Abstract
STATEMENT OF PROBLEM
When veneering composite resin-metal restoration is prepared, the fact that bond strength between Ti and composite resin is relatively weak should be considered.
PURPOSE
The purpose of this study is to evaluate the shear bond strength between the veneering composite resin and commercial pure (CP) Ti / Ti-6Al-4V alloy according to the method of surface treatment.
MATERIAL AND METHODS
The disks were cast by two types of metal. Their surfaces were treated by sandblasting, metal conditioner, TiN coating and silicoating respectively. After surface treatment, the disks were veneered by composite resin (Tescera™, Bisco, USA) which is 5 mm in diameter and 3 mm in thickness. The specimens were stored in water at 25℃ for 24 hours, and then evaluated for their shear bond strength by universal testing machine (STM-5®, United Calibration, USA). These values were statistically analyzed.
RESULTS
1. All methods of surface treatment were used in this study satisfied the requirements of ISO 10477 which is the standard of polymer-based crown and bridge materials. 2. The metal conditioner treated group showed the highest value in shear bond strength of CP Ti, silicoated group, TiN coated group, sandblasted group, in following order. 3. The silicoated group showed the highest value in shear bond strength of Ti-6Al-4V alloy, metal conditioner treated group, sandblasted group, TiN coated group, in following order.
CONCLUSION
Within the limitations of this study, all methods of surface treatment used in this study are clinically available.
Keywords: Shear bond strength, Surface treatment
INTRODUCTION
The interest of patients in better esthetics has increased over the years. This esthetical demand from patients is achieved in a number of instances by veneering the metal base with the esthetic materials such as a resin or porcelain.
Porcelain has excellent abilities of appearance, strength and wear resistance or holds little amount of bacterial plaques. However, it has some disadvantages. Porcelain, that is more brittle and harder than natural enamel, usually wears the opposing natural tooth. Moreover, the manipulation of porcelain takes time and needs skillful technique.
For the features such as abrasion similar to natural tooth structure, biocompatibility and repairablity resin was developed as a solution. Dentists encountered many problems with the composite resin, such as poor esthetics, discoloration, microleakage and relatively low bond strength to metal surface.1-4 However, the demand for resin-veneered crowns, such as those used in the telescope crowns, fixed prostheses or implant protheses, still exists.
Metals used as the dental alloys require sufficient strength that resists the bite force, erosion, and abrasion, and biocompatibility with oral tissue. Recently, because of the high cost of precious metals, the use of non-precious metal alloys has increased. But non-precious metal alloys have some considerably low disadvantages on biocompatibility and bond strength compared with porcelain.5
On the other hand, titanium (Ti) has many advantages as a prosthesis material, including sufficient corrosion resistance, excellent biocompatibility, low density and its suitability for use in patients who have a metal allergy to nickel, cobalt or chromium. The applications of titanium for fixed partial dentures, removable partial dentures and implant prostheses have increased substantially, mainly because of the development of casting and surface treatment techniques.
The strong bonding between titanium and resin plays an important role in the longevity of prosthesis. When titanium is used for a metallic substructure for restoration covered with resin with weak bond strength between layers, microleakage tends to appear on the metal-resin interface leading to failure of prosthodontic prostheses.
Bonding between metalic substructure and composite resin is usually obtained by macro-mechanical retentions such as undercuts, beads, loops, wires, posts and meshes.6-9 However, this process results in bulkier framework, and occurs about 20 µm of gap on the resin-metal interface and leads to discoloration and detachment of resin. To overcome these defects, sandblasting, electrolytic etching technique,10,12 and chemical etching technique6,11,13,14 are used to obtain a micro-roughness on the metals. However, microleakage cannot be solved completely because all the techniques are based on mechanical bonding. Thus chemical bonding is used. Using a metal primer, silicoating,15,16 heat treatment and tin plating are chemical bonding methods and among those, a metal primer and silicoating are clinically preferred.
In dentistry, the use of titanium nitride (TiN) coatings is increasing substantially. The TiN coating improves the natural color of the veneer, the bond between the alloy and composite resin and fabricates biocompatible prostheses.30
Until now, there have been many studies to increase the bonding of titanium and resins. However, surface treatment effect has not been clarified because each study had various bond strengths by various techniques. The purpose of this study is to compare and evaluate the shear bond strength between resin and commercial pure (CP) Ti / Ti-6AL-4V alloy according to the method of surface treatment.
