated both gastric and esophageal varices on upper endoscopy after rebleeding 538 days following TIPS insertion, but did not show stigmata definitely suggestive of acute bleeding. This patient also suffered from complete shunt occlusion (Fig. 2). In this case, bleeding ceased after surgical mesocaval shunt creation. No other cases of shunt dysfunction (stenosis or occlusion) were identified in the other rebleeding patients. Notably, profound coagulopathy was present in two patients with rebleeding.
Fig. 2.
A 52-year-old man with alcoholic cirrhosis and bleeding gastric varices. Portal venogram (a) performed during TIPS insertion demonstrates prominent gastrosplenic (arrowhead) and coronary (arrow) varices. Note right portal vein thrombosis. Post-TIPS portal venogram (b) performed following gastrosplenic variceal embolization shows minimal residual coronary vein opacification (arrow). This vessel was not embolized given significant portosystemic gradient reduction from 25 to 6 mmHg. Patient rebled 538 days post-TIPS due to shunt occlusion, evidenced on venous phase imaging following superior mesenteric arteriogram (c). Note coronary vein and gastric variceal filling (arrows), as well as right portal vein recanalization
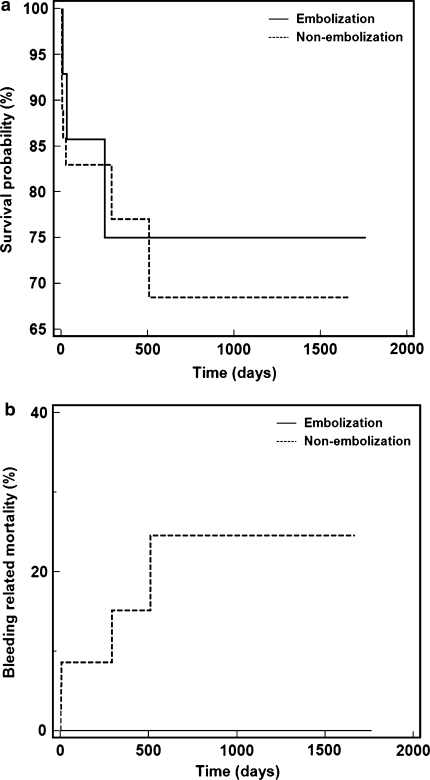
At the time of manuscript composition, 10/52 (19.2%) patients in the study group were known to have expired; 7/37 (18.9%) patients in the TIPS alone group expired, while 3/15 (20%) patients in the TIPS with coil embolization group expired (P = 0.746, Fig. 3). Death was related to recurrent hemorrhage in 5/10 patients, all in the non-embolization group (P = 0.120, Fig. 3).
Fig. 3.
Graphs of Kaplan–Meier estimation of a overall survival probability among patients undergoing TIPS with variceal coil embolization versus TIPS without embolization (P = 0.746). b Bleeding related mortality among patients undergoing TIPS with variceal coil embolization versus TIPS without embolization (P = 0.120)
Discussion
Since it was first introduced more than 20 years ago [19], TIPS has become an established procedure for treatment of complications related to portal hypertension. Accepted indications for TIPS include acute gastroesophageal variceal bleeding that cannot be successfully controlled medically, either with pharmacologic agents or with endoscopic techniques, and recurrent or refractory variceal hemorrhage intolerant to medical management [20]. In cases of TIPS performed using bare metal stents, patients have shown rebleeding rates approximating 20% after 2 years [21], with many cases of recurrent hemorrhage related to shunt dysfunction. The e-PTFE covered Viatorr stent-graft, which was approved by the US Food and Drug Administration in 2004, improves shunt patency by minimizing transmural bile permeation and thereby reducing tissue ingrowth. TIPS primary patency with the Viatorr stent-graft has been found to range between 80 and 90% at 1 year [8, 10, 22, 23] and the use of the Viatorr has shown further reduction in rates of recurrent hemorrhage following TIPS to <10% [10, 23, 24].
Due to the significant morbidity and mortality associated with gastrointestinal variceal hemorrhage [25], consideration of factors that may further reduce post-TIPS rebleeding is important. Systemic venous pressures of more than 15 mmHg and stent dysfunction have been found to represent risk factors for recurrent hemorrhage following TIPS [26]. The use of adjunctive coil embolization of gastroesophageal varices potentially addresses these risk factors by occluding venous collateral channels that may recanalize in the setting of elevated systemic venous pressures or stenotic or occluded shunts. To this end, variceal coil embolization provided a reduction in the post-TIPS rebleeding rate in a large series of patients undergoing TIPS with bare metal stents [12]. In that study, investigators found that variceal embolization prolonged the span of time between recurrent portal hypertension due to shunt insufficiency and clinical rebleeding.
