Abstract
STATEMENT OF PROBLEM
Macroscopic and especially microscopic properties of implant surfaces play a major role in the osseous healing of dental implants. Dental implants with modified surfaces have shown stronger osseointegration than implants which are only turned (machined). Advanced surface modification techniques such as anodic oxidation and Ca-P application have been developed to achieve faster and stronger bonding between the host bone and the implant.
PURPOSE
The purpose of this study was to investigate the effect of surface treatment of titanium dental implant on implant stability after insertion using the rabbit tibia model.
MATERIAL AND METHODS
Three test groups were prepared: sandblasted, large-grit and acid-etched (SLA) implants, anodic oxidized implants, and anodized implants with Ca-P immersion. The turned implants served as control. Twenty rabbits received 80 implants in the tibia. Resonance frequencies were measured at the time of implant insertion, 2 weeks and 4 weeks of healing. Removal torque values (RTV) were measured 2 and 4 weeks after insertion.
RESULTS
The implant stability quotient (ISQ) values of implants for resonance frequency analysis (RFA) increased significantly (P < .05) during 2 weeks of healing period although there were no significant differences among the test and control groups (P > .05). The test and control implants also showed significantly higher ISQ values during 4 weeks of healing period (P < .05). No significant differences, however, were found among all the groups. All the groups showed no significant differences in ISQ values between 2 and 4 weeks after implant insertion (P > .05). The SLA, anodized and Ca-P immersed implants showed higher RTVs at 2 and 4 weeks of healing than the machined one (P < .05). However, there was no significant difference among the experimental groups.
CONCLUSION
The surface-modified implants appear to provide superior implant stability to the turned one. Under the limitation of this study, however, we suggest that neither anodic oxidation nor Ca-P immersion techniques have any advantage over the conventional SLA technique with respect to implant stability.
Keywords: surface treatment, bone to implant contact, removal torque, dental implant
INTRODUCTION
Macroscopic and especially microscopic properties of implant surfaces play a major role in the osseous healing of dental implants. The osseous healing characteristics of various surface structures have been investigated in several experimental and clinical studies.1,2 The surface morphology of dental implants has received increasing attention in recent years.
The development of different dental implants materials, designs, and treatment techniques have not always led to the results expected or desired. Additional studies should be provided about the optimal situation of the connection between an artificial material and the tissues: what type of material gives the best tissue response and what type of surface is preferred by the bone cells or the cells in the soft tissue.
Surface modification methods such as blasting, anodic oxidation, coating, etc. change not only the topography but also the chemistry of the implant surface.3 Dental implants with modified surfaces have shown stronger osseointegration than implants which are only turned (machined). Advanced surface modification techniques such as anodic oxidation 2 and Ca-P application4 have been developed to achieve faster and stronger bonding between the host bone and the implant. They showed that all the modified groups(CMP coated, anodized, blast surface) showed significantly higher bone to implant contact and higher ISQ values than the machined group although there were no significant differences among the groups in removal torque values.
Histomorphometric and removal torque measurements are two representative tests in studying the nature of the implant-tissue interface.5 However, both of them had a disadvantage of being destructive.5 On the other hand, with resonance frequency analysis (RFA), it is now possible to measure the degree of implant stability at any time during the course of implant treatment and loading.6 ISQ values as a parameter to assess the tested implants was noninvasive. It was reported to be a reliable and accurate method for early assessment of the implant stability that is related to the bone implant interface. Using RFA, osseointegration can be periodically evaluated without sacrificing the objects in the animal study.5,7,8
In this study the rabbit tibia model was used and focused on the biomechanical reaction influenced by modified implant surface. This in vivo study was attempted to find substantial differences in various surface modification methods that are applied to titanium dental implants in Korea.
The purpose of this study was to investigate the effect of surface treatment of titanium dental implant on implant stability after implantation using the rabbit tibia model.
MATERIAL AND METHODS
1. Implant Preparation
Eighty machined (screw-shaped) implants (3.5 mm in diameter, 8.0 mm in length) (Dentium Implant, Suwon, Korea) were made of commercially pure (Grade IV) titanium. The implants were divided into control group (machined implants) and 3 experimental groups. Each group comprised twenty implants.
