Abstract
Infection with high-risk types (type 16 or type 18) of human papillomaviruses (HPVs) increases a patient's risk of cervical cancer. Given the importance of the cervix and the severe side effects resulting from traditional cancer therapies, this study aimed to achieve targeted inhibition of viral oncogenes in tumor cells using small interfering RNAs (siRNA). To accomplish this, we developed nine siRNAs against either the E6 or E7 genes of HPV-16 or HPV-18 in several combinations, yielding siRNAs targeting 16E6, 16E7, 18E6 and 18E7. We measured the effectiveness of the siRNAs by examining E6 or E7 mRNA expression after transfection of the siRNAs into HPV-positive CaSki (HPV-16) or HeLa (HPV-18) cell lines. We found that the HPV-siRNAs significantly reduced cell growth and colony formation in both cell lines. Flow cytometry analysis revealed a significant increase in apoptosis. The siRNAs had no effect on cell growth, colony formation or apoptosis in HPV-negative C33A cells, demonstrating a lack of off-target effects. In addition, an in vivo xenograft study showed that intra-tumoral injection of the siRNAs reduced tumor growth in BALB/c nude mice. In conclusion, we have developed highly specific and potent HPV-siRNAs that successfully suppress tumor growth and induce apoptosis in HPV-positive cervical cancer cells. siRNA treatment has potential for further development as an adjuvant therapy for cervical cancer.
Keywords: HPV, E6, E7, cervical cancer, siRNA silencing
Introduction
Cervical cancer is the second most common type of malignancy among women worldwide. It affects nearly half a million women each year, claiming a quarter of a million lives.1, 2 Epidemiological evidence has proven that previous infection with high-risk types of human papillomaviruses (HPVs) is the most significant risk factor for cervical cancer. HPV DNA can be detected in more than 95% of cervical cancer and intraepithelial neoplasia biopsies,3 demonstrating the strong association of this cancer with HPV infection.
HPVs are a group of more than 200 viruses.4 They are small, non-enveloped DNA viruses, 55 nm in diameter.3, 4, 5, 6 They are called papillomaviruses because certain types can cause warts or papillomas, which are benign (non-cancerous) tumors. HPVs that cause common hand and foot warts are different from those that cause growths in the genital area. Some types of HPV, such as type 16 and 18, are associated with cancer development and consequently have been termed high-risk or carcinogenic HPVs.3, 4, 5, 6 High-risk HPVs have different characteristics to those associated with benign lesions. First, the expression of E6 and E7 viral oncogenes is tightly regulated by the E2 protein during the normal life cycle of HPVs; however, in high-risk HPVs, expression of the E2 gene is disrupted during integration of the viral genome into the host cell, leading to aberrant expression of E6 and E7. Furthermore, the E6 and E7 proteins of high-risk HPVs have a much higher affinity to the tumor suppressor proteins p53 and Rb than those of other HPVs. The E6 protein binds to p53 and targets it for accelerated ubiquitin-mediated degradation.3, 4, 5, 6 The E7 protein binds to the hypophosphorylated form of Rb, resulting in the disruption of Rb/E2F complexes and reversal of the G1/S cell-cycle checkpoint.3, 4, 5, 6 Consequently, these viral oncogenes are potential targets for gene therapy of HPV-infected cancers.
RNA interference was first described in animals in 1998 and has now been widely used in functional genomics studies. RNA interference is the post-transcriptional silencing of gene expression by double-stranded RNA molecules.7, 8 Small interfering RNAs (siRNAs) are potentially applicable for the targeting of genes involved in any number of human diseases.9 Although several studies have reported the effects of HPV siRNAs on cervical cancer,10, 11, 12, 13, 14, 15 those reports were only for type-1610, 13, 15 or type-18.11, 14 Furthermore, many of them lack HPV-negative cells as controls11, 13 or short of animal studies.12, 14, 15 In this study, we designed nine HPV-siRNAs against the E6 or E7 oncogenes of the HPV-16 or HPV-18 virus. We investigated their specificity and efficacy on HPV-infected and non-infected cervical cancer cells. The siRNAs demonstrated highly potent and specific inhibition of HPV-positive tumor growth in vitro and in vivo, although they had no effect on HPV-negative cells. We discuss the specific siRNA design for strong suppression of the HPV E6 and E7 genes.
Materials and methods
Construction of siRNA plasmids
The pTOPO-U6 or pTOPO-U6II vector was used to construct HPV-siRNA plasmids as previously described, with few modifications.16, 17 Briefly, a 17∼22 sense and antisense hairpin oligonucleotide was generated complementary to specific mRNA of each gene. This hairpin oligonucleotide was separated by an eight-nucleotide spacer composed of a restriction enzyme cutting site that facilitated cloning into the pTOPO vector. A total of nine HPV-siRNA plasmids were constructed with sequences targeting HPV16-E6 (three plasmids, 16E6-249, −164 and −161), HPV16-E7 (three plasmids, 16E7-666, −629 and −628), HPV18-E6 (one plasmid, 18E6-119) and HPV18-E7 (two plasmids, 18E7-666 and −674) genes. An 18E6-Scramble plasmid that has the same nucleotide composition as 18E6-119 but a randomized sequence was designed as a control. The coding sequences for the HPV-siRNAs were empirically determined and analyzed by BLAST to ensure that they did not have sequence homology with host genes. The siRNA sequences are listed in Table 1.
