Abstract
Functional neuroimaging studies have implicated a number of brain regions, especially the posterior parietal cortex (PPC), as being potentially important for visual–tactile multisensory integration. However, neuroimaging studies are correlational and do not prove the necessity of a region for the behavioral improvements that are the hallmark of multisensory integration. To remedy this knowledge gap, we interrupted activity in the PPC, near the junction of the anterior intraparietal sulcus and the postcentral sulcus, using MRI-guided transcranial magnetic stimulation (TMS) while subjects localized touches delivered to different fingers. As the touches were delivered, subjects viewed a congruent touch video, an incongruent touch video, or no video. Without TMS, a strong effect of multisensory integration was observed, with significantly better behavioral performance for discrimination of congruent multisensory touch than for unisensory touch alone. Incongruent multisensory touch produced a smaller improvement in behavioral performance. TMS of the PPC eliminated the behavioral advantage of both congruent and incongruent multisensory stimuli, reducing performance to unisensory levels. These results demonstrate a causal role for the PPC in visual–tactile multisensory integration. Taken together with converging evidence from other studies, these results support a model in which the PPC contains a map of space around the hand that receives input from both the visual and somatosensory modalities. Activity in this map is likely to be the neural substrate for visual–tactile multisensory integration.
Keywords: hand, intraparietal sulcus, IPS, somatosensory, vision
Introduction
While riding a bicycle, visual information about the road surface combined with tactile information from the handlebars allow us to successfully navigate a slippery route. Multisensory integration is useful because the combination of information from different independent sensory modalities allows for more accurate behavioral decisions. Several studies have shown that vision can enhance touch perception when subjects view the actual hand or arm being touched (Kennett et al., 2001; Maravita et al., 2003; Johnson et al., 2006; Haggard et al., 2007) or just an image of the hand (Tipper et al., 1998; Schaefer et al., 2005; Igarashi et al., 2008).
Human functional neuroimaging studies have implicated a number of brain areas in visual–tactile multisensory integration. Regions of occipital and temporal cortex traditionally classified as unisensory visual cortex also respond to touch (Sathian et al., 1997; Amedi et al., 2001; James et al., 2002; Beauchamp et al., 2007, 2008), whereas regions of anterior and ventral parietal lobe traditionally classified as unisensory somatosensory cortex also respond to visual stimuli, especially videos of touch (Keysers et al., 2004; Blakemore et al., 2005; Schaefer et al., 2005). The posterior parietal cortex (PPC), traditionally classified as association cortex, responds to both visual and tactile stimulation (Bremmer et al., 2001; Saito et al., 2003; Nakashita et al., 2008) and is active during visually-guided grasping (Frey et al., 2005; Culham & Valyear, 2006; Valyear et al., 2007; Filimon et al., 2009).
While neuroimaging studies are invaluable for delineating brain areas involved in a cognitive task, they provide only correlational evidence about whether the brain areas are truly necessary for the task. In order to make inferences about necessity, a brain area must be lesioned and a behavioral deficit demonstrated. Studies of stroke patients have demonstrated that visual stimuli can suppress or enhance the detection of tactile targets (extinction or anti-extinction, respectively), leading to a consensus that the parietal cortex, especially the PPC, plays an important role in extinction and neglect, and may be important for multisensory integration (di Pellegrino et al., 1997; Ladavas et al., 1998; Vandenberghe & Gillebert, 2009).
Another technique, transcranial magnetic stimulation (TMS), allows for the creation of ‘virtual lesions’ by temporarily inactivating a small volume of brain tissue in normal subjects. When combined with magnetic resonance imaging (MRI) data about the anatomical location of a specific brain region, such as the PPC, it can demonstrate a causal link between the PPC and complex cognitive operations, including cross-modal interactions (Ro et al., 2004; Fiorio & Haggard, 2005; Bolognini & Maravita, 2007; Ramos-Estebanez et al., 2007). We performed experiments to assess the causal role played by the PPC in visual–tactile integration using TMS. To accomplish this task, we modulated the behavioural sensations of touch by vision. Then, we used MRI-guided TMS to disrupt brain activity and measured the effects on behavioral multisensory integration.
Materials and methods
Experiments were conducted in accordance with the ethical guidelines of the Declaration of Helsinki and approved by the Committee for the Protection of Human Subjects of the University of Texas Health Science Center at Houston. Eight healthy volunteers (three females, one left handed, mean age 27 years) with no history of neurological or sensory disorders participated in the study. Subjects were screened for the exclusion criteria for MRI and TMS and written informed consent was obtained from each subject prior to experimentation.
