Abstract
Rationale
The bone morphogenetic proteins (BMP), a family of morphogens, have been implicated as mediators of calcification and inflammation in the vascular wall.
Objective
To investigate the effect of altered expression of Matrix Gla Protein (MGP), an inhibitor of BMP, on vascular disease.
Methods and Results
We used MGP transgenic or MGP deficient mice bred to apolipoprotein E mice, a model of atherosclerosis. MGP overexpression reduced vascular BMP activity, atherosclerotic lesion size, intimal and medial calcification, and inflammation. It also reduced expression of the activin-like kinase receptor (ALK)1 and the vascular endothelial growth factor (VEGF), part of a BMP-activated pathway that regulates angiogenesis and may enhance lesion formation and calcification. Conversely, MGP deficiency increased BMP activity, which may explain the diffuse calcification of vascular medial cells in MGP deficient aortas, and the increase in expression of ALK1 and VEGF. Unexpectedly, atherosclerotic lesion formation was decreased in MGP deficient mice, which may be explained by a dramatic reduction in expression of endothelial adhesion molecules limiting monocyte infiltration of the artery wall.
Conclusion
Our results indicate that BMP signaling is a key regulator of vascular disease, requiring careful control to maintain normal vascular homeostasis.
Keywords: Bone morphogenetic protein, Matrix Gla protein, Atherosclerosis, Vascular calcification, Inflammation
Introduction
Inflammation and atypical cell differentiation are hallmarks of atherosclerotic lesion development and are regulated by systemic and local factors. Bone morphogenetic proteins (BMP)-2 and -4, multifunctional growth factors and morphogens, have been implicated as mediators of endothelial inflammation in response to proatherogenic oscillatory shear stress, oxidative stress and proinflammatory cytokines 1, 2. Several BMPs have also been detected in calcified atherosclerotic plaques 3, 4, and BMP-2 promotes calcification of aortic myofibroblasts 5, consistent with the ability of BMPs to induce bone differentiation 6. Thus, the morphogenetic activities of BMP-2 and -4 might directly influence lesion progression through endothelial inflammation and cell differentiation.
Gene deletion in mice previously identified Matrix Gla protein (MGP) as an essential inhibitor of arterial calcification 7, which prevents osteochondrogenic lineage reprogramming of smooth muscle cells (SMC) 8. Excessive MGP expression, on the other hand, has no discernable effect on the aortic vascular wall, but leads to growth inhibition of the pulmonary vascular tree through interference with BMP-4 signaling 9. MGP antagonizes BMP signaling 10, 11 through direct protein-protein interaction involving essential proline and gamma-carboxylated glutamate residues in MGP 12. It acts as a negative feedback regulator in a BMP-activated pathway that is triggered by interaction between BMP-2 or -4 and the activin-like kinase receptor (ALK)2, first stimulating expression of the activin-like kinase receptor (ALK)1, and then expression of the vascular endothelial growth factor (VEGF) and MGP itself 9, 11, 13. This regulatory pathway is likely to play an important role in embryonic development since ALK1 and VEGF are essential for angiogenesis 13, 14, BMP and MGP affects pattern formation in vascular mesenchymal cells 15, and MGP promotes SMC differentiation 8. Here, we hypothesize that perturbations in BMP-signaling play important roles in the development of vascular disease.
Methods
Animals and Diets
Apoe-/- mice on a C57BL/6J background were purchased from Jackson Laboratory (Bar Harbor ME). MGPtg/wt mice, generated in our laboratory on a C57BL/6J background 9, were crossed with Apoe-/- mice to generate MGPtg/wt;Apoe-/- mice. We used the MGPtg/wt mice since the phenotype was apparent in MGPtg/wt mice, and a low birth rate of MGPtg/tg mice made it difficult to obtain MGPtg/tg mice 9. MGP+/- mice on C57BL/6J background 7 were obtained from Dr. Gerard Karsenty (Columbia University, New York, NY) and crossed with the Apoe-/- mice to generate MGP-/-;Apoe-/- mice. All mice were maintained on standard chow (Diet 8604, Harlan Teklad, Laboratory, Madison, WI). At 8 to 10 weeks of age, littermate Apoe-/- and MGPtg/wt;Apoe-/- mice were either continued on standard chow or switched to a high-fat/high-cholesterol diet (Western diet) (Research Diets, New Brunswick, NJ, diet #D12108, containing 21% fat [w/w], 1.25% cholesterol [w/w]) for 16 weeks. The studies were reviewed by the Institutional Review Board and conducted in accordance with the animal care guidelines set by the University of California, Los Angeles.
See Supplemental Methods for details on animals, lipid and phosphate analyses, lesion and calcification quantification, validation of antibodies, immunoblotting, RNA analysis, immunohistochemistry and immunofluorescence, ELISA, complete blood count, and statistical analysis.
Results
Reduced aortic atherosclerotic lesion formation in MGPtg/wt;Apoe-/- mice
To explore the ability of BMP signaling to influence vascular disease, we took advantage of the availability of transgenic mice expressing human MGP (MGPtg/wt) and mice with MGP gene deletion (MGP-/-) 7, 9. These were crossbred with Apolipoprotein E null (Apoe-/-) mice, a common model of atherosclerosis and lesion calcification.
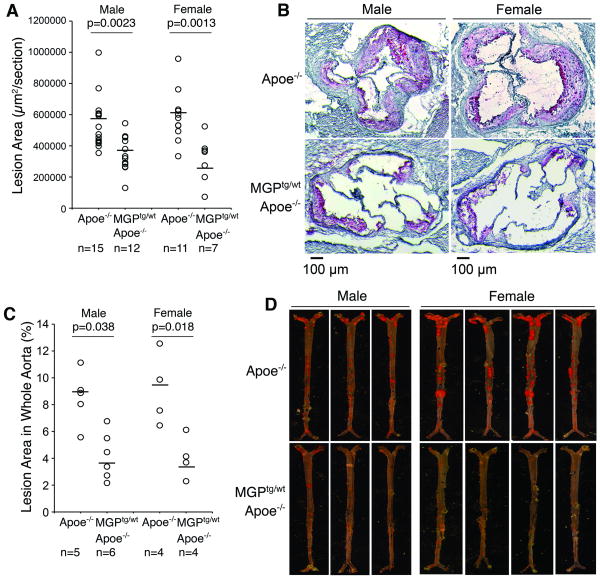
We first determined the effect of increased MGP expression on atherosclerotic lesions and arterial calcification by comparing Apoe-/- and MGPtg/wt;Apoe-/- mice after 16 weeks on Western diet. The hypercholesterolemia of the Apoe-/- and MGPtg/wt;Apoe-/- mice was exacerbated by the Western diet, but there were no statistically significant differences in body mass or lipid levels in male or female mice, respectively (Online Table I). Cross-sectional analysis of the atherosclerotic lesions showed statistically significant differences between the two strains. The Apoe-/- mice had an average aortic sinus lesion area of 540,117 μm2 and 589,159 μm2 in males (n=15) and females (n=11), respectively, whereas MGPtg/wt;Apoe-/- mice had an average area of 338,312 μm2 and 216,890 μm2 in males (n=12, p=0.0023) and females (n=7, p=0.0013), respectively (Fig. 1A, B). The lower number of females was due to difficulties in obtaining sufficient females carrying the MGP transgene 9. En face analysis of whole aortas showed similar differences between the two strains. The Apoe-/- mice had an average aortic lesion area of 8.5% and 9.1% in males (n=5) and females (n=4), respectively, whereas MGPtg/wt;Apoe-/- mice had an area of 4.2% and 4.1% in males (n=6, p=0.038) and females (n=4, p=0.018), respectively (Fig. 1C, D). Thus, enhanced MGP expression limited formation of atherosclerotic lesions.