MATERIAL AND METHODS
1. Preparation of cast metal disk
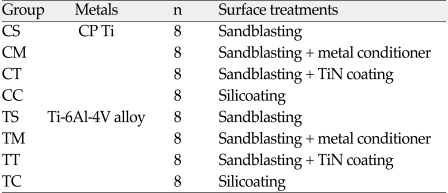
The materials used in this study are summarized in Table I. Disk-shaped acryl patterns (10.0 mm in diameter and 2.5 mm in thickness) of CP Ti Grade II (Kobe Steel Co., Japan), Ti-6Al-4V Grade V alloy (Kobe Steel Co., Japan) were cast using a magnesia-based investment (Selevest CB, Selec Co., Japan) in an argon arc-centrifugal casting machine (Ticast Super R®, Selec Co., Japan) according to the manufacturer's instructions. Thirty two cast disks were made of each alloy.
Table I.
Materials used for this study
All specimens were polished with a Labopol-2® grinding machine (Struers, Denmark) by the process of wet-grinding. The finishing coarse grain was #1000. Then, all specimens were washed ultrasonically with acetone for 10 minutes and ethanol for 10 minutes respectively using ultrasonic cleaner (Ultraschall®, Krupp Co, Germany). The cleaned disks were dried for 24 h.
2. Preparation of specimens for shear testing
The specimens were divided into eight experimental groups consisted of eight specimens for each group according to a surface treatment to CP Ti / Ti-6Al-4V alloy listed in Table II.
Table II.
Experimental groups
We used three surface treatment methods. First method is using sandblasting only, second, metal conditioner treatment after sandblasting, and third, TiN coating and silicoating after sandblasting.
CS, CM, CT, TS, TM and TT groups were blasted by 250 µm grain-sized aluminum oxide for 10 seconds using a grit blaster (Micro sand blaster®, Phoenix electric Co, Korea). The emission pressure was 2.8 kgf/cm2 with the nozzle positioned approximately 5 mm from the surface of the metal. The grit-blasted cast disks were washed ultrasonically with acetone for 10 minutes and ethanol for 10 minutes respectively using ultrasonic cleaner (Ultraschall®, Krupp Co, Germany). CS and TS groups were left uncoated while CM and TM groups were coated with metal conditioner (bonding agent) and CT and TT groups were coated with TiN. ONE-STEP® (Bisco, Schaumbrug IL, USA) as a bonding agent was used. Rocatec™ system (3M ESPE AG, Germany) as method for silicoating was used. CC and TC groups were blast-cleaned (10 seconds) with 110 µm Al203 corundum (Rocatec Pre, 3M ESPE AG, Germany) at a pressure 2.8 kgf/cm2. In the second step, the surface was tribochemically coated using Rocatec Plus (3M ESPE AG, Germany) at a pressure of 2.8 kgf/cm2 for 13 seconds. And then, silane coupling agent (3M™ ESPE™ Sil; 3M ESPE AG, Germany) was applied and then allowed to dry for 5 minutes.
3. Arc Ion Plating coating
Arc Ion Plating coating (AIP) of TiN on the cleaned metal surface was performed by means of multi-purpose coating system (ATS-MC-STD-300®, Atec system, Korea). Before coating on it, the working chamber was evacuated to 5 mm Torr and kept at chamber temperature 300℃ for 2 h. During AIP of TiN, a reactive gas mixture of argon (Ar) and nitrogen (N2) was used for deposition onto the metal plate. The mixture of gas was maintained by infusion of Ar and N2 gas at a ratio of 9:1 (infusion volume; Ar: 27 sccm and N2: 3 sccm) (sccm stands for standard cc/min). Based on the rate and duration of deposition, we estimate 1.7 µm for the thickness of the TiN coating layer, which were produced on the metal plate.
4. Composite resin veneering
As composite resins for veneering, Tescera™ (Bisco, Schaumburg IL, USA) was used in this study. Composite resin system was used to veneer metal according to the manufacture's recommendation.
A thin layer of flowable resin (Flowable Composite®, Bisco, Schaumburg IL, USA) was placed on metal surface and light-cured for 10 seconds with the SmartLite™PS (Dentsply DeTrey GmbH, Germany). After that, an additional layer of flowable resin (Flowable Composite®, Bisco, USA) was placed on metal surface and light-cured for 10s each with the SmartLite™PS (Dentsply DeTrey GmbH, Germany). Then, a plastic ring (5 mm×5 mm×3 mm) was placed on the metal surface. The ring was filled with the body portion of the Tescera™ (Bisco, Schaumburg IL, USA). Resin was light-exposed for 2 minutes and heat-exposed for 20 minutes respectively in the polymerization unit (TESCERA™ ATL™, Bisco, Schaumburg IL, USA).