Table 1. Sequence design of HPV-siRNAs.
| HPV-siRNA | Nucleotides | siRNA sequence |
|---|---|---|
| 16E6-249 (249-267) | 19 | 5′-TTATGCATAGTATATAGAGgaagcttgCTCTATATACTATGCATAA-3′ |
| 16E6-164 (164-183) | 20 | 5′-CTAGGCAAACAACTATACATGATAgaagcttgTATCATGTATAGTTGTTTGC-3′ |
| 16E6-161 (161-178) | 18 | 5′-CTAGCTGCAAACAACTATACATgaagcttgATGTATAGTTGTTTGCAG-3′ |
| 16E7-666 (666-682) | 17 | 5′-GAAATAGATGGTCCAGCgaagcttgGCTGGACCATCTATTTC-3′ |
| 16E7-629 (629-645) | 17 | 5′-CTAGCTCTACTGTTATGAGCAgaagcttgTGCTCATAACAGTAGAG-3′ |
| 16E7-628 (628-649) | 22 | 5′-CTAGTCTCTACTGTTATGAGCAATTAgaagcttgTAATTGCTCATAACAGTAGAGA-3′ |
| 18E6-119 (119-125) | 17 | 5′-CCAACACGGCGACCCTAgaagcttgTAGGGTCGCCGTGTTGG-3′ |
| 18E6-Scramble | 17 | 5′-CACCACATACCGCACGGgaagcttgCCGTGCGGTATGTGGTG-3′ |
| 18E7-666 (666-682) | 17 | 5′-CTAGTTCTATGTCACGAGCAAgaagcttgTTGCTCGTGACATAGAA-3′ |
| 18E7-674 (674-690) | 17 | 5′-CTAGCACGAGCAATTAAGCGAgaagcttgTCGCTTAATTGCTCGTG-3′ |
Abbreviations: HPV, human papillomaviruse; siRNA, small interfering RNA.
The position of each siRNA target sequence from the transcriptional start site is shown in parentheses. All siRNAs sequences have been confirmed by BLAST to have no homology to any part of the human genome. The nucleotide sequences in lowercase letters indicate the spacer for the hairpin folding area.
Cell lines and plasmid transfection
Cervical cancer cell lines HeLa (HPV18-positive), CaSki (HPV16-positive) and C33A (HPV-negative) were used in this study. These cells were cultured in minimum essential medium, Roswell Park Memorial Institute Medium-1640 and Dulbecco's Modified Eagel Medium for HeLa, Caski and C33A cells, respectively (Gibco BRL, Rockville, MD). All media were supplemented with 10% FCS and 1% antibiotics and maintained at 37 °C in a humidified incubator containing 5% CO2. To determine the transfection efficacy, the pGFP plasmid (BD Biosciences Clotech, CA) was used. For plasmid transfection, cells were seeded at a density of 1 × 106 in a 100-mm2 dish and cultured for 16 h. When cell confluence reached 60%, they were transfected with 6 μg of an HPV-siRNA plasmid or control vector using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) in OPTI-MEM medium (Invitrogen) for 6 h. The medium was then replaced with fresh medium and normal cell culture was resumed.
RNA extraction and reverse transcription (RT-PCR) analysis
The mRNA expression of E6 and E7 was determined by RT-PCR, similar to previous descriptions.18 In brief, total RNA was extracted with TRIzol reagent (Gibco BRL). The complementary DNA was synthesized by incubation of samples in 37 °C for 1 h in a buffer containing 50 m Tris, pH 8.4, 75 m KCl, 3 m MgCl2, 300 ng of RNA, 0.2 μg oligo-dT, 10 m DTT, 0.5 m dNTP, 10 units of RNase inhibitor and 50 units of reverse transcriptase. For each PCR run, a master mixture was prepared with 1x TaqMan buffer, 5 m MgCl2, 200 μ dNTP, 300 n of each primer, 1 unit of AmpliTaq Gold DNA Polymerase and 3 μl of complementary DNA solution in a total of 30 μl. Primer sequences for each gene are listed in Table 2. PCR reactions were carried out with specific primers and the following settings: 30 cycles at 94 °C for 40 s, 56 °C for 40 s and 72 °C for 1 min, using a Tpersonal thermocycler (Biometra, Gottingen, Germany). The PCR products were analyzed by 2% agarose gel electrophoresis, stained with ethidium bromide, photographed at 254 nm and the density of each band was measured. For quantification, the density of each gene was calibrated to the control vector after normalization to the internal actin level.