Figure 1. Increased MGP expression limits the development of atherosclerotic lesions.
Apoe-/-and MGPtg/wt;Apoe-/-mice were maintained on Western diet for 16 weeks.
(A-D) Mean lesion area (A), representative Oil Red O stained aortic sinus section (B), aortic lesion coverage in en face specimens (C), and representative Sudan IV stained aortas (D) of male and female Apoe-/-and MGPtg/wt;Apoe-/-mice.
Statistically significant differences were determined by the Mann-Whitney rank sum test.
Reduced aortic atherosclerotic lesion calcification in MGPtg/wt;Apoe-/- mice
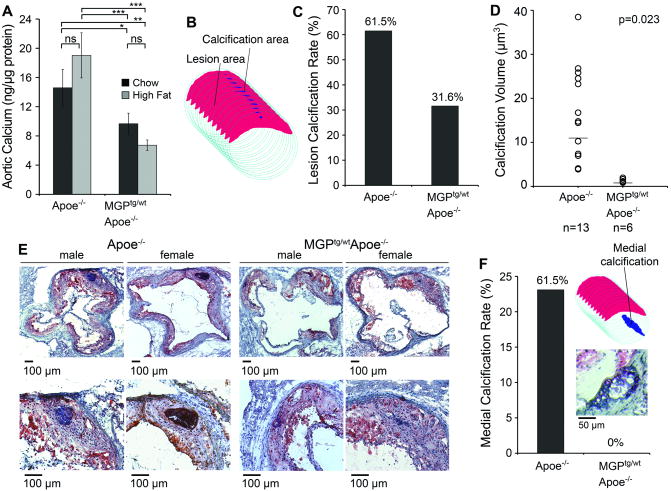
Because of MGP's well-defined role as an inhibitor of vascular calcification, we examined the effect of enhanced MGP expression on calcification. We first compared total calcium in aortic tissue in Apoe-/- and MGPtg/wt;Apoe-/- mice after 16 weeks of regular chow diet (CH) or Western diet (high fat diet, HF). The results showed no significant effect of the Western diet in the respective strain (Fig. 2A). However, total calcium was significantly decreased in both chow and fat-fed MGPtg/wt;Apoe-/- mice compared to the similarly fed Apoe-/- mice (Fig. 2A). We next compared lesion calcification by cross-sectional analysis (schematically illustrated in figure 2B) in the Apoe-/- and MGPtg/wt;Apoe-/- mice that were fed a Western diet. This showed that both the rate and amount of lesion calcification per animal were significantly lower in MGPtg/wt;Apoe-/- mice than in Apoe-/- mice. The rate of calcification was 61.5% and 31.6% in the Apoe-/- and the MGPtg/wt;Apoe-/- mice, respectively (Fig. 2C), and the average amount of calcification was 16.7 μm3 (n=13) in Apoe-/- mice but only 1.2 μm3 in MGPtg/wt;Apoe-/- (n=6) (Fig. 2D, E). Only animals that exhibited calcification were analyzed, and males and females were analyzed together due to the low calcification rate in the MGPtg/wt;Apoe-/- mice. Thus, enhanced MGP expression limited total aortic calcium and lesion calcification. Similar to previous reports 16, we observed that the Apoe-/- mice developed cartilage metaplasia and medial calcification in parts of the media without lesions. We found that 61.5% of the Apoe-/- mice, but none of the MGPtg/wt;Apoe-/- mice, had medial calcification (Fig. 2F), suggesting that MGP also limits medial calcification. Because of the important role of phosphate in stimulating vascular calcification, we compared serum phosphate levels in the different mice, but there were no statistically significant differences (Online Table II).
Figure 2. Increased MGP expression limits lesion calcification and medial (non-lesion) calcification.
Apoe-/-and MGPtg/wt;Apoe-/-mice were maintained on Western diet for 16 weeks.
(A) Total calcium in aortic tissues (n=4) as compared to chow-fed mice.
(B) Schematic representation of analysis of lesion calcification.
(C) Calcification rate in Apoe-/-and MGPtg/wt;Apoe-/-mice.
(D) Mean lesion calcification in Apoe-/- and MGPtg/wt;Apoe-/-mice that exhibited calcification (male and female mice were analyzed together).
(E) Representative Oil Red O stained aortic sinus sections with calcification from male and female Apoe-/-and MGPtg/wt;Apoe-/-mice.
(F) Medial calcification in Apoe-/-and MGPtg/wt;Apoe-/-mice. Medial calcification was analyzed as schematically shown in insert. Representative section of Oil Red O stained medial calcification.
Asterisks indicate statistically significant differences *<0.05, **<0.01, ***<0.001, Tukey's test.
In panel D, statistically significant differences were determined by the Mann-Whitney rank sum test.
Diminished vascular BMP activity in MGPtg/wt;Apoe-/- mice
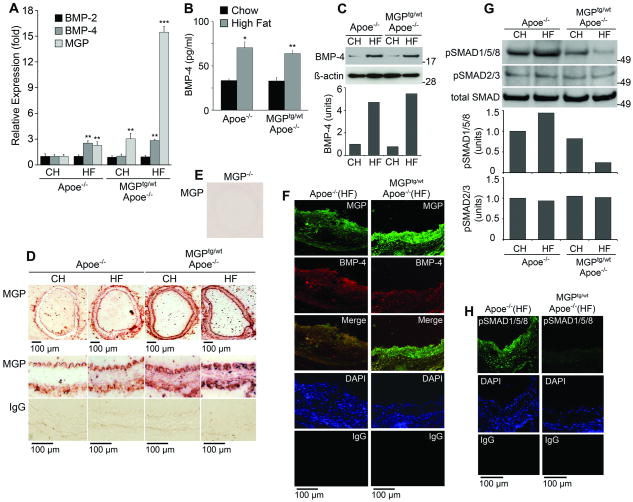
To examine BMP signaling in atherosclerotic vessels and its response to enhanced MGP expression, we first compared expression of BMP-2, -4 and MGP in Apoe-/- and MGPtg/wt;Apoe-/- mice after 16 weeks of chow or Western diet. Aortic expression of BMP-4 increased about 3-fold in both groups of mice on the Western diet compared to chow, whereas expression of BMP-2 remained the same (Fig. 3A). Glyceraldehyde-3-phosphate dehydrogenase was used as reference gene for the PCR reactions. BMP-4 protein in serum and aorta also increased, as determined by ELISA and immunoblotting with quantification by densitometry (Fig. 3B, C). ß-Actin was used as loading control. Aortic expression of MGP was about 3-fold higher in chow-fed MGPtg/wt;Apoe-/- mice than in Apoe-/- mice. It increased about 3-fold in the Apoe-/- mice and 15-fold in the MGPtg/wt;Apoe-/- mice on the Western diet (Fig. 3A), suggesting a synergistic effect of the transgene and the Western diet.