5. Measurements of shear bond strength
Each specimen was seated in a shear-testing jig. Shear bond strength was determined with a screw-driven universal testing machine (STM-5®, United Calibration, USA) at a crosshead speed of 1.0 mm/min. The schematic diagram and testing machine is shown on the Fig. 1. and 2. The amounts of the shear bond strength were calculated according to the formula:
Fig. 1.
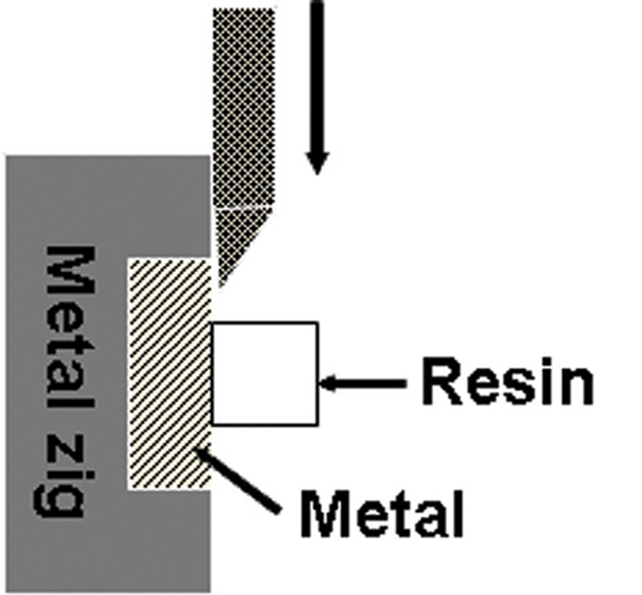
Schematic diagram of test design.
Fig. 2.
Universal testing machine (STM-5®, United Calibration, USA).
B = F / S
B: Shear bond strength (MPa), F: load at fracture (N), and S: bonded surface area (mm2)
6. Examination of interface
The observation of surface-treated metals was performed with the use of an optical microscope (BX51 TRF®, Olympus, Japan). After shear bond strength test, the fracture surfaces of the specimens were observed using an optical microscope at ×200 magnification to assess the types of bond failure.
These analyses were used to assess the mechanisms of failure as well as the nature of the interface between the surface treatment and a veneering composite resin.
7. Statistical analysis
The means and standard deviations (SD) of the shear bond strength (n = 8) were calculated and statistically analyzed with a one-way analysis of variance (ANOVA) (P < .05).
RESULTS
1. Testing of shear bond strength
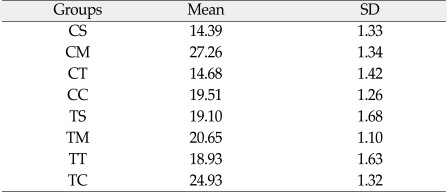
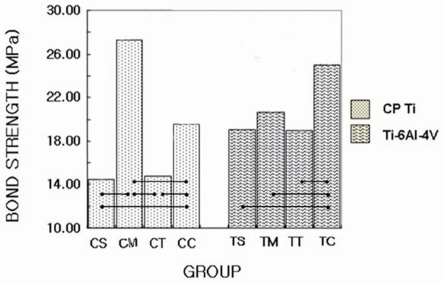
The values of the mean shear bond strengths and standard deviations for each experimental group are shown in Table III and Fig. 3.
Table III.
Mean shear bond strengths (MPa) and standard deviations (SD) of composite veneering resin to CP Ti and Ti-6Al-4V alloy
Fig. 3.
Graphic representation of bond strength values of all groups.
Tested surface treatment passed the requirements of ISO 10477.17 The requirements noted on ISO 10477, the standard of polymer-based crown and bridge materials, are that shear bond strength should be more than 5 MPa.
The mean and standard deviations of shear bond strength of CP Ti ranged from 14.39 MPa (1.33) to 27.26 MPa (1.44). The CM group (27.26 MPa) showed the highest value in shear bond strength of CP Ti, CC group (19.51 MPa), CT group (14.68 MPa), CS group (14.39 MPa), in the following order. CS and CT group were not significantly different statistically (P > .05). There were statistically significant differences between the remaining groups.