Table 2. List of primer sequences used for the analysis of HPV E6 or E7 gene expressions by RT-PCR.
| HPV gene | Primer sequences |
|---|---|
| HPV16E6 | F: 5′-AATGTTTCAGGACCCTACGG-3′ R: 5′-TCAGGACACAGTGGCTTTTG-3′ |
| HPV16E7 | F: 5′-TTTGCAACCAGAGACAACTGA-3′ R: 5′-GCCCATTAACAGGTCTTCCA-3′ |
| HPV18E6 | F: 5′-GCGACCCTACAAGCTACCTG-3′ R: 5′-GTTGGAGTCGTTCCTGTCGT-3′ |
| HPV18E7 | F: 5′-GCATGGACCTAAGGCAACAT-3′ R: 5′-TGTTGCTTACTGCTGGGATG-3′ |
| Actin | F: 5′-GTGGGGCGCCCCAGGCACCA-3′ R: 5′-CTCCTTAATGTCACGCACGATTTC-3′ |
Abbreviations: HPV, human papillomaviruse; RT-PCR, reverse transcription PCR.
Cell growth and colony formation assay
To determine cell growth, cell number and viability were determined daily by staining with 0.25% trypan blue and counting with a hemocytometer. For the colony formation assay, a total of 1000 cells, transfected with either a HPV-siRNA or control vector plasmid, were seeded on six-well plates and allowed to grow for 10 days in complete culture medium containing 30% fetal bovine serum. The cell colonies were then stained with 5% crystal violet (Sigma, St Louis, MO) for 15 min, washed with double distilled dH2O and counted. For quantification, Sorenson's solution (0.03 m tri-sodium citrate, 0.0195 N HCl, 45% ethanol) was added to each well to remove the dye from cells. After a 30-min incubation, the solution was transferred to a well in a 96-well plate and its absorbance was measured at 570 nm by a spectrometer.
Flow cytometry
Cells treated with an HPV-siRNA or control vector plasmid were harvested and washed with phosphate-buffered saline (PBS). The pellets were fixed with ethyl alcohol and PBS at −20 °C (EtOH/PBS=3/1). The pellets were then suspended and incubated with 0.5% Triton X-100 and 0.05% RNase in PBS at 37 °C for 1 h. The pellets were then incubated with 40 mg ml–1 propidium iodide for 30 min. Samples were analyzed by flow cytometry (Becton Dickinson, San Jose, CA). The cell-cycle distribution was determined using Cell Quest Pro and ModiFit softwares.
Xenografts
BALB/c AnN-Foxn1nu/CrlNarl nude mice were used. A 4∼6-week-old nude mouse was subcutaneously injected with 5 × 106 HeLa cells at each side of the upper hind limb region to generate solid tumors at both sites. Three days after tumor cell injection, one tumor was injected with an HPV-siRNA plasmid in 50 μl PBS, following by boosters of 25 μg plasmid in 25 μl PBS twice a week for a total of 30 days. The other tumor was injected with a control vector plasmid following the same schedule as the experimental group. The tumor dimensions were measured every 2∼3 day with calipers and calculated as length × width × height.
Statistical analysis
A two-ay ANOVA test was used to examine the association of siRNA and control plasmids. All P-values were two-sided, and the significance level was set at a P<0.05.
Results
Inhibition of E6 and E7 expression by HPV-siRNAs
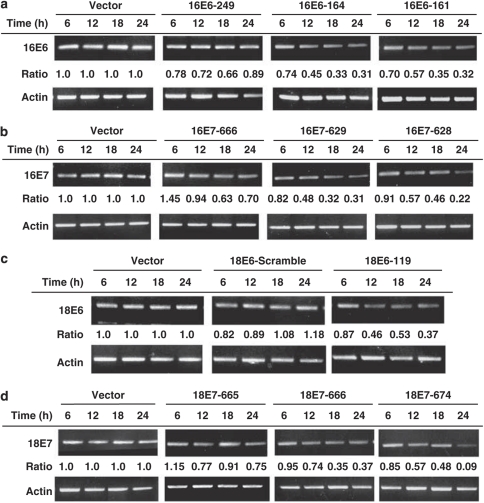
CaSki (HPV16-positive) or HeLa (HPV18-positive) cells were used to examine the effects of E6 or E7 gene knockdown by HPV-siRNAs. A total of nine HPV-siRNAs were designed and their effects on E6 or E7 gene expression were determined by RT-PCR analysis after transfection of each specific siRNA into cells. Results are shown in Figure 1. The siRNAs 16E6-164 and 16E6-161 showed effective knockdown of HPV16-E6 gene expression: 31 and 32% of control levels, respectively, at 24 h. Similarly, the siRNAs 16E7-629 and 16E7-628 showed effective knockdown of HPV16-E7 gene expression: 31 and 22% of control levels, respectively, at 24 h (Figure 1b). The siRNA 18E6-119 showed effective knockdown of HPV18-E6 gene expression: 37% of control levels at 24 h (Figure 1c). The siRNAs 18E7-666 and 18E7-674 showed effective knockdown of HPV18-E7 gene expression: 37 and 9% of control levels, respectively, at 24 h (Figure 1d). We, therefore, obtained at least one effective siRNA for each viral oncogene in each type of HPV.
Figure 1.