Figure 3. Fat-feeding enhances and increased MGP expression limits aortic BMP-signaling.
Apoe-/-and MGPtg/wt;Apoe-/-mice fed chow (CH) or Western (HF) diet were examined.
(A) Aortic expression of BMP-2, BMP-4 and MGP, as determined by real-time PCR.
(B) Serum levels of BMP-4, as determined by ELISA.
(C) Aortic levels of BMP-4, as determined by immunoblotting with densitometry.
(D) MGP expression and localization, as determined by immunohistochemistry, in aortic segments without atherosclerotic lesions.
(E) Control experiment using MGP-/- aorta and the same MGP-antibodies as in panel D.
(F) BMP-4 and MGP expression, as determined be immunofluorescence, in lesions of in Apoe-/-and MGPtg/wt;Apoe-/-mice fed a Western diet.
(G) BMP activity in total aorta, as determined by pSMAD1/5/8 immunoblotting with densitometry.
(H) BMP activity in atherosclerotic lesions from Apoe-/-and MGPtg/wt;Apoe-/-mice fed a Western diet, as determined by immunofluorescence.
Asterisks indicate statistically significant differences compared to control (Apoe-/- mice on chow). **<0.01, ***<0.001, Tukey's test.
We next examined MGP in the vascular wall by immunochemistry. In aortic segments without obvious atherosclerotic lesions, MGP was detected mostly in proximity to the endothelium and the adventitia in chow-fed Apoe-/- mice (Fig. 3D). As Western diet and the transgene increased MGP expression, the staining in these areas intensified and MGP staining also appeared in the media (Fig. 3D). Control experiments using MGP-/- aorta and the same MGP-antibodies showed no staining (Fig. 3E). In atherosclerotic lesions, MGP and BMP-4 were detected in the same areas (Fig. 3F), but the MGP expression was higher relative to BMP-4 in the MGPtg/wt;Apoe-/- mice than in the Apoe-/- mice (Fig. 3F).
We predicted that BMP signaling activity would be less in the aortic walls of the MGPtg/wt;Apoe-/- mice because of the enhanced MGP expression. To test this, we determined the levels of activated, phosphorylated (p)SMAD1/5/8 in aortas from Apoe-/- and MGPtg/wt;Apoe-/- mice fed chow or a Western diet. The level of pSMAD1/5/8 was lower in the MGPtg/wt;Apoe-/- aortas than in the Apoe-/- aortas as determined by immunoblotting with densitometry (Fig. 3G) and immunohistochemistry (Online Fig. II). Total SMAD and pSMAD2/3, which mediate TGF-ß signaling, were included for comparison and showed no change. Furthermore, pSMAD1/5/8 levels were diminished in atherosclerotic lesions from MGPtg/wt;Apoe-/- mice compared to Apoe-/- mice as determined by immunofluorescence (Fig. 3H). Thus, the enhanced MGP expression in MGPtg/wt;Apoe-/- mice diminished BMP signaling.
Increased MGP expression suppresses expression of ALK1 and VEGF
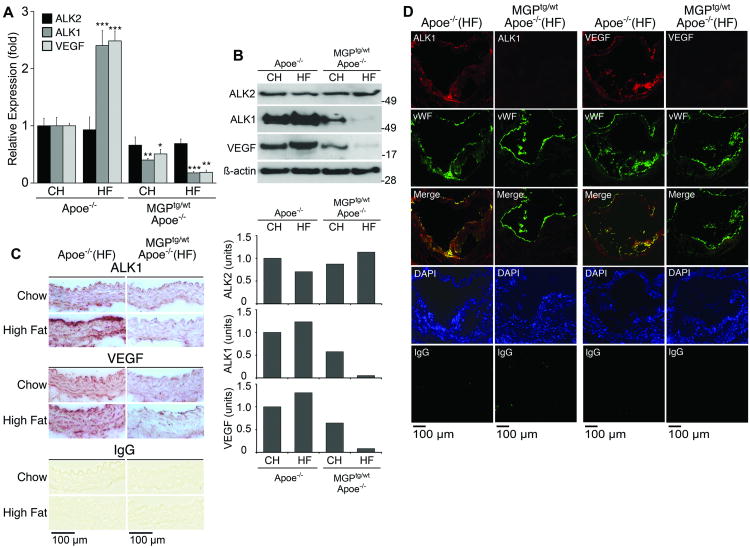
Expression of ALK1 is stimulated by BMP 11, an effect that is mediated by the ALK2 receptor 17. ALK1 is essential for normal angiogenesis, and has a dual role in VEGF regulation. ALK1 suppresses VEGF expression when stimulated by BMP-9, a circulating BMP that has been referred to as a vascular “quiescence” factor 18. Yet, ALK1 also enhances VEGF levels by inducing the ALK5 receptor, which reacts with TGF-ß1 to increase VEGF expression 19. To examine this pathway, we determined aortic expression of ALK2, ALK1 and VEGF. The aortic expression of ALK1 and VEGF was increased in Apoe-/- mice on Western diet compared to chow, and suppressed in MGPtg/wt;Apoe-/- mice on both chow and Western diet as determined by real-time PCR (Fig. 4A), immunoblotting with densitometry (Fig. 4B), and immunohistochemistry of aortas without atherosclerotic lesions (Fig. 4C). There was no significant change in ALK2 expression. In atherosclerotic lesions, expression of both ALK1 and VEGF was strongly enhanced in Apoe-/- mice on Western diet compared to MGPtg/wt;Apoe-/- mice (Fig. 4D). ALK1 and VEGF co-localized to a large extent with the von Willebrand (vWF), a marker for vascular endothelium, in the MGPtg/wt;Apoe-/- mice (Fig. 4D). Thus, diminished BMP signaling led to suppression of aortic ALK1 and VEGF, suggesting that the same signaling pathway is active in vascular disease and in angiogenesis. To determine if other BMP inhibitors contributed to the decreased BMP activity, we also examined the expression of crossveinless 2 (CV2), Noggin and Chordin 20. The results showed a stimulation of Noggin and Chordin in both types of mice on the Western diet, whereas the expression of CV2 correlated with BMP activity and was inhibited in MGPtg/wt;Apoe-/- mice (Online Fig. III). Consequentely, none of these inhibitors selectively contributed to the decreased BMP activity in the MGPtg/wt;Apoe-/- mice.