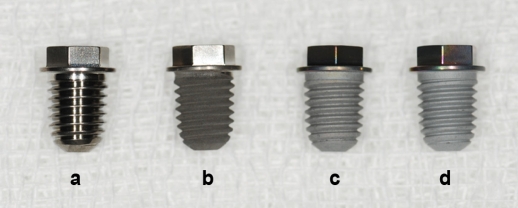
The test groups were designated sandblasted, large-grit and acid-etched (SLA) implants, anodic oxidized implants, and anodized implants with Ca-P immersion : according to surface treatment methods (Fig. 1).
Fig. 1.
Root form screw shaped implants with a. machined surface, b. SLA surface, c. Ca-P surface, and d. anodized surface.
2. Surface Modification (Dentium Implant, Suwon, Korea)
Machined group: a smooth, turned surface used as control.
SLA group: S.L.A (Sand-blasted with large grit acid-etched) Ti specimens (Grade IV) were treated by sandblasting using alumina particle (50 - 100 µm) and washed in an ultrasonic cleaner and dried. The sandblasted Ti specimens further etched by a warm hydrochloric acid, and rinsed and cleaned by ultrasonic cleaner and dried.
Anodized group: Anodizing of the specimen was carried out in an aqueous electrolyte, by applying a pulsed DC field to the specimen. The frequency and duty of the pulsed DC power were 660 Hz and 10%, respectively. The electrolyte was prepared by dissolving 0.15 mol calcium acetate monohydrate {Ca(CH3COO)2·H2O} and 0.02 mol calcium glycerophosphate (CaC3H7O6P) in de-ionized water. To obtain oxide layers, 270 volt were applied to the specimens with each treatment lasting 3 minutes. All of the anodizing processing was carried out in a water-cooled bath made of stainless steel, and a Ti plate was used as the counter electrode.
Ca-P group: Calcium phosphate thin film (- 300 nm) was deposited on the anodized Ti by the electron-beam deposition system, and followed by heat treatment at 350℃ for 1 hour. Evaporants of calcium phosphate were prepared by sintering the mixed powder of hydroxyapatite (Alfa Aesar, Johnson Matthey, London, England) and calcium oxide (Sigma-Aldrich, USA) at 1000℃ for 2 h.
The implant surfaces were modified with three different techniques by Dentium® implant Co. (Suwon, Korea). One was sandblasted with large grit and acid-etched (SLA) surface, which is released in the market. The other surfaces are under development.
3. Animals and surgical procedure
This study was approved by the Animal Research Committee of Seoul National University and all experiments were done in accordance with the Institute of Laboratory Animal Resources guidelines of Seoul National University, Seoul, Korea. Twenty New Zealand white mature male rabbits, weighing 2.5 - 3.5 kg, were used in this study. Prior to surgery, the shaved skin in the proximal tibia area was washed with betadine and a preoperative antibiotic, 0.12 g kanamycin IM, was administered prophylactically. The lidocaine (Yu-han Co., Seoul, Korea, 1:100,000 epi.) was injected locally into surgical sites. The anesthetic procedures, preparation of the surgery site, and drilling for implant insertion were performed as described in previous studies.9-11 Each rabbit received four implants, two in each tibia. The position (proximal vs. distal and right vs. left) for each of the implants was randomly assigned. The implants penetrated the first cortical layer only. The periosteum and fascia were sutured with chromic gut, and the skin was sutured with silk. Each rabbit recovered without complications and received 0.06 g kanamycin IM per day for 3 days postoperatively. One rabbit died after 3 days of insertion and another rabbit was inserted next schedule. Following 2 and 4 weeks of healing, the rabbits were anesthetized and sacrificed with an intravenous administration of KCl. The healing periods of 4 weeks was chosen to investigate a fairly early healing phage, and 2 weeks to investigate an immediate healing phage.
4. Resonance frequency analysis
Twenty rabbits were used for resonance frequency analysis (RFA) in order to measure changes in implant stability during healing period. For RFA, the implants were measured at the time of insertion and removal. The method was previously described by Meredith et al.5 yield quantitative data on the bone-implant interface stiffness. The top of the implant was exposed after the cover screw was removed. Then, implant stability quotient (ISQ) values were measured by Osstell™ (Integration Diagnostics AB, Sweden). Because different ISQ values could obtain in different directions, transducer was positioned in the same direction. Insertion values were compared to the values at sacrifice, each implant site separately.