In the current investigation, we successfully treated patients suffering from gastroesophageal variceal hemorrhage with TIPS with or without variceal coil embolization, which was performed at the discretion of the primary operating interventional radiologist. Among patients who underwent TIPS for acute or recent variceal hemorrhage, herein, patients who were treated with TIPS creation alone showed clinical evidence of recurrent hemorrhage with an approximately 15% greater incidence than those who received concurrent variceal embolization. While not statistically significant, these results represent a statistical trend that resulted in considerable morbidity among patients who did not undergo embolization, requiring hospitalization, blood product transfusion, endoscopy and additional angiography with embolization. A subgroup analysis of patients who underwent TIPS creation for treatment of acute variceal hemorrhage showed a 25% greater incidence of rebleeding in patients who were treated with TIPS creation alone that approached statistical significance. Furthermore, a trend toward increased bleeding-related mortality was observed in the non-embolization cohort. These results corroborate equivalent findings reported for TIPS created with bare stents [12]. Notably, no cases of rebleeding in the non-embolization group were found to be related to shunt dysfunction or occlusion, indicating that TIPS patency was not a significant factor in triggering rebleeding, and that patients may bleed from fragile patent varices despite an open shunt and reduced portosystemic gradient. This is in contradistinction to the embolization group, where the single case of repeat hemorrhage was related to shunt occlusion. This case highlights the importance of complete variceal embolotherapy.
While coil embolization provides an expected measure of protection against early recurrent hemorrhage from the occluded gastroesophageal varices, there is little literature on the mechanism of late rebleeding in this setting and the degree of security provided by embolization against late rebleeding. To this end, 9/15 patients in the embolization cohort had at least 140 days of follow-up (median 701 days, range 141–1,763 days), and only one showed evidence of recurrent hemorrhage. However, this case was associated with bleeding from a non-embolized coronary vein (patient had a gastrosplenic varix embolized) in the setting of an occluded shunt. These results lend support to the notion that coil embolization provides protection from late recurrent hemorrhage.
The use of adjunctive embolotherapy during the TIPS procedure is generally safe and well tolerated [12]. Reports of potential complications associated with coil embolization, such as coil migration with non-target embolization, are anecdotal. When coil embolization of gastroesophageal varices is pursued, there are benefits to doing so prior to stent insertion. Embolization prior to TIPS insertion allows for better angiographic filling and visualization of collateral vessels, which may decompress after shunt creation. Furthermore, a patent shunt represents a channel for systemic embolization of misplaced coils into the pulmonary circulation, should an episode of poor coil deployment and migration arise. The primary advantage of post-stent coil embolization is the ability to observe the effect of portosystemic gradient reduction on angiographic collateral vessel filling. It should be noted that the use of extensive adjunctive variceal embolotherapy theoretically allows for the use of a smaller shunt diameter, which may lower rates of post-procedure encephalopathy. This hypothesis was not tested in this investigation, as nearly all patients received 10-mm diameter shunts. It is noteworthy to mention that balloon retrograde transvenous obliteration (BRTO) of gastrorenal shunts has been shown to be effective in the management of gastric variceal hemorrhage [27] and may be useful in selected cases to avoid encephalopathy that may occur as a result of TIPS. Finally, the benefits of coil embolization during TIPS should be weighed against the increases in procedure duration, patient and operator radiation exposure, and procedure costs.
The overall rebleeding rate following TIPS in this study was slightly higher than that reported in literature for Viatorr TIPS [10, 23, 24]. The reason for this is uncertain, as no noteworthy differences in patient demographics, liver disease characteristics or procedure technical factors were identified. Notably, three patients in the non-embolization group who suffered rebleeding did not have angiographically visible varices on initial direct portal venography. However, initial direct portograms in these patients were performed from the midportion of the portal vein or from the superior mesenteric vein rather than the splenic vein, potentially reducing the likelihood of opacifying gastroesophageal varices arising from the splenic vein.
There were several important limitations to this study. First, this study was retrospective and was not randomized. Specifically, embolization was performed at the discretion of the operating interventional radiologist. Despite this potential bias, portal pressures were similar prior to and following TIPS for the two groups, making it unlikely that patients with less favorable characteristics were selected to undergo TIPS alone. Second, our investigation was conducted at a single institution and the sample size was limited. Moreover, the number of patients in the embolization and non-embolization cohorts was disproportionate, with slightly more than double the patients in the non-embolization group. Third, minor technical differences in TIPS placement, such as diameter of post-stent deployment balloon angioplasty, may have been present in the two study groups. However, experience indicates that the self-expanding Viatorr stent may enlarge to its nominal diameter over time despite under expansion following deployment. Finally, endoscopic confirmation of rebleeding sources was not available in several patients with recurrent hemorrhage following TIPS.