Figure 4. Increased MGP expression attenuates aortic expression of ALK1 and VEGF.
(A-C) Aortic expression of ALK2, ALK1 and VEGF in Apoe-/-and MGPtg/wt;Apoe-/-mice fed chow (CH) or Western (HF) diet, as determined by real-time PCR (A), immunoblotting with densitometry (B), and immunohistochemistry (C).
(D) Expression of ALK1 and VEGF colocalize with expression of vWF in atherosclerotic lesions from Apoe-/-and MGPtg/wt;Apoe-/-mice fed a Western diet.
Asterisks indicate statistically significant differences compared to control (Apoe-/- mice on chow). **<0.01, ***<0.001, Tukey's test.
Increased MGP expression suppresses gene expression associated with inflammation
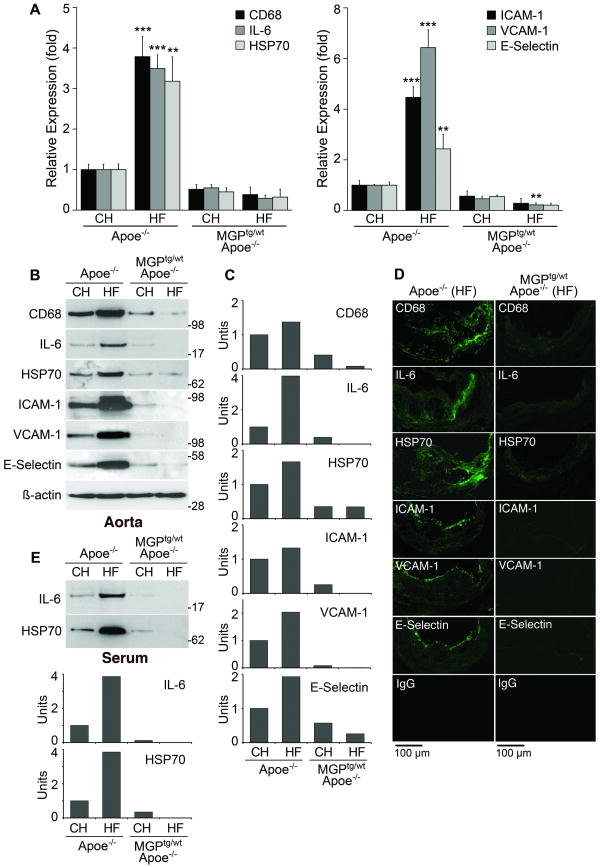
Inflammation is a hallmark of atherosclerotic lesions and is partly mediated by BMP-2 and -4 1, 2. To examine the effect of MGP overexpression on inflammatory activity, we compared aortic expression of CD68, a macrophage marker 21, interleukin (IL)-6 22, heat shock protein (HSP)70, a stress-induced protein 23, and the endothelial adhesion molecules intercellular adhesion molecule (ICAM)-1, vascular cell adhesion molecule (VCAM)-1 and E-selectin in Apoe-/- and MGPtg/wt;Apoe-/- mice on regular chow or Western diet. The results showed that aortic expression of all proteins increased with Western diet in the Apoe-/- mice, but not in the MGPtg/wt;Apoe-/- mice as determined by real-time PCR and immunoblotting with densitometry (Fig. 5A-C). The real-time PCR results in the mice on Western diet were also normalized to lesion area, which did not significantly alter the results (Online Fig. IV). The serum levels of IL-6 and HSP70 reflected the changes in the aortic expression (Fig. 5D). Furthermore, expression of all proteins decreased in lesions of MGPtg/wt;Apoe-/- mice compared to Apoe-/- mice as determined by immunofluorescence (Fig. 5E). Thus, the results suggested that inflammation is reduced by MGP overexpression.
Figure 5. Increased MGP expression suppresses inflammatory activity.
(A-D) Aortic expression of CD68, IL-6, HSP70, ICAM-1, VCAM-1 and E-selectin in Apoe-/-and MGPtg/wt;Apoe-/-mice fed chow (CH) or Western (HF) diet, as determined by real-time PCR (A), immunoblotting with densitometry (B, C), and immunofluorescence (D). (E) Serum levels of IL-6 and HSP70, as determined by immunoblotting with densitometry. Asterisks indicate statistically significant differences. **<0.01, ***<0.001, Tukey's test.
Reduced formation of atherosclerotic lesions in MGP-/-;Apoe-/- mice
MGP-/-mice are known to develop arterial calcification 7, 8, and our results showed increased aortic pSMAD1/5/8 levels (Online Fig. V), suggesting increased BMP activity. As previously reported, the calcification also caused structural changes in the aortic wall (see H&E staining, Online Fig. VA, top) 7, 8.
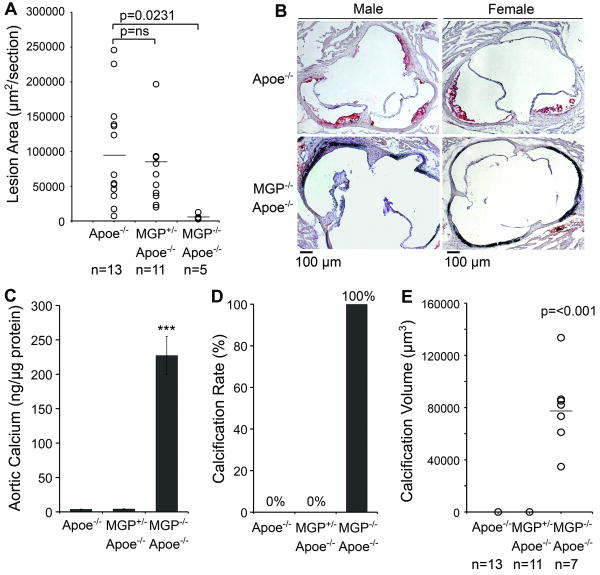
To determine if lesion formation occurred in MGP deficiency, we compared MGP-/-;Apoe-/- mice to Apoe-/- and MGP+/-;Apoe-/- mice. The MGP-/-;Apoe-/- mice were unable to tolerate a Western diet due to its severe phenotype, which was indistinguishable from that of MGP-/- mice 7. All mice were therefore maintained on a chow diet for 18 weeks. As expected, the MGP-/-;Apoe-/- mice were smaller than the Apoe-/- mice 7, but there were no significant differences in lipid levels (Online Table I). Cross-sectional analysis showed that the average aortic sinus lesion area was 99,981 μm2 in chow-fed Apoe-/- mice (n=13), whereas lesion formation was minimal, 3,857 μm2 in MGP-/-;Apoe-/- mice (n=5, p=0.0231) (Fig. 6A, B). Males and the females were combined in the analysis due to the limited number of MGP-/-;Apoe-/- mice that survived 18 weeks. There were no significant differences between the Apoe-/- and the MGP+/-;Apoe-/- mice (Fig. 6A). The MGP-/-;Apoe-/- mice demonstrated the same altered aortic wall structure as the MGP-/- mice, which prevented en face analysis.