Knockdown of HPV-E6 or HPV-E7 gene expression by transfection of specific HPV-siRNAs. The silencing ability of each siRNA was determined by RT-PCR analysis after transfection of 6 μg of each siRNA plasmid for 6–24 h. The identity of each siRNA is indicated at the top of the bands. Actin gene expression was used as a control for each gene. The relative density of each sample is indicated at the bottom of each band, normalized to the actin level in each individual sample. (a) Differential inhibition of HPV-16 E6 gene expression in Caski cells. (b) Differential inhibition of HPV-16 E7 gene expression in Caski cells. (c) Differential inhibition of HPV-18 E6 gene expression in HeLa cells. (d) Differential inhibition of HPV-18 E7 gene expression in HeLa cells.
Suppression of HPV-positive cell growth by HPV-siRNAs
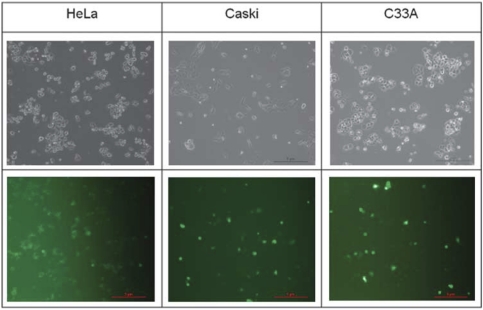
We examined the potential alteration of cellular phenotypes after E6 or E7 knockdown by transfection of these siRNA plasmids. The transfection efficacy was determined by the evaluation of green fluorescent protein expression in pGFP-transfected cells. Results were shown in the Figure 2. As shown, the fluorescent light presented in any of the cell types (HeLa, CaSki or C33A) was comparable, with approximately 70% of the total cells. These results suggest that the plasmid delivery ability among these three cell types was similar.
Figure 2.
Comparable transfection efficacy among cells of HeLa, CaSki and C33A. A total of 1 × 106 cells (Caski or HeLa or C33A) were seeded into a 100-mm2 dish and incubate for 16 h. The cells were then transfected with 6 μg of pGFP plasmid for 12 h. The medium was then replaced with fresh medium and normal cell culture was resumed. After 24 h, the cells were examined by a fluorescent microscopy (Nikon, Eclipse, TE300, Nikon Precision Taiwan, Taipei, Taiwan) with visible light (upper panel) or fluorescent light (lower panel).
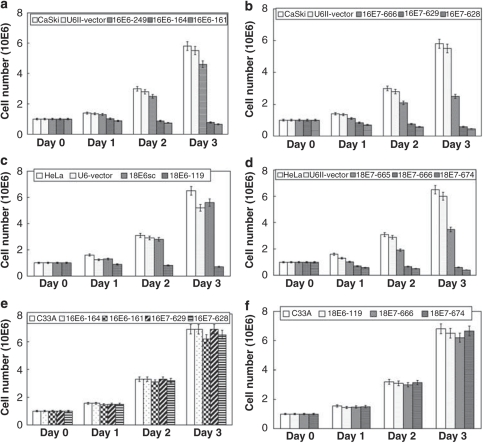
Figure 3 shows the effect on cell growth. As shown in Figure 3a, consistent with the observed knockdown of gene expression (Figure 1a), the two specific 16E6-siRNAs (16E6-164, 16E6-161) caused significant inhibition of cell growth, with approximately 70% reduction at day 2 and 85% at day 3 after transfection. Similarly, two of the specific 16E7-siRNAs (17E7-629, 16E7-628) caused substantial inhibition of cell growth, with approximately 75% reduction at day 2 and 90% at day 3 (Figure 3b). The specific 18E6-siRNA, 18E6-119, also caused substantial inhibition of cell growth, with 74 and >90% reduction at day 2 and day 3, respectively (Figure 3c). Two specific 18E7-siRNAs (18E7-666 and 18E7-674) caused substantial growth inhibition, with >80% reduction at day 2 and >90% at day 3 (Figure 3d). These results are consistent with the level of knockdown caused by each specific HPV-siRNA (Figure 1).
Figure 3.
Cell growth was inhibited in HPV-positive cervical cancer cells by HPV-siRNAs. A total of 1 × 106 cells (Caski or HeLa or C33A) were seeded into a 100-mm2 dish. After transfection of a specific HPV-siRNA, cell numbers were counted every day for up to 3 days. (a) Differential inhibition of cell growth by HPV-16E6-siRNAs in Caski cells. (b) Differential inhibition of cell growth by HPV-16E7-siRNAs in Caski cells. (c) Differential inhibition of cell growth by HPV-18E6-siRNAs in HeLa cells. (d) Differential inhibition of cell growth by HPV-18E7-siRNAs in HeLa cells. (e) Minimal effect of cell growth by HPV-16-siRNAs in non-HPV-infected C33A cells. (f) Minimal effect of cell growth by HPV-18-siRNAs in non-HPV-infected C33A cells.