In summary, treatment of gastroesophageal variceal hemorrhage with TIPS alone was associated with relatively high rebleeding rates in this study, whereas TIPS performed with concurrent variceal coil embolization resulted in a negligible recurrent hemorrhage rate. Despite the limitations of this investigation, these findings support the need for a large prospective randomized trial to confirm the benefit of variceal coil embolization during TIPS performed for variceal hemorrhage as opposed to portosystemic pressure gradient reduction alone.
References
- 1.ter Borg PC, Hollemans M, Buuren HR, et al. Transjugular intrahepatic porto-systemic shunts: long-term patency and clinical results in a patient cohort observed for 3–9 years. Radiology. 2004;231:537–545. doi: 10.1148/radiol.2312021797. [DOI] [PubMed] [Google Scholar]
- 2.Boyer TD. Transjugular intrahepatic portosystemic shunt in the management of complications of portal hypertension. Curr Gastroenterol Rep. 2008;10:30–35. doi: 10.1007/s11894-008-0006-y. [DOI] [PubMed] [Google Scholar]
- 3.Berzigotti A, García-Pagán JC. Prevention of recurrent variceal bleeding. Dig Liver Dis. 2008;40:337–342. doi: 10.1016/j.dld.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 4.LaBerge JM, Somberg KA, Lake JR, et al. Two-year outcome following transjugular intrahepatic portosystemic shunt for variceal bleeding: results in 90 patients. Gastroenterology. 1995;108:1143–1151. doi: 10.1016/0016-5085(95)90213-9. [DOI] [PubMed] [Google Scholar]
- 5.Sahagun G, Benner KG, Saxon R, et al. Outcome of 100 patients after transjugular intrahepatic portosystemic shunt for variceal hemorrhage. Am J Gastroenterol. 1997;92:1444–1452. [PubMed] [Google Scholar]
- 6.Nazarian G, Ferral H, Castaneda-Zuniga W, et al. Development of stenoses in transjugular intrahepatic porto-systemic shunts. Radiology. 1994;192:231–234. doi: 10.1148/radiology.192.1.8208944. [DOI] [PubMed] [Google Scholar]
- 7.Haskal Z, Pentecost M, Soulen M, Shlansky-Goldberg R, Baum R, Cope C. Transjugular intrahepatic porto-systemic shunt stenosis and revision: early and midterm results. AJR Am J Roentgenol. 1994;163:439–444. doi: 10.2214/ajr.163.2.8037046. [DOI] [PubMed] [Google Scholar]
- 8.Vignali C, Bargellini I, Grosso M, et al. TIPS with expanded polytetrafluoroethylene-covered stent: results of an Italian multicenter study. AJR Am J Roentgenol. 2005;185:472–480. doi: 10.2214/ajr.185.2.01850472. [DOI] [PubMed] [Google Scholar]
- 9.Maleux G, Nevens F, Wilmer A, et al. Early and long-term clinical and radiological follow-up results of expanded-polytetrafluoroethylene-covered stent-grafts for transjugular intrahepatic porto-systemic shunt procedures. Eur Radiol. 2004;14:1842–1850. doi: 10.1007/s00330-004-2359-4. [DOI] [PubMed] [Google Scholar]
- 10.Hausegger KA, Karnel F, Georgieva B, et al. Transjugular intrahepatic portosystemic shunt creation with the Viatorr expanded polytetrafluoroethylene-covered stent-graft. J Vasc Interv Radiol. 2004;15:239–248. doi: 10.1097/01.rvi.0000116194.44877.c1. [DOI] [PubMed] [Google Scholar]
- 11.Jung HS, Kalva SP, Greenfield AJ, et al. TIPS: Comparison of shunt patency and clinical outcomes between bare stents and expanded polytetrafluoroethylene Stent-Grafts. J Vasc Intervent Radiol. 2009;20:180–185. doi: 10.1016/j.jvir.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Tesdal IK, Filser T, Weiss C, Holm E, Dueber C, Jaschke W. Transjugular intrahepatic portosystemic shunts: adjunctive embolotherapy of gastroesophageal collateral vessels in the prevention of variceal rebleeding. Radiology. 2005;236:360–367. doi: 10.1148/radiol.2361040530. [DOI] [PubMed] [Google Scholar]
- 13.Martin M, Zajko AB, Orons PD, et al. Transjugular intrahepatic portosystemic shunt in the management of variceal bleeding: indications and clinical results. Surgery. 1993;114:719–726. [PubMed] [Google Scholar]