Figure 6. Reduced lesion formation in extensively calcified arteries from in MGP-/-;Apoe-/- mice.
Apoe-/-, MGP+/-;Apoe-/- and MGP-/-;Apoe-/-mice were maintained on chow for 18 weeks. Male and female mice were analyzed together.
(A) Mean lesion area.
(B) Representative Oil Red O stained aortic sinus section from male and female Apoe-/-and MGP-/-;Apoe-/-mice.
(C) Total calcium in aortic tissues (n=4).
(D) Calcification rate.
(E) Mean lesion calcification.
Asterisks indicate statistically significant differences. ***<0.001, Tukey's test.
In panel A and D, statistically significant differences were determined by the Mann-Whitney rank sum test.
Furthermore, total calcium in aortic tissue was highly increased in the MGP-/-;Apoe-/- mice (Fig. 6C), and cross-sectional analysis of the aortas demonstrated the extensively calcified media in the MGP-/-;Apoe-/- mice (Fig. 6D, E) compared to the Apoe-/- mice that had minimal calcification in both types of analysis, also when compared to Apoe-/- mice fed a Western diet (Fig. 1). The rate of calcification was 100% in the MGP-/-;Apoe-/- mice compared to 0% in the Apoe-/- mice (Fig. 6D). The average amount of calcification in the aorta was 73,136 μm3 (n=7, 30 μm aortic segments) in MGP-/-;Apoe-/- mice, and 0 μm3 in the Apoe-/- mice (n=13) (Fig. 6C) consistent with previous reports 7, 8. There were no significant differences between the Apoe-/- and the Apoe-/-;MGP+/- mice. In addition calcification without lesion formation was observed in the aortic valves of the MGP-/-;Apoe-/- mice, suggesting that the valvular BMP response was similar to that of the aortic wall. There were no significant differences in serum phosphate levels (Online Table II).
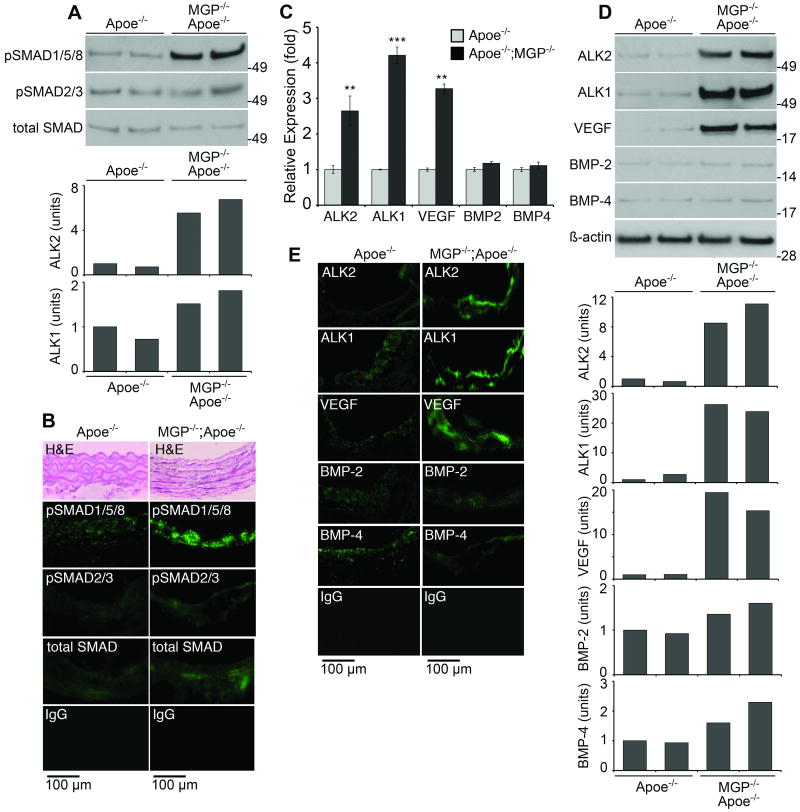
We then compared SMAD1/5/8 signaling and expression of ALK2, ALK1, VEGF, BMP-2 and -4 in the MGP-/-;Apoe-/- and the Apoe-/- mice. Again, the MGP-deficiency resulted in increased levels of pSMAD1/5/8, as determined by immunoblotting with densitometry and immunofluorescence (Fig. 7A,B). Expression of ALK2, ALK1 and VEGF, but not BMP-2 and -4, was also enhanced in the MGP-/-;Apoe-/- mice as determined by real-time PCR, immunoblotting with densitometry and immunofluorescence (Fig. 7C-E). Furthermore, expression of CV2, but not Noggin and Chordin, was significantly increased (Online Fig. VI), supporting that CV2 is responsive to changes in BMP activity.
Figure 7. Increased aortic BMP-signaling and expression of ALK1 and VEGF in MGP-/-;Apoe-/- mice.
(A,B) BMP activity in aortas of Apoe-/- and MGP-/-;Apoe-/- mice as determined by immunoblotting with densitometry for pSMAD1/5/8 (A), and in lesions as determined by immunofluorescence (B) Structural changes in aortas of MGP-/-;Apoe-/- mice are visible in the H&E-stained sections (B, top).
(C-E) Expression of ALK2, ALK1, VEGF, BMP-2 and -4 in aortas from Apoe-/- and MGP-/-;Apoe-/- mice as determined by real-time PCR (C) and immunoblotting with densitometry (D), and in lesions as determined by immunofluorescence (E).
Asterisks indicate statistically significant differences. **<0.01, ***<0.001, Tukey's test.
Diminished inflammatory response in MGP-/-;Apoe-/- mice
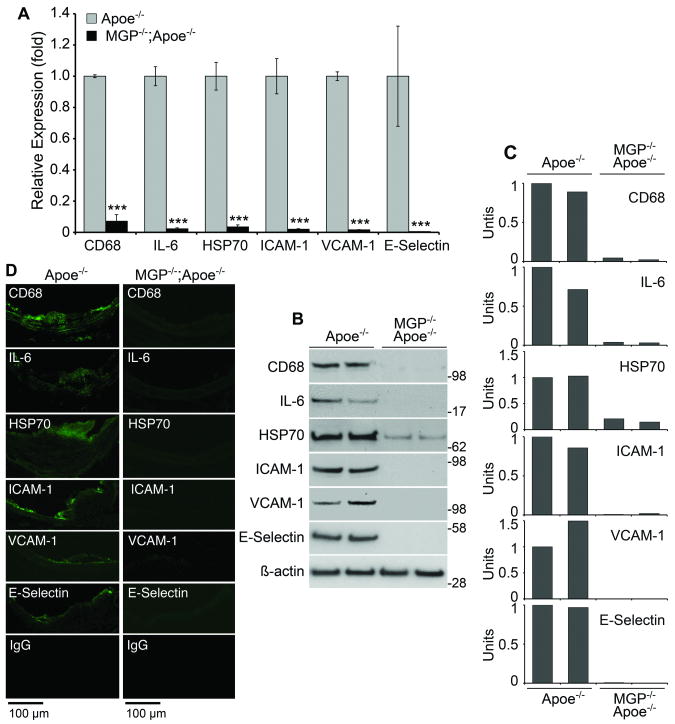
To examine if changes in the inflammatory response are involved in reducing lesion formation, we examined the expression of CD68, IL-6, HSP70 and endothelial adhesion molecules in the MGP-/-;Apoe-/- and the Apoe-/- mice. Expression of all proteins was dramatically reduced in the aortas of the MGP-/-;Apoe-/- mice compared to the Apoe-/- mice, as determined by real-time PCR, immunoblotting with densitometry and immunofluorescence (Fig. 8A-D). No differences were detected in levels of circulating monocytes and other white blood cells as determined by standard methodology (Online Table III). The results suggest that reduced expression of endothelial adhesion molecules explains, at least in part, the decrease in lesion formation.