The mean and standard deviations of shear bond strength of Ti-6Al-4V alloy ranged from 18.93 MPa (1.63) to 24.93 MPa (1.32). The TC group (24.93 MPa) showed the highest value in shear bond strength of Ti-6Al-4V alloy and then TM group (20.65 MPa), TS group (19.10 MPa), TT group (18.93 MPa), in the following order. The TC group was the only group that had statistically significant differences compared with another groups. There were no statistically significant differences (P > .05) among TS group, TM group and TT group.
2. The examination of interface
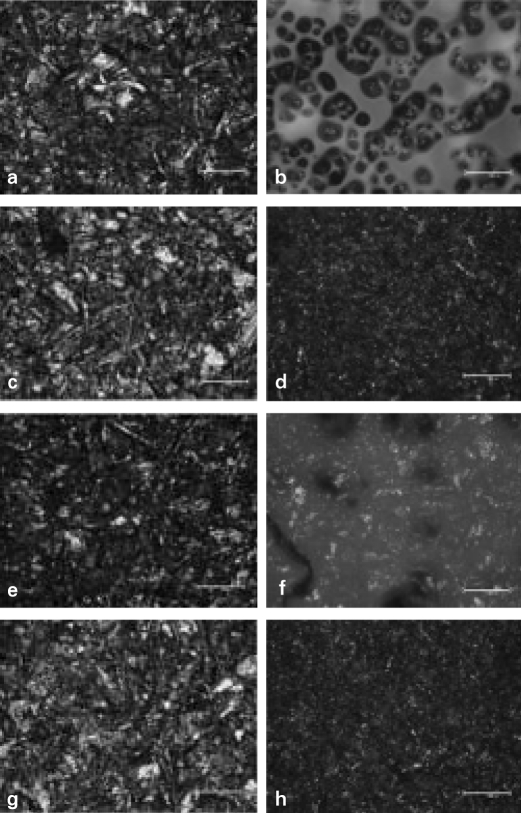
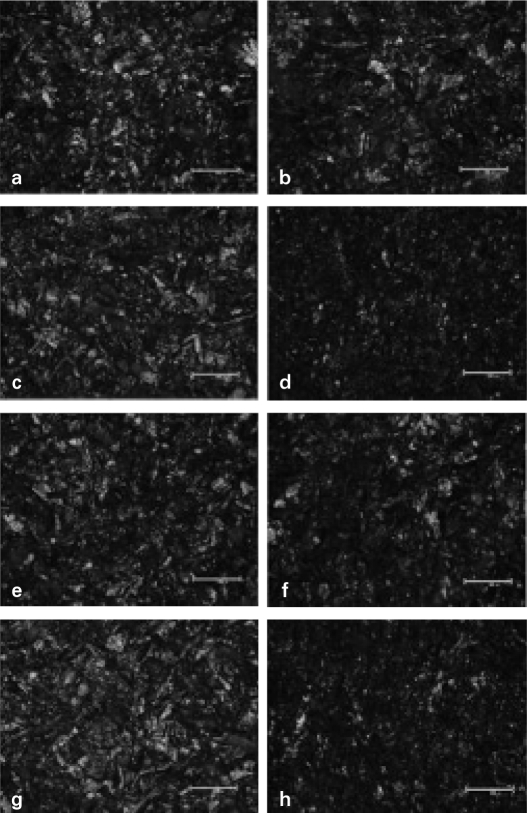
The surface treated specimens were observed using an optical microscope (BX51 TRF®, Olympus, Japan) at × 200 magnification (Fig. 4). Optical microscope demonstrated different topography resulted from the various treatments.
Fig. 4.
Optical microscope photomicrographs of surface treated metal specimens (× 200). a. CS, b. CM, c. CT, d. CC, e. TS, f. TM, g. TT, h. TC. CS, TS, CT and TT group showed irregular aspect with many undercuts. The milky thick membrane was found in CM and TM group. CC and TC group showed less irregular aspect with more undercuts than CS and TS groups and also had milky thin membrane.
Each method of surface treatment has similar characteristics under the optical microscope in CP Ti and Ti-6Al-4V alloy. Surface roughness increased in all specimens. CS, TS, CT and TT group showed irregular aspect which formed many undercuts. There was milky thick membrane in CM and TM group. CC and TC group showed less irregular aspect formed by many undercuts than CS, TS group and had milky thin membrane.