To examine whether these siRNAs specifically inhibit growth in HPV-infected cells, we examined the growth of HPV-negative cervical cancer cells (C33A) after transfection with the siRNAs. All four siRNAs against HPV-16 (16E6-164, 16E6-161, 16E7-629 and 16E7-628) (Figure 3e) or the three siRNAs against HPV-18 (18E6-119, 18E7-666 and 18E7-674) (Figure 3f) had minimal effect (<10% inhibition) on cell growth. These results suggest that these siRNAs have high specificity for HPV genes and have almost no effect on cells not infected with HPV.
Suppression of HPV-positive colony formation by HPV-siRNAs
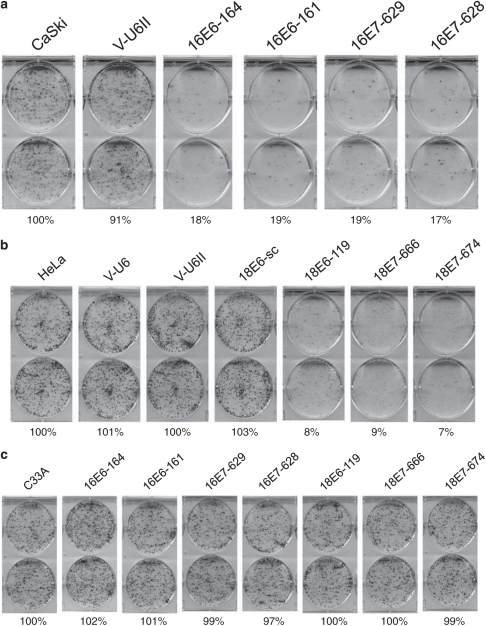
To validate the cell growth results, we tested the same siRNAs for their effect on colony formation. As shown in Figure 4a, after transfection of the specific siRNA plasmids, all the four HPV-16-siRNAs (16E6-164, 16E6-161, 16E7-629 and 16E7-628) caused significant inhibition (>80%) of colony formation compared with the control vector (Figure 4a). Similarly, all the three HPV-18-siRNAs (18E6-119, 18E7-666 and 18E7-674) caused significant inhibition (>90%) of colony formation (Figure 4b). These siRNAs were also transfected into the non-HPV-infected cells to test their specificity. As shown, there was no significant effect (< 5%) on colony formation in C33A cells by any of the seven HPV-siRNAs (Figure 4c).
Figure 4.
Colony formation was inhibited in HPV-positive cervical cancer cells by HPV-siRNAs. A total of 1000 cells (Caski or HeLa or C33A) transfected with a specific HPV-siRNA plasmid were seeded in six-well plates and allowed to grow for 10 days. The identity of each HPV-siRNA is indicated at the top of each plate. Relative levels of colony formation were quantified and are shown below each plate. (a) Suppression of colony formation by HPV-16-siRNAs in Caski cells. (b) Suppression of colony formation by HPV-18-siRNAs in Caski cells. (c) Minimal effect of colony formation by HPV-siRNAs in non-HPV infected C33A cells.
Induction of apoptosis by HPV-siRNAs
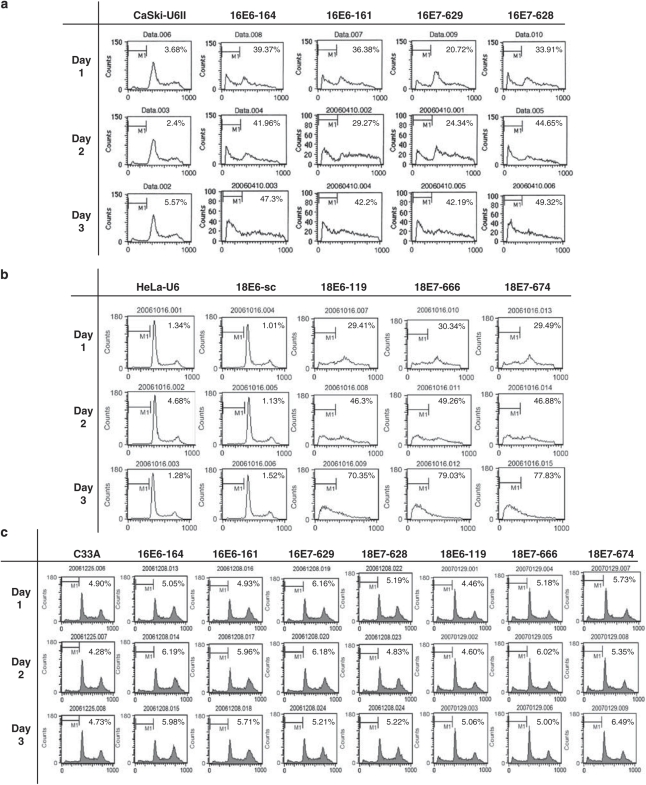
Because the targets of viral oncogenes E6 and E7 are p53 and pRb, which participate in cell cycle and apoptotic regulatory pathways, we examined whether any of these HPV-siRNAs have an effect on cell-cycle or apoptosis. Cells were subjected to flow cytometry after transfection by the specific HPV-siRNAs. As shown in Figure 5a, the cells transfected with the control vector (Caski-U6II) have a distinct peak at the G1 phase and a smaller peak at the G2/M phase. In cells transfected with any of the HPV-16-siRNAs (16E6-164, 16E6-161, 16E7-629 and 16E7-628), both the G1 and G2/M peaks were diminished, whereas the apoptotic fraction (M1 phase) was significantly increased. At day 3, over 40% of cells transfected with any of the four HPV-16-siRNAs were apoptotic. Similarly, a substantial increase in the number of apoptotic cells was induced by any of the three HPV-18-siRNAs (18E6-119, 18E7-666 and 18E7-674) (Figure 5b). At day 3, over 70% of cells transfected with these siRNAs were apoptotic. By contrast, there was almost no apoptosis (<2%) in cells transfected with the scrambled plasmid (Figure 5b). As for the specificity of these siRNAs, none of the seven HPV-siRNAs had a significant effect on apoptosis in non-HPV-infected cells (<2% of difference compared with the non-transfected control) (Figure 5c).