Figure 8. Increased aortic BMP-signaling but reduced expression of inflammatory proteins in MGP-/-;Apoe-/- mice.
(A-D) Expression of CD68, IL-6, HSP70, ICAM-1, VCAM-1 and E-selectin in aortas of Apoe-/- and MGP-/-;Apoe-/- mice as determined by real-time PCR (A), immunoblotting with densitometry for pSMAD1/5/8 (B,C), and in lesions as determined by immunofluorescence (D).
Asterisks indicate statistically significant differences. ***<0.001, Tukey's test.
Discussion
BMP signaling in vertebrate development is essential for regulation of gastrulation, patterning, and organogenesis 24, whereas postnatal BMP signaling regulates injury repair, remodeling and inflammation 1, 2, 25, 26. In this study, we show that BMP signaling is a connecting link between hyperlipidemia, an inflammatory stimulus, and the atherogenic response in the arterial wall. We also present evidence that a BMP-activated regulatory pathway, which was identified in vitro and includes ALK2, ALK1, MGP and VEGF 11, 17, is active in vivo during the development of vascular disease.
The presence of the MGP transgene led to more efficient reduction in BMP activity and lesion formation in response to a high fat diet in Apoe-/- mice. It also led to less expression of ALK1 and VEGF, the precise role of which is likely dependent on location and timing. Suppression of ALK1 and VEGF in lesions may limit neoangiogenesis and reduce intraplaque hemorrhages and calcification 27, although a baseline expression of VEGF is required for maintenance and repair of a healthy endothelium 28. It is also known that VEGF is required for normal ossification during endochondral bone formation 29, and high VEGF levels might actually promote calcification. The beneficial effect of BMP inhibition, however, has to be weighed against the effect of limiting BMP in vascular growth considering that excess MGP inhibits angiogenesis and pulmonary vascular growth 9, 23. It illustrates the delicate balance in BMP signaling that directs growth and homeostasis of different vascular beds.
We detected enhanced MGP expression close to the endothelium, in the media and in atherosclerotic lesions. It is consistent with previous reports of increased MGP expression in calcified media and atherosclerotic lesions 3, 30-32. It suggests that MGP has a dual role in limiting BMP-induced events in both the endothelium and the media, including endothelial inflammation 1, 2 and atypical differentiation of medial cells 8. We also observed changes in expression of CV2, Noggin, and Chordin, especially in the endothelium. Interestingly, CV2 expression was enhanced by increased BMP activity, whereas Noggin and Chordin expression was induced by high fat diet, suggesting distinct gene regulation.
The findings in this and other studies 7, 8, 33 suggest that the response in developing arteries to loss of MGP and highly enhanced BMP activity is calcification of medial cells. Speer et al. 8 recently showed that the calcification of the MGP-/- aorta involves an osteochondrogenic lineage reprogramming of SMC, suggesting that such transdifferentiation of SMC is driving calcification in the vascular wall. This effect appears to be dependent on locally expressed MGP given that only MGP expression targeted to SMC, but not circulating MGP, is able to rescue the MGP-/- mouse 33. Our results further suggest that it occurs in absence of inflammatory activation. A similar calcific response may be triggered by increased BMP signaling in the atherosclerotic lesions, supporting the general concept that developmental programs can be reactivated in vascular disease.
Only BMP-4 was found to increase in response to the high fat diet even though both BMP-2 and -4 have been implicated in pro-inflammatory effects in the endothelium and vascular calcification 1, 2, 5, 34. Although we cannot exclude local variations of BMP-2 not detected in the analysis, it suggests that BMP-4 more readily initiates atherosclerotic development in mice. The expression of endothelial adhesion molecules and proteins associated with inflammation and cellular stress correlated well with increased BMP activity and lesion formation in the Apoe-/- and MGPtg/wt;Apoe-/- aortas. Despite a high BMP activity, expression of adhesion molecules and other inflammatory proteins was dramatically reduced in the MGP-deficient mice. The low expression of adhesion molecules likely prevented monocyte infiltration of the artery wall, thereby explaining, at least in part, the reduction in lesions. The reason for the lack of induction of endothelial adhesion molecules is still unclear. We speculate that the uncontrolled BMP activity affects EC differentiation, which in turn causes abnormal EC responses. However, further studies are required to clarify this. Other factors that may affect the inflammatory response include potential defects in monocyte function and the severe phenotype of the MGP-/- mice.
Interestingly, HDL, a well-known anti-atherogenic factor, is able to induced expression of ALK2, which in turn allows induction of ALK1, VEGF and MGP 17. Indeed, the Apo-AI transgenic mice, which have high HDL and minimal atherosclerosis, showed enhanced aortic expression of all these factors 17, which suggests that HDL protects against inflammation and atherosclerosis in part by influencing BMP signaling. However, it is not clear if the protective effect derives from ALK2 activating several protective pathways or from early induction of ALK1, MGP and VEGF prior to lesion formation.
In this study, we have focused on MGP's role as an inhibitor of BMP signaling. We have not evaluated other aspects of its role as a matrix protein, including potential changes in extracellular location and binding of calcium and other proteins in the extracellular matrix. For example, MGP may attach to vitronectin through its C-terminus 35, which could alter its ability to bind BMP. The function of MGP is affected by factors that interfere with gamma-carboxylation. For example warfarin, a Vitamin K antagonist increases the levels of uncarboxylated, non-functioning MGP and may enhance calcification 12, 36, 37. It is also known that the reduction of Vitamin K is less efficient in vascular SMC than in the liver 36. Furthermore, MGP contains phosphorylated serines, which contribute to its function as a calcification inhibitor 38. However, it is not clear if there is a relationship between the phosphorylation status of MGP and its effect on BMP signaling.