5. Removal torque test
For removal torque measurement fourteen rabbits (seven at 2 and 4 weeks of healing, respectively) were used. Full thickness mucoperiosteal flaps were reflected to expose the underlying implants. The implanted sites were clinically examined. The cover screw was removed and an implant mount was securely engaged to the implant. The jaws of the RT tester (Model BTG60CN, Tohnichi Corporation, Tokyo, JAPAN) firmly grabbed the mount (Fig. 2) and then reverse torque was applied. Measurements of peak torque to initiate reverse rotation were recorded and mean torque values were calculated for each implant surface.
Fig. 2.
A photograph of the removal torque tester holding the sample. The mount is connected to the implant and the jaws of the RT tester firmly grab the mount.
6. Statistics
Repeated One-way analysis of variance (ANOVA) was used for statistical analysis of the RFA and for post hoc comparison, Duncan's test was executed. For removal torque tests, One-way ANOVA and Kruskal-Wallis test was executed.
RESULTS
1. Resonance frequency analysis (RFA)
RFA is a non-invasive test method designed to make quantitative measurements of the implant stability as a function of the bone/implant interface as described by Meredith.7 A mean value was calculated from the measurements performed parallel to the long axis of the rabbit femur. The mean resonance frequency values for the four investigates implant surface modifications are summarized in Fig. 4 and Fig. 5.
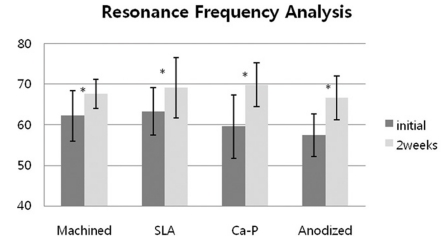
Fig. 4.
Resonance Frequency Analysis of implants after 2 weeks healing periods. *: statistically significant (P < .05).
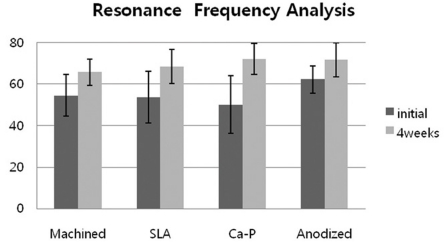
Fig. 5.
Resonance Frequency Analysis of implants after 4 weeks healing periods. *: statistically significant (P < .05).
The mean ISQ values at the time of first surgery were 62.2 for machined surface, 63.3 for SLA, 59.6 for Ca-P, and 57.4 for anodized group. The mean ISQ values at the time of 2 weeks of healing were 67.6 for machined surface, 69.1 for SLA, 69.8 for Ca-P and 66.7 for anodized. The mean ISQ values were increased significantly at 2 weeks of healing periods (P < .05). However, there were no significant differences between the test groups and the control group (P > .05).
The mean ISQ values at the time of 4 weeks of healing periods were 65.8 for machined surface, 68.4 for SLA, 72.1 for Ca-P and 71.7 for anodized group. The test and control implants also showed significant ISQ values at 4 weeks of healing (P < .05). No significant differences, however, were found among all the groups.
All the groups found no significant differences in ISQ values between 2 and 4 weeks after implant insertion (P > .05).
2. Removal torque test
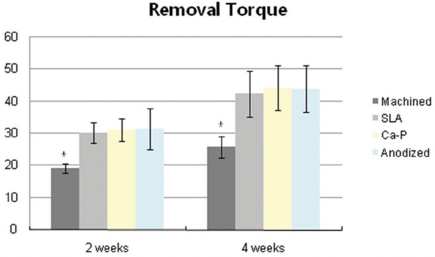
In removal torque testing, all the implants were stable and anchored by bone after 2 and 4 weeks of healing periods. The mean resistance to removal torque for the Ca-P group at second and fourth weeks was 31.2 and 44.1 Ncm, respectively. The mean removal torque values for the anodized group were 31.4 Ncm at 2 weeks and 44.0 Ncm at 4 weeks. For the SLA group, the mean removal torque value increased from 30.0 to 42.5 Ncm. The mean removal torque values for machined group were 19.3 and 25.8 Ncm respectively. The Fig. 6 shows the means and standard deviations of the tested groups.