After testing them, the fracture surfaces of the specimens were observed using an optical microscope at ×200 magnification. Adhesive failure was observed in all specimens (Fig. 5). All specimens showed little resin fragment remaining on the metal surface.
Fig. 5.
Optical microscope photomicrographs of metal specimens after shear bond strength test (× 200). a. CS, b. CM, c. CC, d. CR e. TS, f. TM, g. TT, h. TC. Adhesive failure was observed in all specimens. It was observed that some resin fragments were remained on the surface of all specimens.
DISCUSSION
A composite resin is classified into both direct composite resins and indirect composite resins. The first generation of indirect composite resins was developed in the 1980s.18 The first generation of indirect composite resins has higher abrasion resistance than direct composite resins.19 However, they have drawbacks such as low bending strength, elastic modulus, abrasion resistance. These disadvantages resulted in marginal fracture, abrasion, and discoloration.20
To solve these problems, ceramic polymer was introduced as the second generation of composite resins. The ceramic optimized polymer (ceromer) is composed of a large number of ceramic particles.18 Various modes of polymerization (light polymerization, heat and pressure polymerization, argon polymerization, and vacuum polymerization) were used to improve mechanical properties such as compressive strength, bending strength, elastic modulus, abrasion resistance and to decrease polymerization shrinkage.18,21 They are used in veneered crowns (partial or complete veneers), pontics for fixed partial dentures, removable partial dentures and implant prostheses.22,23 Body portion of Tescera™ (Bisco, Schaumbrug IL, USA) which included 72 vol% hybrid filler was used in this study.
Alloys containing metals such as Cr, Co, Mo and Ni may cause local hypersensitivity responses and even systemic problems.24 Ti was used as one of the alternative materials to eliminate these problems. CP Ti and Ti-6Al-4V alloys have similar desirable mechanical and physical properties. Ti-6AL-4V alloys can be manufactured into the cast titanium framework which is stronger than CP Ti. Thus, to compare with CP Ti, Ti-6AL-4V alloy was used in this study.
Both chemical and mechanical bonding techniques have been proposed to avoid a detachment of the ceromer materials from the alloys. Rose et al.2 reported that greater shear bond strengths were created with retentive beads than with the chemical bonding materials. But, all of resin specimens with retentive beads demonstrated gaps at the resin-metal interfaces. On the other hand, there were methods of surface treatment such as sandblasting with aluminium oxide, chemical polishing, wire electrical discharge machining, and anodizing oxidation on CP Ti to increase the bond strength. However these methods were reported with insufficient bonding strength to hold. There also was a research on chemically increasing the Ti affinity using the primer, or increasing Ti bonding using silane.25,26
The aim of this study was to evaluate shear bond strength of a ceromer according to surface treatment of CP Ti and Ti-6Al-4V alloys. CP Ti and Ti-6Al-4V alloys brought various results according to methods of surface treatment.
Masami et al.4 and Tanaka et al.13 reported that the bond strength between composite resins and alloys was clearly improved by applying only sandblasting. May et al.27 reported that the polymethyl methacrylate (PMMA) established a greater shear bond strength to CP Ti pretreated with air abrasive than the untreated one by more than 3.7 times. El-Sherif et al.7 reported that retainer surfaces prepared by air abrading with 250 µm aluminum oxide were superior in retention to those made by the electrochemical etching techniques. In this study, only treatment of sandblasting offered enough bonding strength. The reason may be on using 250 µm grain-sized aluminum oxide for 10 seconds from the distance of approximately 5 mm from the surface of the metal.
Katsuhiko et al.28 reported that metal surface modification by coated TiN significantly improves the bond strength between the Au-Pd-Ag alloy and resin composite material in clinical applications. When they observed surface of treated specimen with the use of an electron probe microanalyser, TiN coating using sputtering technique presented filled micro-gap between metal and veneering composite resin.
TiN coating has often been applied to dental alloys in order to improve the properties of dental alloys, giving the alloys a high degree of hardness, wear resistance, discoloration resistance, esthetics, corrosion resistance and good biocompatibility.29 One of the major characteristics of TiN-coated surface is its low frictional coefficient,30 and this characteristic possibly affects its adhesion to resin materials. However, in this study, TiN coating surface treatment obtained approximately the same shear bond strength value as that of sandblasting surface treatment. There was no significant difference observed between TiN coating group and sandblasting group. For the study, AIP methods were used for TiN coating. It is a method which coats the surface by particle deposition. This differs with conventional sputtering method that forms several coating membranes on the surface. It is assumed that using the AIP method results in bonding strength that was not up to the expectation.