Figure 5.
Induction of apoptosis in HPV-positive cervical cancer cells by HPV-siRNAs. A total of 1 × 106 cells (Caski or HeLa or C33A) were transfected with a specific HPV-siRNA for 24 h. The cells were then synchronized to G0 phase by replacing the culture medium with serum-free medium for 12 h. Cells were then continuously cultured in regular medium and subjected to flow cytometric analysis every 24 h up to 72 h. The M1 fraction in each graph indicates apoptotic cells, and the percentage of M1 cells is indicated in each figure. (a) Increase of apoptotic fractions by HPV-16-siRNAs in Caski cells. (b) Increase of apoptotic fractions by HPV-18-siRNAs in HeLa cells. (c) Minimal effect of apoptosis by HPV-siRNAs in C33A cells.
Suppression of tumor growth in vivo by HPV-siRNAs
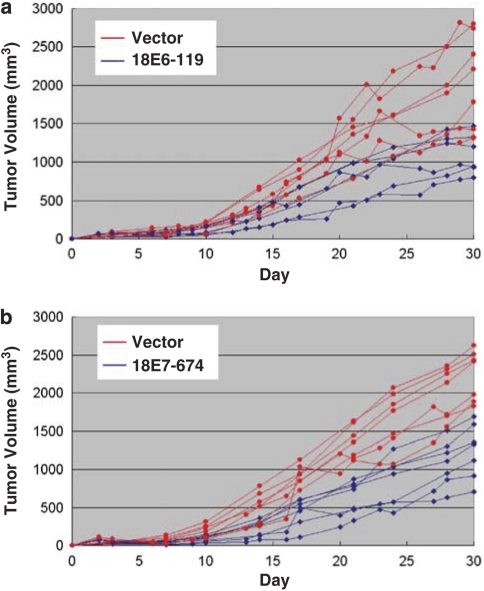
To investigate the effect of HPV-siRNAs on tumor growth in vivo, two of the potent siRNAs against the HPV-E6 and -E7 genes (18E6-119, 18E7-674) were used in a xenograft model in BALB/C nude mice. Cancer cells were subcutaneously injected in the upper portion of both rear limbs. After 3 days, an HPV-siRNA was administered to one tumor site and a control vector plasmid was administered to the other to compare the effect on tumor formation. Tumor sizes were continuously monitored every 2–3 days for a total of 30 days. Results are shown in the Figure 6. Although tumor size varied between mice, we consistently found that tumor growth was inhibited in the HPV-siRNA-treated cells. With the HPV-18E6-119 siRNA, tumor growth was decreased to 49% of the average at day 17 (P<0.001) and to 56% of the average at day 30 (P<0.001) (Figure 6a). Similarly, tumor growth was inhibited by the HPV-18E7-666 siRNA, to 48% of the average at day 17 (P=0.0018) and 54% of the average at day 30 (P<0.001) (Figure 6b). These results demonstrate the effective suppression of tumor growth in vivo by these HPV-siRNAs.
Figure 6.
Suppression of tumor growth in xenografts of HPV-positive cervical cancer cells by HPV-siRNAs. BALB/c null mice (seven in each group) were injected with 5 × 106 HeLa cells subcutaneously to generate solid tumors at two sites. After 3 days, 30 μg of either an HPV-18-siRNA or control vector plasmid in 30 μl of normal saline solution was intra-tumorally injected, followed by booster injections of 15 μg of plasmid twice a week for 2 weeks. The tumors were monitored for a total of 30 days. The tumor volume was calculated as the length x width x height. Red lines: tumor growth status in the mice injected with vector plasmid. Blue lines: tumor growth status in the mice injected with HPV18-siRNA. (a) Effect of 18E6-119 siRNA on tumor growth. (b) Effect of 18E6-674 siRNA on tumor growth.
Discussion
Cervical cancer is the second most common cancer in women worldwide and the majority of cases are caused by high-risk types of human papillomavirus (HPV-16 and -18), which possess the E6 and E7 oncogenes. The concurrent expression of E6 and E7 protein is a prerequisite for cancer development and required to maintain malignant phenotypes. Although the pap smear screening test has recently become popular as a method to reduce the incidence rate of cervical cancer, effective therapy for cervical cancer is still urgently needed in developing countries.