In summary, our results support the concept that modulation of BMP signaling through alterations in MGP expression regulates vascular disease as well as the development of a normal arterial wall. A regulatory pathway triggered by BMP and involving ALK2, ALK1, VEGF and MGP is central for vascular development, disease and homeostasis. Understanding the role of vascular BMP may reveal new approaches to treat and prevent vascular disease.
Novelty and Significance.
What is known?
Bone morphogenetic protein (BMP)-2 and -4 are multifunctional growth factors that mediate endothelial inflammation in response to proatherogenic stimuli in vitro.
BMP-2 and -4 have bone-inducing properties and are detected in calcified areas of atherosclerotic lesions.
Matrix Gla protein (MGP) binds and inhibits BMP-2 and -4. MGP deficiency leads to chondrogenic transdifferentiation and calcification of vascular smooth muscle cells (SMC).
BMP-2 and -4 induce expression of the activin-like kinase receptor 1 (ALK1) is essential for angiogenesis and in turn regulates the vascular endothelial growth factor (VEGF).
What new information does this article contribute?
Increased levels of MGP efficiently limit BMP activity, endothelial inflammation, atherosclerosis and lesion calcification.
BMP activity regulates expression of ALK1 and VEGF in atherosclerotic vessels.
The vascular endothelium is abnormal in MGP deficiency; it lacks expression of endothelial adhesion molecules, which lead to less monocyte infiltration and atherosclerotic lesion formation.
Chondrogenic transdifferentiation and calcification of SMC occurs in the absence of inflammation in MGP deficient aortas.
Summary
Inflammation promotes atherosclerotic lesion development and atypical cell differentiation. The BMPs, a family of multifunctional growth factors and morphogens, enhance inflammation in the vascular wall. BMPs also regulate expression of VEGF through the ALK1 receptor. MGP antagonizes BMP activity through direct protein-protein interaction. We demonstrate that overexpression of MGP limits BMP activity, endothelial inflammation, atherosclerotic lesion formation and lesion calcification in a mouse model of atherosclerosis. In addition, the increased MGP limits expression of ALK1 and VEGF in atherosclerotic lesions. Conversely, we demonstrate that loss of MGP results in high BMP activity. Paradoxically, the loss of MGP results in an abnormal endothelium with dramatic reductions in endothelial adhesion proteins, monocyte infiltration, and atherosclerotic lesion formation. However, the chondrogenic transdifferentiation and calcification of SMC occurs in absence of inflammation. Thus, BMP signaling is a key regulator of vascular disease, requiring careful control to maintain normal vascular homeostasis.
Supplementary Material
Acknowledgments
Funding: This work was funded in part by NIH grants HL30568 and HL81397, and the American Heart Association (Western Affiliate).
Non-Standard Abbreviations and Acronyms
- ALK
activin-like kinase receptor
- Apoe
Apolipoprotein E
- BMP
Bone morphogenetic protein
- CH
Chow diet
- CV2
Crossveinless-2
- ELISA
Enzyme-linked immunosorbent assay
- Gla
gamma-carboxyglutamic acid
- HF
High fat (Western) diet
- HSP
Heat shock protein
- ICAM-1
Intercellular adhesion molecule-1
- IgG
Immunoglobulin G
- IL
Interleukin
- MGP
Matrix Gla protein
- pSMAD
Phosphorylated SMAD
- SMAD
Homolog of the drosophila protein, mothers against decapentaplegic (MAD) and the C. elegans protein SMA
- TGF-ß
Transforming growth factor ß
- VCAM-1
Vascular cell adhesion molecule-1
- VEGF
Vascular endothelial growth factor
Footnotes
Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Csiszar A, Smith KE, Koller A, Kaley G, Edwards JG, Ungvari Z. Regulation of bone morphogenetic protein-2 expression in endothelial cells: role of nuclear factor-kappaB activation by tumor necrosis factor-alpha, H2O2, and high intravascular pressure. Circulation. 2005;111:2364–2372. doi: 10.1161/01.CIR.0000164201.40634.1D. [DOI] [PubMed] [Google Scholar]
- 2.Sorescu GP, Song H, Tressel SL, Hwang J, Dikalov S, Smith DA, Boyd NL, Platt MO, Lassegue B, Griendling KK, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res. 2004;95:773–779. doi: 10.1161/01.RES.0000145728.22878.45. [DOI] [PubMed] [Google Scholar]
- 3.Dhore CR, Cleutjens JP, Lutgens E, Cleutjens KB, Geusens PP, Kitslaar PJ, Tordoir JH, Spronk HM, Vermeer C, Daemen MJ. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001;21:1998–2003. doi: 10.1161/hq1201.100229. [DOI] [PubMed] [Google Scholar]
- 4.Schluesener HJ, Meyermann R. Immunolocalization of BMP-6, a novel TGF-beta-related cytokine, in normal and atherosclerotic smooth muscle cells. Atherosclerosis. 1995;113:153–156. doi: 10.1016/0021-9150(94)05438-o. [DOI] [PubMed] [Google Scholar]
- 5.Al-Aly Z, Shao JS, Lai CF, Huang E, Cai J, Behrmann A, Cheng SL, Towler DA. Aortic Msx2-Wnt calcification cascade is regulated by TNF-alpha-dependent signals in diabetic Ldlr-/- mice. Arterioscler Thromb Vasc Biol. 2007;27:2589–2596. doi: 10.1161/ATVBAHA.107.153668. [DOI] [PubMed] [Google Scholar]
- 6.Canalis E, Economides AN, Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev. 2003;24:218–235. doi: 10.1210/er.2002-0023. [DOI] [PubMed] [Google Scholar]
- 7.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 8.Speer MY, Yang HY, Brabb T, Leaf E, Look A, Lin WL, Frutkin A, Dichek D, Giachelli CM. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res. 2009;104:733–741. doi: 10.1161/CIRCRESAHA.108.183053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao Y, Nowak S, Yochelis A, Garfinkel A, Bostrom KI. Matrix GLA Protein, an Inhibitory Morphogen in Pulmonary Vascular Development. J Biol Chem. 2007;282:30131–30142. doi: 10.1074/jbc.M704297200. [DOI] [PubMed] [Google Scholar]
- 10.Wallin R, Cain D, Hutson SM, Sane DC, Loeser R. Modulation of the binding of matrix Gla protein (MGP) to bone morphogenetic protein-2 (BMP-2) Thromb Haemost. 2000;84:1039–1044. [PubMed] [Google Scholar]
- 11.Yao Y, Zebboudj AF, Shao E, Perez M, Bostrom K. Regulation of bone morphogenetic protein-4 by matrix GLA protein in vascular endothelial cells involves activin-like kinase receptor 1. J Biol Chem. 2006;281:33921–33930. doi: 10.1074/jbc.M604239200. [DOI] [PubMed] [Google Scholar]
- 12.Yao Y, Shahbazian A, Bostrom KI. Proline and gamma-carboxylated glutamate residues in matrix Gla protein are critical for binding of bone morphogenetic protein-4. Circ Res. 2008;102:1065–1074. doi: 10.1161/CIRCRESAHA.107.166124. [DOI] [PubMed] [Google Scholar]