In this study, after polishing metal by wet-grinding, surface roughness was measured using DIAVITE DH-7® (Asmeto AG, Switzerland). Values of RA were approximately 0.27 µm in CP titanium and 0.24 µm in Ti-6Al-4V alloy. After sandblasting and TiN coating the roughness was analyzed and then showed no significant differences between the group: 10.25 µm in CS group, 10.23 µm in CT group, 11.90 µm in TS group, 12.16 µm in TT group. Mean shear bond strength of Ti-6Al-4V alloy is higher than that of CP Ti. The reason may be the difference of roughness. Mean roughness of Ti-6Al-4V alloy is higher than that of CP Ti.
The surface treatment methods obtained the maximum bonding strength according to metal were different. There were significant differences between the groups using a Rocatec™ system (ESPE GmbH, Seefeld/Oberbay, Germany) and the group treated by only the action of sandblasting, but the group using ONE-STEP® (Bisco, Schaumbrug, IL, USA) as a metal conditioner showed highest bond strength on CP Ti.
Izchak et al.6 reported that chemical and mechanochemical bonding techniques displayed the higher shear bond strengths. The mechanical techniques yielded lower shear bond strength values. Chemical bonding minimized gap formation at the composite-metal interface.
On the other hand, Ti-6Al-4V alloys showed no significant differences among the sandblasting group, the metal conditioner group, and the TiN coating group. There were significant differences only between silicoating group and the other groups. The group using Rocatec™ system as silicoating method showed the highest bond strength on Ti-6Al-4V alloys.
Silicoating is a method using ceramic interfacial bonding that chemically combines metal oxide film and 0.5 mm thick Sioxc layer produced on the metallic surface and provides Si-OH or Al-OH group that makes it possible of metal-resin chemical bond. May et al.31 reported that the bond shear values of heat-processed PMMA bonded to CP Ti Grade II with 110 µm alumina air abrasion alone did not increase over untreated specimens. Silicoat silane coating enhanced the shear bond strength of PMMA more than 60% when compared with no pretreatment. Other studies reported that the application of Rocatec™ system (ESPE GmbH, Seefeld/Oberbay, Germany) increased bond shear strength by 68% when the surface was veneered with conventional heat-activated PMMA.32 ONE-STEP® (Bisco, Schaumbrug, IL, USA) used in this study, contains Biphenyl dimethacrylate (BPDM). OLA16 reported that silicoating increased the bond strength for titanium alloy.
All methods of surface treatment were used in this study passed the requirements of ISO 10477 (> 5 MPa) which is the standard of polymer-based crown and bridge materials. The use of metal primer offers the following advantages: (1) simple procedure, (2) non-necessity of a proprietary apparatus and (3) reduced cost performance. On the results of this study, application of metal primer is recommended over silicoating method which requires complicated procedures and expensive appliances.
Only adhesive failure was observed at the metal-resin interface of all specimens. It was observed that some resin fragments still were found on the surface of all specimens. Tescera ATL™ (Bisco, Schaumbrug IL, USA) which was an indirect resin used in this study showed that the bond strength in resin was higher than that of metal-resin interface. According to manufacture's MSDS (Material Safety Data Sheet), shear bond strength of Tescera ATL™ (Bisco, Schaumbrug, IL, USA) is 64 MPa. Further study is needed on the surface treatment method that could result in better metal-resin bonding strength.
CONCLUSION
This study evaluated bond strength between the veneering composite resin and surface treated CP Ti and Ti-6AL-4V alloy.
The results are as follows:
1. All methods of surface treatment were used in this study passed the requirements of ISO 10477 (> 5 MPa) which is the standard of polymer-based crown and bridge materials.
2. The metal conditioner treated group (27.26 MPa) showed the highest value in shear bond strength of CP Ti, silicoated group (19.51 MPa), TiN coated group (14.68 MPa), sandblasted group (14.39 MPa), in following order.
3. The silicoated group (24.93 MPa) showed the highest value in shear bond strength of Ti-6Al-4V alloy, metal conditioner treated group (20.65 MPa), sandblasted group (19.10 MPa), TiN coated group (18.93 MPa), in following order.
Within the limitations of this study, all methods of surface treatment were considered to be clinically available.