In the present study, we designed several siRNA plasmids against the E6 or E7 viral oncogenes in HPV-16 and HPV-18 and found that all of them effectively inhibit the expression of E6 or E7 in the HPV-infected cervical cancer cells (Figure 1). Transfection of these siRNAs caused significant suppression of both cell growth (Figures 3a-d) and colony formation (Figures 4a and b) in HPV-positive cells, whereas they had no effect in HPV-negative cells (Figures 3e, f and 4c), demonstrating the effectiveness and specificity of these siRNAs. In examining cell-cycle status, we found that these HPV-siRNAs promoted the induction of apoptosis in the HPV-infected cells (Figure 5). These results are consistent with recent findings that the reduction of E6 or E7 expression can induce apoptosis in HPV-positive cells.14, 15 The mechanism by which E6 or E7 knockdown induces apoptosis is unclear. Presumably, silencing of E6 or E7 leads to an accumulation of p53 or pRB, respectively, either of which may induce senescence or apoptosis.14, 15, 19 It is of note that the effect of these siRNAs was specific to HPV-infected cells, as the non-infected C33A cells remained unaltered in response to siRNA transfection. These results demonstrate the efficacy of our siRNA sequence design.
The design of an effective siRNA sequence is an important issue. It is unfortunate that, no highly effective, simple protocol exists thus far. The first requirement for an effective siRNA sequence is that the siRNA must target part of the open reading frame of the gene, however, not all sites are effective. For example, in the HPV16-E6 gene, the siRNAs 16E-164 or -161 are more effective at silencing than 16E6-249. These results indicate that the targeting sequences located 161–183 nucleotides from the transcriptional start codon have stronger effects than sequences located 249–267 nucleotides away (Table 1). In the HPV16-E7 gene, the siRNAs 16E7-629 and −628 are more effective at silencing than 16E7-666. Apparently, siRNAs targeted to sites 628–649 nucleotides from the transcriptional start codon are more effective than siRNAs targeted to sites 666–682 away (Table 1). Similarly, siRNAs targeted to nucleotides 119–125 and 666–690 are effective for HPV18 E6 and E7 genes, respectively (Table 1). The second requirement for an effective siRNA sequence is that the length must be between 17–22 nucleotides, however, there appears to be no preference for a specific length within that range. This can be demonstrated by comparing the knockdown of two siRNAs against the HPV16 E7 gene. The siRNA 16E7-629 (17 nucleotides, targeting to 629–645) has a similar silencing effect as the siRNA 16E7-628 (22 nucleotides, targeting 628–649). As comparing the silencing effect of E6 and E7 genes, as E7 and E6 are transcribed bicistronically,20 an siRNA targeting to E7 may silence E6 as well, leading to a greater phenotypic effect. In the present study, however, we found comparable effects on growth inhibition, apoptotic induction and tumor suppression by either E6 or E7 knockdown (Figures 3, 4, 5 and 6). This may be due to effective siRNA design and optimized dosage, resulting in maximal gene suppression. Although the aim of this study was to discover candidate siRNAs, the ultimate aim will be the application of the high potent siRNAs to HPV-infected cancer patients. For this, other siRNA delivery strategy, such as lentiviral vector, may be good to use.
Consistent with our in vitro results, we found that knockdown of HPV E6 or E7 expression by siRNA delivery has potent anti-tumor effects, as measured by significant suppression of xenograft tumor growth (Figures 6a and b). As the E6 and E7 HPV oncogenes share no sequence homology to human genes, silencing of these oncogenes may be a highly effective molecular therapy for cervical cancer without causing high toxicity in normal cells. In conclusion, we have developed a series of potent HPV-siRNAs that successfully suppress tumor growth and induce apoptosis in HPV-positive cervical cancer cells, while having minimal effect in HPV-negative human cells. Given the immense interest in RNA interference as a potential therapeutic, these siRNAs may be further developed as an adjuvant therapy for cervical cancer.
Acknowledgments
This work was supported by National Science Research Grant of Taiwan (NSC 96-2628-B-182A-098-MY3 to JTC and 98-2320-B-182-006-MY3 to AJC).
The authors declare no conflict of interest.