- 14.Haskal ZJ, Scott M, Rubin RA, et al. Intestinal varices: treatment with the transjugular intrahepatic portosystemic shunt. Radiology. 1994;191:183–187. doi: 10.1148/radiology.191.1.8134568. [DOI] [PubMed] [Google Scholar]
- 15.Patel N. Transjugular Intrahepatic Portosystemic Shunt. In Portal Hypertension: Diagnosis and Intervention, 2nd ed. Fairfax, VA: Society of Cardiovascular & Interventional Radiology; 2001; 31
- 16.Tesdal IK, Jaschke W, Buhler M, et al. Transjugular intrahepatic portosystemic shunting with balloon-expandable and self-expanding stents: technical and clinical aspects after 3 1/2 years’ experience. Cardiovasc Intervent Radiol. 1997;20:29–37. doi: 10.1007/s002709900105. [DOI] [PubMed] [Google Scholar]
- 17.Kessler J, Trerotola SO. Use of the Amplatzer Vascular Plug for embolization of a large retroperitoneal shunt during transjugular intrahepatic portosystemic shunt creation for gastric variceal bleeding. J Vasc Intervent Radiol. 2006;17:135–140. doi: 10.1097/01.RVI.0000186958.59457.10. [DOI] [PubMed] [Google Scholar]
- 18.Abi-Jaoudeh N, Matsumoto AH, Angle FJ, et al. TIPS rebleeding rates for different subtypes of varices in the era of covered stents. J Vasc Intervent Radiol. 2008;19:S77–S78. doi: 10.1016/j.jvir.2007.12.225. [DOI] [Google Scholar]
- 19.Colapinto RF, Stronell RD, Gildiner M, et al. Formation of intrahepatic portosystemic shunts using a balloon dilatation catheter: preliminary clinical experience. AJR Am J Roentgenol. 1983;140:709–714. doi: 10.2214/ajr.140.4.709. [DOI] [PubMed] [Google Scholar]
- 20.Boyer TD, Haskal ZJ. American Association for the Study of Liver Disease practice guidelines: the role of transjugular intrahepatic portosystemic shunt creation in the management of portal hypertension. J Vasc Intervent Radiol. 2005;16:615. doi: 10.1097/01.RVI.0000157297.91510.21. [DOI] [PubMed] [Google Scholar]
- 21.Colombato L. The role of transjugular intrahepatic portosystemic shunt (TIPS) in the management of portal hypertension. J Clin Gastroenterol. 2007;41:S344–S351. doi: 10.1097/MCG.0b013e318157e500. [DOI] [PubMed] [Google Scholar]
- 22.Angermayr B, Cejna M, Koenig F, et al. Survival in patients undergoing transjugular intrahepatic porto-systemic shunt: ePTFE-covered stent-grafts versus bare stents. Hepatology. 2003;38:1043–1050. doi: 10.1053/jhep.2003.50423. [DOI] [PubMed] [Google Scholar]
- 23.Charon JP, Alaeddin FH, Pimpalwar SA, et al. Results of a retrospective multicenter trial of the Viatorr expanded polytetrafluoroethylene-covered stent-graft for transjugular intrahepatic portosystemic shunt creation. J Vasc Intervent Radiol. 2004;15:1219–1230. doi: 10.1097/01.RVI.0000137434.19522.E5. [DOI] [PubMed] [Google Scholar]
- 24.Tripathi D, Ferguson J, Barkell H, et al. Improved clinical outcome with transjugular intrahepatic portosystemic stent-shunt utilizing polytetrafluoroethylene-covered stents. Eur J Gastroenterol Hepatol. 2006;18:225–232. doi: 10.1097/00042737-200603000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Burroughs AK, Triantos CK, O’Beirne J, Patch D. Predictors of early rebleeding and mortality after acute variceal hemorrhage in patients with cirrhosis. Nat Clin Pract Gastroenterol Hepatol. 2009;6:72–73. doi: 10.1038/ncpgasthep1336. [DOI] [PubMed] [Google Scholar]
- 26.Sanyal AJ, Freedman AM, Luketic VA, et al. The natural history of portal hypertension after transjugular intrahepatic portosystemic shunts. Gastroenterology. 1997;112:889–898. doi: 10.1053/gast.1997.v112.pm9041251. [DOI] [PubMed] [Google Scholar]
- 27.Maruyama H, Yokosuka O. Current management of gastric varices. JNMA J Nepal Med Assoc. 2007;46:143–150. [PubMed] [Google Scholar]