References
- 1.Staffanou RS, Hembree JH, Jr, Rivers JA, Myers ML, Kilgore JL. Leakage study of three esthetic veneering materials. J Prosthet Dent. 1985;54:204–206. doi: 10.1016/0022-3913(85)90289-6. [DOI] [PubMed] [Google Scholar]
- 2.Jones RM, Moore BK, Goodacre CJ, Munoz-Viveros CA. Microleakage and shear bond strength of resin and porcelain veneers bonds to cast alloys. J Prosthet Dent. 1991;65:221–228. doi: 10.1016/0022-3913(91)90165-s. [DOI] [PubMed] [Google Scholar]
- 3.Petridis H, Hirayama H, Kugel G, Habib C, Garefis P. Shear bond strength of techniques for bonding esthetic veneers to metal. J Prosthet Dent. 1999;82:608–614. doi: 10.1016/s0022-3913(99)70062-4. [DOI] [PubMed] [Google Scholar]
- 4.Mukai M, Fukui H, Hasegawa J. Relationship between sandblasting and composite resin-alloy bond strength by a silica coating. J Prosthet Dent. 1995;74:151–155. doi: 10.1016/s0022-3913(05)80178-7. [DOI] [PubMed] [Google Scholar]
- 5.Kelly JR, Rose TC. Nonprecious alloys for use in fixed prosthodontics: literature review. J Prosthet Dent. 1983;49:363–370. doi: 10.1016/0022-3913(83)90279-2. [DOI] [PubMed] [Google Scholar]
- 6.Barzilay I, Myers ML, Cooper LB, Graser GN. Mechanical and chemical retention of laboratory cured composite to metal surfaces. J Prosthet Dent. 1988;59:131. doi: 10.1016/0022-3913(88)90001-7. [DOI] [PubMed] [Google Scholar]
- 7.El-Sherif MH, El-Messery A, Halhoul MN. The effecs of alloy surface treatments and resins on the retention of resin bonded retainers. J Prosthet Dent. 1991;65:782. doi: 10.1016/s0022-3913(05)80012-5. [DOI] [PubMed] [Google Scholar]
- 8.Dunny JA, King GE. Minor connector designs for anterior acrylic resin bases: a preliminary study. J Prosthet Dent. 1975;34:496–502. doi: 10.1016/0022-3913(75)90035-9. [DOI] [PubMed] [Google Scholar]
- 9.Brown DT, Desjardins RP, Chao EYS. Fatigue failure in acrylic resin retaining minor connectors. J Prosthet Dent. 1987;58:329–335. doi: 10.1016/0022-3913(87)90051-5. [DOI] [PubMed] [Google Scholar]
- 10.Livaditis GJ. A chemical etching system for creating micromechanical retention in resin bonded retainers. J Prosthet Dent. 1986;56:181–188. doi: 10.1016/0022-3913(86)90468-3. [DOI] [PubMed] [Google Scholar]
- 11.Doukoudakis A, Cohen B, Tsoutsos A. A new chemical method for etching metal frameworks of the acid etched prothesis. J Prosthet Dent. 1987;58:421–423. doi: 10.1016/0022-3913(87)90267-8. [DOI] [PubMed] [Google Scholar]
- 12.Zurasky JE, Duke ES. Improved adhesion of acrylic resins to base metal alloy. J Prosthet Dent. 1987;57:520–524. doi: 10.1016/0022-3913(87)90028-x. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka T, Fujiyama E, Shimizu H, Tanaki A, Atsuta M. Surface treatment of nonprecious alloys for adhension-fixed partial dentures. J Prosthet Dent. 1986;55:456. doi: 10.1016/0022-3913(86)90176-9. [DOI] [PubMed] [Google Scholar]
- 14.Barzilay I, Mayers ML, Cooper LB, Graser GN. Mechanical and chemical retention of laboratory cured composite to metal surfaces. J Prosthet Dent. 1988;59:131–137. doi: 10.1016/0022-3913(88)90001-7. [DOI] [PubMed] [Google Scholar]
- 15.Bahannan SA, Connelly ME, Mueninghoff LA. Application of silica coating technique for removable prosthodontics. A clinical report. J Prosthet Dent. 1991;65:1. doi: 10.1016/0022-3913(91)90037-w. [DOI] [PubMed] [Google Scholar]
- 16.Hansson O. Strength of bond with Comspan Opaque to three silicoated alloys and titanium. Scand J Dent Res. 1990;98:248. doi: 10.1111/j.1600-0722.1990.tb00969.x. [DOI] [PubMed] [Google Scholar]