Fig. 6.
Removal torque values (Ncm) of implants after 2 weeks and 4 weeks healing periods. *: statistically significant (P < .05).
The removal torque values of experimental groups showed more significant difference than in control group (P < .05) at the second and the fourth weeks after insertion. There was no significant difference in RTV among the experimental groups although the anodized group showed the highest value.
In this study, at the macroscopic level, the fracture after removal torque test most likely occurred at the interface between the implant surface and the bone. Another finding was that more bone was found in the outfolded area than inside the threads, independent of the surface modification method.
DISCUSSION
Bone response was evaluated using removal torque measurement and resonance frequency analysis.
Meredith12 demonstrated that ISQ values of implants with higher initial ISQ values can reach a plateau in shorter healing periods than with lower initial ISQ values. They concluded that such a method can serve as a useful research technique and may prove to be valuable in studying the behavior of implants in surrounding bone.12
In addition, clinical observations indicated that the final healing time was affected by individual differences and operation conditions. During the process of osseointegration, the increasing rate of the ISQ values of about 300 Hz per week. Also, when the surgery was not successful, the ISQ value showed a 12% reduction during the first 2 weeks of healing.8
Experimental implants had a significantly higher ISQ values at 2 weeks after insertion but there were no statistical significantly differences among each group. There were no statistical significant differences of the ISQ values at 4 weeks of healing among experimental groups. However, in Duncan analysis considering P = 0.059, Ca-P group showed higher ISQ values, and was interpreted as more stable compare to SLA group and anodized group respectively.
All the groups found no significant differences in ISQ values between 2 and 4 weeks after implant insertion (P > .05). Such surface-modified implants showed increased or maintained ISQ values during the initial healing period, which was interpreted as more rapid and favorable bone reaction for early loading.
In this study, ISQ values were overpassed to our expectations that we had much difficulty in analyzing the results. In general, the increasing values after 2 and 4 weeks of healing period were noticed. In addition, tested groups had increased ISQ values compare to machined group. If the discrepancy were bigger than individual different effect to results, the statistics showed the differences among the groups.
Removal torque forces have been used as a biomechanical measure of anchorage or osseointegration in which the greater forces required to remove implants may be interpreted as an increase in the strength of osseointegration.
Consequently, removal torque testing might not be the best test for the evaluation of implant fixation or the amount of bone around the implant. The underlying biomechanical phenomena in torque testing are very complex. Most of all, the shear stress condition at the interface is important. Removal torque measurements are invasive biomechanical tests that provide information on the rigidity of the implant in the bone marrow. As removal torque testing measures the shear forces of the interface between bone and implant, the results do not always show a direct relation with bone response or surface roughness. Thus, it is also necessary to measure bone to implant contact. Measurements of removal torque and percentage of bone implant contact require destruction of the study specimens; therefore, it is impossible to make direct inference of a possible threshold value to clinical success and survival rates.
At 2 weeks after insertion, the removal torque values of experimental groups showed significant difference compare to control group (P < .05). Anodized group showed the highest removal torque value, but was not significantly different compare to others. At 4 weeks, there was no significant difference (P = 0.060) in One-way ANOVA analysis, but there were significant differences between experimental and control groups in Kruscal-Wallis test. No significant difference was found among experimental groups. Maybe the difference was so little that it was a definitive factor for each introduced method.
The removal torque values of the experimental groups increased to 50% at 2 weeks, compared with machined group at 4 weeks. We inferred that surface treatment affected osteoblast reaction and the osseointegration speed.
The higher removal torque value in the experimental implants with rough surface character is in accordance with the results of the previous studies.
Like in this study, the chemically etched implant surface conferred 4× greater resistance to reverse torque rotation(in this study, 2×) as compared to the machined implant surface 2 months post-surgery in the rabbit femur.13
Ivanoff et al. reported that the removal torque was closely related with the bone-implant contact and amount of bone inside the threads.14
Greater torque rotation forces required to remove implants may be interpreted as an increase in the bone-implant contact leading to higher strength of osseointegration.