- 13.Shao ES, Lin L, Yao Y, Bostrom KI. Expression of vascular endothelial growth factor is coordinately regulated by the activin-like kinase receptors 1 and 5 in endothelial cells. Blood. 2009;114:2197–2206. doi: 10.1182/blood-2009-01-199166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.David L, Feige JJ, Bailly S. Emerging role of bone morphogenetic proteins in angiogenesis. Cytokine Growth Factor Rev. 2009;20:203–212. doi: 10.1016/j.cytogfr.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Garfinkel A, Tintut Y, Petrasek D, Bostrom K, Demer LL. Pattern formation by vascular mesenchymal cells. Proc Natl Acad Sci U S A. 2004;101:9247–9250. doi: 10.1073/pnas.0308436101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rattazzi M, Bennett BJ, Bea F, Kirk EA, Ricks JL, Speer M, Schwartz SM, Giachelli CM, Rosenfeld ME. Calcification of advanced atherosclerotic lesions in the innominate arteries of ApoE-deficient mice: potential role of chondrocyte-like cells. Arterioscler Thromb Vasc Biol. 2005;25:1420–1425. doi: 10.1161/01.ATV.0000166600.58468.1b. [DOI] [PubMed] [Google Scholar]
- 17.Yao Y, Shao ES, Jumabay M, Shahbazian A, Ji S, Bostrom KI. High-density lipoproteins affect endothelial BMP-signaling by modulating expression of the activin-like kinase receptor 1 and 2. Arterioscler Thromb Vasc Biol. 2008;28:2266–2274. doi: 10.1161/ATVBAHA.108.176958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.David L, Mallet C, Keramidas M, Lamande N, Gasc JM, Dupuis-Girod S, Plauchu H, Feige JJ, Bailly S. Bone morphogenetic protein-9 is a circulating vascular quiescence factor. Circ Res. 2008;102:914–922. doi: 10.1161/CIRCRESAHA.107.165530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shao ES, Lin L, Yao Y, Bostrom KI. Expression of vascular endothelial growth factor is coordinately regulated by the activin-like kinase receptors 1 and 5 in endothelial cells. Blood. 2009;114:2197–2206. doi: 10.1182/blood-2009-01-199166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Umulis D, O'Connor MB, Blair SS. The extracellular regulation of bone morphogenetic protein signaling. Development. 2009;136:3715–3728. doi: 10.1242/dev.031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hudkins KL, Le QC, Segerer S, Johnson RJ, Davis CL, Giachelli CM, Alpers CE. Osteopontin expression in human cyclosporine toxicity. Kidney Int. 2001;60:635–640. doi: 10.1046/j.1523-1755.2001.060002635.x. [DOI] [PubMed] [Google Scholar]
- 22.Abeywardena MY, Leifert WR, Warnes KE, Varghese JN, Head RJ. Cardiovascular biology of interleukin-6. Curr Pharm Des. 2009;15:1809–1821. doi: 10.2174/138161209788186290. [DOI] [PubMed] [Google Scholar]
- 23.Yao Y, Watson AD, Ji S, Bostrom KI. Heat Shock Protein 70 Enhances Vascular Bone Morphogenetic Protein-4 Signaling by Binding Matrix Gla Protein. Circ Res. 2009 doi: 10.1161/CIRCRESAHA.109.202333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao GQ. Consequences of knocking out BMP signaling in the mouse. Genesis. 2003;35:43–56. doi: 10.1002/gene.10167. [DOI] [PubMed] [Google Scholar]
- 25.Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, Einhorn T, Tabin CJ, Rosen V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006;38:1424–1429. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- 26.Corriere MA, Rogers CM, Eliason JL, Faulk J, Kume T, Hogan BL, Guzman RJ. Endothelial Bmp4 is induced during arterial remodeling: effects on smooth muscle cell migration and proliferation. J Surg Res. 2008;145:142–149. doi: 10.1016/j.jss.2007.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, Wrenn SP, Narula J. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2005;25:2054–2061. doi: 10.1161/01.ATV.0000178991.71605.18. [DOI] [PubMed] [Google Scholar]
- 28.Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5:623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 30.Canfield AE, Farrington C, Dziobon MD, Boot-Handford RP, Heagerty AM, Kumar SN, Roberts IS. The involvement of matrix glycoproteins in vascular calcification and fibrosis: an immunohistochemical study. J Pathol. 2002;196:228–234. doi: 10.1002/path.1020. [DOI] [PubMed] [Google Scholar]
- 31.Engelse MA, Neele JM, Bronckers AL, Pannekoek H, de Vries CJ. Vascular calcification: expression patterns of the osteoblast-specific gene core binding factor alpha-1 and the protective factor matrix gla protein in human atherogenesis. Cardiovasc Res. 2001;52:281–289. doi: 10.1016/s0008-6363(01)00375-3. [DOI] [PubMed] [Google Scholar]
- 32.Shanahan CM, Cary NR, Metcalfe JC, Weissberg PL. High expression of genes for calcification-regulating proteins in human atherosclerotic plaques. J Clin Invest. 1994;93:2393–2402. doi: 10.1172/JCI117246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murshed M, Schinke T, McKee MD, Karsenty G. Extracellular matrix mineralization is regulated locally; different roles of two gla-containing proteins. J Cell Biol. 2004;165:625–630. doi: 10.1083/jcb.200402046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hruska KA, Mathew S, Saab G. Bone morphogenetic proteins in vascular calcification. Circ Res. 2005;97:105–114. doi: 10.1161/01.RES.00000175571.53833.6c. [DOI] [PubMed] [Google Scholar]
- 35.Nishimoto SK, Nishimoto M. Matrix Gla protein C-terminal region binds to vitronectin. Co-localization suggests binding occurs during tissue development. Matrix Biol. 2005;24:353–361. doi: 10.1016/j.matbio.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Wallin R, Cain D, Sane DC. Matrix Gla protein synthesis and gamma-carboxylation in the aortic vessel wall and proliferating vascular smooth muscle cells--a cell system which resembles the system in bone cells. Thromb Haemost. 1999;82:1764–1767. [PubMed] [Google Scholar]
- 37.Price PA, Faus SA, Williamson MK. Warfarin causes rapid calcification of the elastic lamellae in rat arteries and heart valves. Arterioscler Thromb Vasc Biol. 1998;18:1400–1407. doi: 10.1161/01.atv.18.9.1400. [DOI] [PubMed] [Google Scholar]
- 38.Schurgers LJ, Spronk HM, Skepper JN, Hackeng TM, Shanahan CM, Vermeer C, Weissberg PL, Proudfoot D. Post-translational modifications regulate matrix Gla protein function: importance for inhibition of vascular smooth muscle cell calcification. J Thromb Haemost. 2007;5:2503–2511. doi: 10.1111/j.1538-7836.2007.02758.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.