References
- Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci. 2006;110:525–541. doi: 10.1042/CS20050369. [DOI] [PubMed] [Google Scholar]
- Goldie S. A public health approach to cervical cancer control: considerations of screening and vaccination strategies. Int J Gynecol Obstet. 2006;94:95–105. doi: 10.1016/S0020-7292(07)60016-2. [DOI] [PubMed] [Google Scholar]
- Munger K, Baldwin A, Edwards K, Hayakawa H, Nguyen C, Owens M, et al. Mechanisms of human papillomavirus-induced oncogenesis. J Virol. 2004;78:11451–11460. doi: 10.1128/JVI.78.21.11451-11460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrmann F, Laimins L. Human papillomaviruses: targeting differentiating epithelial cells for malignant transformation. Oncogene. 2003;22:5201–5207. doi: 10.1038/sj.onc.1206554. [DOI] [PubMed] [Google Scholar]
- Munger K, Howley P. Human papillomavirus immortalization and transformation functions. Virus Res. 2002;89:213–228. doi: 10.1016/s0168-1702(02)00190-9. [DOI] [PubMed] [Google Scholar]
- Snijders PJF, Steenbergen RDM, Heideman DAM, Meijer CJLM. HPV-mediated cervical carcinogenesis: concepts and clinical implications. J Pathol. 2006;208:152–164. doi: 10.1002/path.1866. [DOI] [PubMed] [Google Scholar]
- Zaratiegui M, Irvine DV, Martienssen RA. Noncoding RNAs and gene silencing. Cell. 2007;128:763–776. doi: 10.1016/j.cell.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Bayne EH, Allshire RC. RNA-directed transcriptional gene silencing in mammals. Trends Genet. 2005;21:370–373. doi: 10.1016/j.tig.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Kim DH, Rossi JR. Strategies for silencing human disease using RNA interference. Genetics. 2007;8:173–184. doi: 10.1038/nrg2006. [DOI] [PubMed] [Google Scholar]
- Yamato K, Tamada T, Kizaki M, Ui-Tei K, Natori Y, Fujino M, et al. New highly potent and specific E6 and E7 siRNAs for treatment of HPV16 positive cervical cancer. Cancer Gene Ther. 2008;15:140–153. doi: 10.1038/sj.cgt.7701118. [DOI] [PubMed] [Google Scholar]
- Gu W, Putral L, Hengst K, Minto K, Saunders NA, Leggatt G, et al. Inhibition of cervical cancer cell growth in vitro and in vivo with lentiviral- vector delivered short hairpin RNA targeting human papillomavirus E6 and E7 oncogenes. Cancer Gene Ther. 2006;13:1023–1032. doi: 10.1038/sj.cgt.7700971. [DOI] [PubMed] [Google Scholar]
- Jiang M, Milner J. Selective silencing of viral gene E6 and E7 expression in HPV-positive human cervical carcinoma cells using small interfering RNAs. Methods Mol Biol. 2005;292:401–420. doi: 10.1385/1-59259-848-x:401. [DOI] [PubMed] [Google Scholar]
- Jonson AL, Rogers LM, Ramakrishnan S, Downs LS., Jr Gene silencing with siRNA targeting E6/E7 as a therapeutic intervention in a mouse model of cervical cancer. Gynecol Oncol. 2008;11:356–364. doi: 10.1016/j.ygyno.2008.06.033. [DOI] [PubMed] [Google Scholar]
- Lea JS, Sunaga N, Sato M, Kalahasti G, Miller DS, Minna JD, et al. Silencing of HPV 18 oncoproteins with RNA interference causes growth inhibition of cervical cancer cells. Report Sci. 2007;14:10–18. doi: 10.1177/1933719106298189. [DOI] [PubMed] [Google Scholar]
- Sima N, Wang W, Kong D, Deng D, Xu Q, Zhou J, et al. RNA interference against HPV16 E7 oncogene leads to viral E6 and E7 suppression in cervical cancer cells and apoptosis via upregulationof Rb and 53. Apoptosis. 2008;13:273–280. doi: 10.1007/s10495-007-0163-8. [DOI] [PubMed] [Google Scholar]
- Chiu CC, Lin CY, Lee LY, Chen YJ, Kuo TF, Chang JTC, et al. Grp78 regulates multiple malignant phenotypes in head-neck cancer and may serve as a molecular target of therapeutic intervention. Mol Cancer Ther. 2008;7:2788–2797. doi: 10.1158/1535-7163.MCT-08-0172. [DOI] [PubMed] [Google Scholar]
- Tseng CP, Huang CL, Huang CH, Cheng JC, Stem A, Tseng CH, et al. Disabled-2 small interfering RNA modulates cellular adhesive function and MAPK activity during megakaryocytic differentiation of K562 cells. FEBS Lett. 2003;541:21–27. doi: 10.1016/s0014-5793(03)00281-3. [DOI] [PubMed] [Google Scholar]
- Chang JTC, Chan SH, Lin CY, Lin TY, Wang HM, Liao CT, et al. Differentially expressed genes in radioresistant nasopharyngeal cancer cells: gp96 and GDF15. Mol Cancer Ther. 2007;6:2271–2279. doi: 10.1158/1535-7163.MCT-06-0801. [DOI] [PubMed] [Google Scholar]
- DeFilippis RA, Goodwin EC, Wu L, DiMaio D. Endogenous human papillomavirus E6 and E7 Proteins differentially regulate proliferation, senescence, and apoptosis in HeLa cervical carcinoma cells. J Virol. 2003;77:1551–1563. doi: 10.1128/JVI.77.2.1551-1563.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S, Tao M, McCoy JP, Jr, Zheng ZM. The E7 oncoprotein is translated from spliced E6*I transcripts in high-risk human papillomavirus type 16- or type 18-positive cervical cancer cell lines via translation reinitiation. J Virol. 2006;80:4249–4263. doi: 10.1128/JVI.80.9.4249-4263.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]