Sennerby et al.15 found no difference in removal torque values for screw-shaped implants inserted in rabbit tibiae for 6 weeks, 3 months, and 6 months; in cancellous bone (femoral intra-articular implants), there was no difference between removal torque values at 3 and 6 months.
The results in this study support the idea that modified surface has a positive effect on osseointegration and early loading of the implant. Other study about removal torque using the rabbit tibia showed the similar results.8,16
In this study, a conventional device as hand-controlled torque may introduce an operator error. And the implants had cutting edges for self-tapping and sometimes cortical bone grew to this cutting edge. Removal torque value can be very high in this situation. Much attention was paid to achieve monocortical fixation into the rabbit tibiae.
Removal torque testing might not be the best test for the evaluation of implant fixation or the amount of bone around the implant.
The bone response was measured with the removal torque test to determine the ability of the surface properties of surface treated implants to influence bone response.
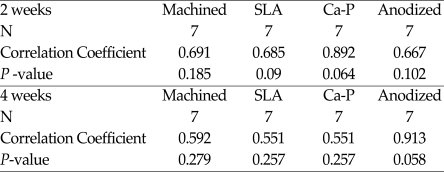
In this study, between ISQ value and removal torque value, there was not constant ratio, but relative correlationships. Spearman's analysis was used for statistical analysis on correlationship (Table I). ISQ values were relative related to removal torque value in SLA, anodized groups. However, there were not significant differences among them (P > .05).
Table 1.
Nonparametic Correlations on ISQ values and removal torque values
If, Correlatation coefficient > 0.5, A is related to B, The mean difference is significant at the 0.05 level.
According to previous reports, the anodized surface showed higher removal torque values than blasted surface, in contrast to this study.17,18
Anodized surface may have various properties according to manufacturing methods. They had the thick oxide layers formed by anodization process. That process could change the oxide thickness of titanium implants and make the biological effect. The surface changes from an amorphous metal surface with a noncrystalline oxide to a polycrystalline metal surface with a crystalline oxide layer. The surface is heterogenous with mainly smooth area of thick oxide but separated with porous regions on a nanometer level.19
Heterogeneity of the data can be caused by variation in the in vivo animal study as well as implant location. For example, local bone conditions vary significantly between various animals. This will have a very serious effect on the results of implant bone response studies.
We concluded that surface modifications of SLA, Ca-P and anodized surface showed faster osseointegration and bone healing than machined surface. Under the limitation of this study, however, we suggest that neither anodic oxidation nor Ca-P immersion techniques have any advantage over the conventional SLA technique with respect to implant stability.
Fig. 3.
A photograph of the Ostell tester holding the sample.
References
- 1.Weinlaender M, Kenney EB, Lekovic V, Beumer J, 3rd, Moy PK, Lewis S. Histomorphometry of bone apposition around three types of endosseous dental implants. Int J Oral Maxillofac Implants. 1992;7:491–496. [PubMed] [Google Scholar]
- 2.Sul YT, Johansson CB, Petronis S, Krozer A, Jeong Y, Wennerberg A, Albrektsson T. Characteristics of the surface oxides on turned and electrochemically oxidized pure titanium implants up to dielectric breakdown: the oxide thickness, micropore configurations, surface roughness, crystal structure and chemical composition. Biomaterials. 2002;23:491–501. doi: 10.1016/s0142-9612(01)00131-4. [DOI] [PubMed] [Google Scholar]
- 3.Ellingsen JE. Surface configurations of dental implants. Periodontol 2000. 1998;17:36–46. doi: 10.1111/j.1600-0757.1998.tb00121.x. [DOI] [PubMed] [Google Scholar]