- 17.ISO 10477. Dentistry - polymer-based crown and bridge materials. 2004(E). [Google Scholar]
- 18.Touati B, Aidan N. Second generation laboratory composite resins for indirect restorations. J Esthet Dent. 1997;9:108–118. doi: 10.1111/j.1708-8240.1997.tb00928.x. [DOI] [PubMed] [Google Scholar]
- 19.Staffanou RS, Hembree JH, Jr, Rivers JA, Myers ML. Abrasion resistance of three types of esthetic veneering materials. J Prosthet Dent. 1985;53:309–310. doi: 10.1016/0022-3913(85)90498-6. [DOI] [PubMed] [Google Scholar]
- 20.Ameye C, Lambrechts P, Vanherle G. Conventional and microfilled composite resins. Part I: color and marginal adaptation. J prosthet Dent. 1981;46:623–630. doi: 10.1016/0022-3913(81)90068-8. [DOI] [PubMed] [Google Scholar]
- 21.Kakaboura A, Rahiotis C, Zinelis S, Al-Dhamadi YA, Silikas N, Watts DC. In vitro characterization of two laboratory-processed resin composites. Dent Mater. 2003;19:393–398. doi: 10.1016/s0109-5641(02)00082-9. [DOI] [PubMed] [Google Scholar]
- 22.Vojvodic D, Jerolimov V, Celebic A, Catovic A. Bond strength of silicoated and acrylic resin bonding system to metal. J Prosthet Dent. 1999;81:1–6. [PubMed] [Google Scholar]
- 23.Yoshida K, Kamada K, Taira Y, Atsuta M. Effect of three adhesive primers on the bond strengths of four light-activated opaque resins to noble alloy. J Oral Rehabil. 2001;28:168–173. doi: 10.1046/j.1365-2842.2001.00662.x. [DOI] [PubMed] [Google Scholar]
- 24.Moffa JP. Biological effects of nickel-containing dental alloys. Council on dental materials, instruments and equiment. J Am Dent Assoc. 1982;104:501–505. doi: 10.14219/jada.archive.1982.0223. [DOI] [PubMed] [Google Scholar]
- 25.Ekstrand K, Ruyter IE, Oysad H. Adhesion to titanium of methacrylate-based polymer materials. Dent Mater. 1988;4:111–115. doi: 10.1016/s0109-5641(88)80002-2. [DOI] [PubMed] [Google Scholar]
- 26.Matsumura H, Yoshida K, Tanaka T, Atsuta M. Adhesive bonding of titanium with a titanate coupler and 4-META/MMA-TBB opaque resin. J Dent Res. 1990;69:1614–1621. doi: 10.1177/00220345900690091601. [DOI] [PubMed] [Google Scholar]
- 27.May KB, Van Putten MC, Bow DA, Lang BR. 4-META polymethyl methacrylate shear bond strength to titanium. Oper Dent. 1997;22:37–40. [PubMed] [Google Scholar]
- 28.Tanaka T, Kimoto K, Sawada T, Toyoda M. Shear bond strength of veneering composite resin to titanium nitride coating alloy deposited by radiofrequency sputtering. J Dent. 2006;34:277–282. doi: 10.1016/j.jdent.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Mezger PR, Creugers NH. Titanium nitride coatings in clinical dentistry. J Dent. 1992;20:342–344. doi: 10.1016/0300-5712(92)90021-4. [DOI] [PubMed] [Google Scholar]
- 30.Steele JG, McCabe JF, Barnes IE. Properties of a titanium nitride coating for dental instruments. J Dent. 1991;19:226–229. doi: 10.1016/0300-5712(91)90123-g. [DOI] [PubMed] [Google Scholar]
- 31.May KB, Russell MM, Razzoog ME, Lang BR. The shear strength of polymethyl methacrylate bonded to titanium partial denture framework material. J Prosthet Dent. 1993;70:410–413. doi: 10.1016/0022-3913(93)90076-z. [DOI] [PubMed] [Google Scholar]
- 32.May KB, Fox J, Razzoog ME, Lang BR. Silane to enhance the bond between polymethyl methacrylate and titanium. J Prosthet Dent. 1995;73:428–431. doi: 10.1016/s0022-3913(05)80070-8. [DOI] [PubMed] [Google Scholar]