- 4.You C, Yeo IS, Kim MD, Eom TK. Characterization and in vivo evaluation of calcium phosphate coated cp-titanium by dip-spin method. Curr Appl Phys. 2005;5:501–506. [Google Scholar]
- 5.Meredith N. Assessment of implant stability as a prognostic determinant. Int J Prosthodont. 1998;11:491–501. [PubMed] [Google Scholar]
- 6.Meredith N, Alleyne D, Cawley P. Quantitative determination of the stability of the implant-tissue interface using resonance frequency analysis. Clin Oral Implants Res. 1996;7:261–267. doi: 10.1034/j.1600-0501.1996.070308.x. [DOI] [PubMed] [Google Scholar]
- 7.Meredith N. On the clinical measurement of implant stability, osseointegration, PhD thesis. Sweden: Department of Handicap Research, Goteborg University; 1997. [Google Scholar]
- 8.Huang HM, Chiu CL, Yeh CY, Lin CT, Lin LH, Lee SY. Early detection of implant healing process using resonance frequency analysis. Clin Oral Implants Res. 2003;14:437–443. doi: 10.1034/j.1600-0501.2003.00818.x. [DOI] [PubMed] [Google Scholar]
- 9.Kim YH, Koak JY, Chang IT, Wennerberg A, Heo SJ. A histomorphometric analysis of the effects of various surface treatment methods on osseointegration. Int J Oral Maxillofac Implants. 2003;18:349–356. [PubMed] [Google Scholar]
- 10.You C, Yeo IS, Kim MD, Eom TK. Characterization and in vivo evaluation of calcium phosphate coated cp-titanium by dip-spin method. Curr Appl Phys. 2005;5:501–506. [Google Scholar]
- 11.Yeo IS, Han JS, Yang JH. Biomechanical and Histomorphometric Study of Dental Implants with different surface characteristics. J Biomed Mater Res B Appl Biomater. 2008;87:303–311. doi: 10.1002/jbm.b.31104. [DOI] [PubMed] [Google Scholar]
- 12.Meredith N, Shagaldi F, Alleyne D, Sennerby L, Cawley P. The application of resonance frequency measurement to study the stability of titanium implants during healing in the rabbit tibia. Clin Oral Implants Res. 1997;8:234–243. doi: 10.1034/j.1600-0501.1997.080310.x. [DOI] [PubMed] [Google Scholar]
- 13.Zhu X, Ong JL, Kim S, Kim K. Surface characteristics and structure of anodic oxide films containing Ca and P on a titanium implant material. J Biomed Mater Res. 2002;60:333–338. doi: 10.1002/jbm.10105. [DOI] [PubMed] [Google Scholar]
- 14.Ivanoff CJ, Sennerby L, Lekohm U. Influence of mono and bicortical anchorage on the titanium implants. A study in rabbit tibia. Int J Oral Maxillofac Surg. 1996;25:229–235. doi: 10.1016/s0901-5027(96)80036-1. [DOI] [PubMed] [Google Scholar]
- 15.Sennerby L, Thomsen P, Ericsson LE. A histomorphometric and biomechanical comparison of titanium implants inserted in rabbit cortical and cancellous bone. Int J Oral Maxillofac Implants. 1992;7:62–71. [PubMed] [Google Scholar]
- 16.Oh SH, Lee DJ, Jeong JH, Kim YK. Cytotoxicity of calcium metaphosphate coated on cp-titanium. Key Eng Mater. 2002;218-220:256–258. [Google Scholar]
- 17.Choi JW, Heo SJ, Koak JY, Kim SK, Lim YJ, Kim SH, Lee JB. Bone responses of anodized titanium implants under different current voltages. J Oral Rehabil. 2006;33:889–897. doi: 10.1111/j.1365-2842.2006.01669.x. [DOI] [PubMed] [Google Scholar]
- 18.Larsson C, Thomsen P, Aronsson BO, Rodahl M, Lausmaa J, Kasemo B, Ericson LE. Bone response to surface-modified titanium implants: Studies on the early tissue response to machined and electropolished implants with different oxide thicknesses. Biomaterials. 1996;17:605–616. doi: 10.1016/0142-9612(96)88711-4. [DOI] [PubMed] [Google Scholar]
- 19.Shalabi MM, Gortemaker A, Van't Hof MA, Jansen JA, Creugers NH. Implant surface roughness and bone healing: a systematic review. J Dent Res. 2006;85:496–500. doi: 10.1177/154405910608500603. [DOI] [PubMed] [Google Scholar]
- 20.Wennerberg A, Ohlsson R, Rosen BG, Andersson B. Characterizing three-dimensional topography of engineering and biomaterial surfaces by confocal laser scanning and stylus techniques. Med Eng Phys. 1996;18:548–556. doi: 10.1016/1350-4533(95)00005-4. [DOI] [PubMed] [Google Scholar]