Task and stimuli
Subjects reported the location of a weak mechanical touch that was delivered to either the index (D2) or little (D5) finger on their right hand at 1.0 s after trial onset (Fig. 1). The mechanical touches were delivered with piezoelectric benders (Piezo Systems, Woburn, MA, USA; Beauchamp et al., 2007, 2009) attached to the tips of D2 and D5. The benders were actuated with a 150-ms Gaussian-modulated sine wave voltage, delivered under computer control, which produced a physical deflection in the bender that peaked 75 ms after onset and resulted in the percept of a faint tap. Because multisensory integration is strongest with weak unisensory stimuli (Stein & Meredith, 1993) we determined the deflection required to produce a peri-threshold tactile stimulus was determined separately for D2 and D5 in each subject. Beginning with a suprathreshold stimulus, the stimulus intensity was reduced in 1-dB steps until the subject reported being unable to detect the touch. The stimulus intensity 1 dB above this level was used for the main experiment. The perithreshold intensity corresponded to a mean mechanical deflection of 0.75 ± 0.25 μm for D5 and 0.99 ± 0.32 μm for D2, consistent with previous studies on human vibrotactile thresholds (Brisben et al., 1999).
Fig. 1.
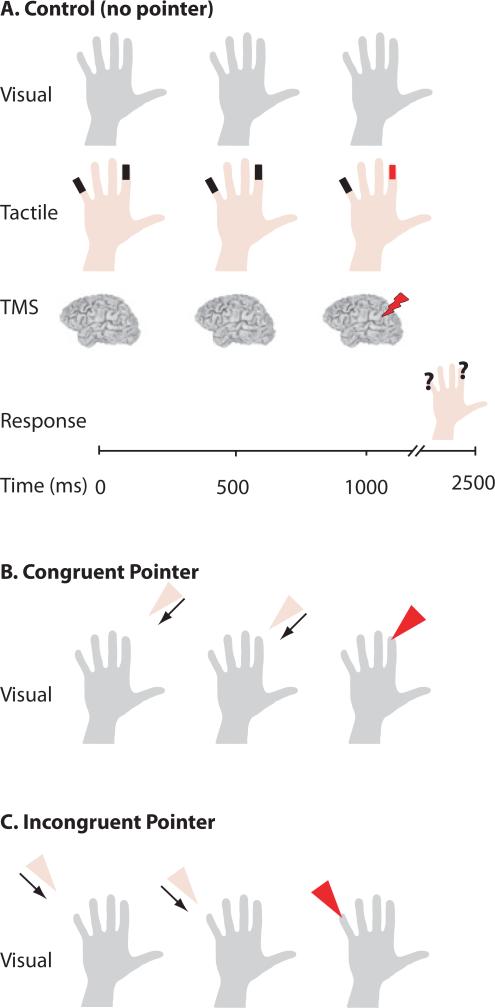
Experimental design. (A) Control (No Pointer) trial. Top row: subjects viewed a static image of a hand throughout the trial. Second row: tactile stimulators (black rectangles) were attached to the tips of D2 and D5. A mechanical tap (red rectangle) was delivered to either D2 or D5 1.0 s after the start of the trial. Third row: on some trials, TMS was delivered 1.0 s after the start of the trial. Fourth row: subjects reported the location of the tap. (B) Congruent Pointer trial. The visual display contained a moving pointer that contacted either D2 or D5 (the same finger that received the tactitle stimulation) 1.0 s after trial start (arrows for illustration only). All other trial events are identical to control trials. (C) Incongruent Pointer trial. The moving pointer contacted the fingertip that did not receive tactile stimulation.
The visual stimulus consisted of a centrally-presented static image of an actor's right hand (palm facing towards the subject) that appeared at trial onset and remained visible throughout the duration of each trial. In the ‘Congruent Pointer’ condition, the visual display also contained an animated triangular shape (the pointer) that appeared in the upper part of the display at trial onset and moved continuously, making contact with either D2 or D5 on the still image of the hand 1.0 s after trial onset. The same finger received both visual pointer contact and the mechanical touch. In the ‘Incongruent Pointer’ condition, the animated pointer appeared at trial onset but 1.0 s after trial onset it contacted the finger that did not receive the mechanical touch. In the ‘No Pointer’ condition the visual stimulus consisted of only the still image of the hand with no animated pointer. In all conditions, the subjects’ task was to indicate the finger receiving the mechanical touch as soon as possible after the touch occurred, using their left hand to signal their response with a two-button computer mouse.
MRI
Anatomical MRI scans were obtained from each subject using a 3-tesla whole-body MR scanner (Phillips Medical Systems, Bothell, WA, USA). Images were collected using a magnetization-prepared 180° radio-frequency pulses and rapid gradient-echo (MP-RAGE) sequence optimized for gray–white matter contrast with 1-mm-thick sagittal slices and an in-plane resolution of 0.938 × 0.938 mm. AFNI software (Cox, 1996) was used to analyze MRI data. Three-dimensional cortical surface models were created with FreeSurfer (Fischl et al., 1999) and visualized in SUMA (Argall et al., 2006). Cortical surfaces were partially inflated using 500 iterations of a smoothing algorithm to better visualize the deeper sulcal areas (Van Essen, 2004). Finally, to allow reporting of the stimulation sites in standard coordinates, each individual brain was normalized to the N27 atlas brain (Mazziotta et al., 2001).
Experimental apparatus
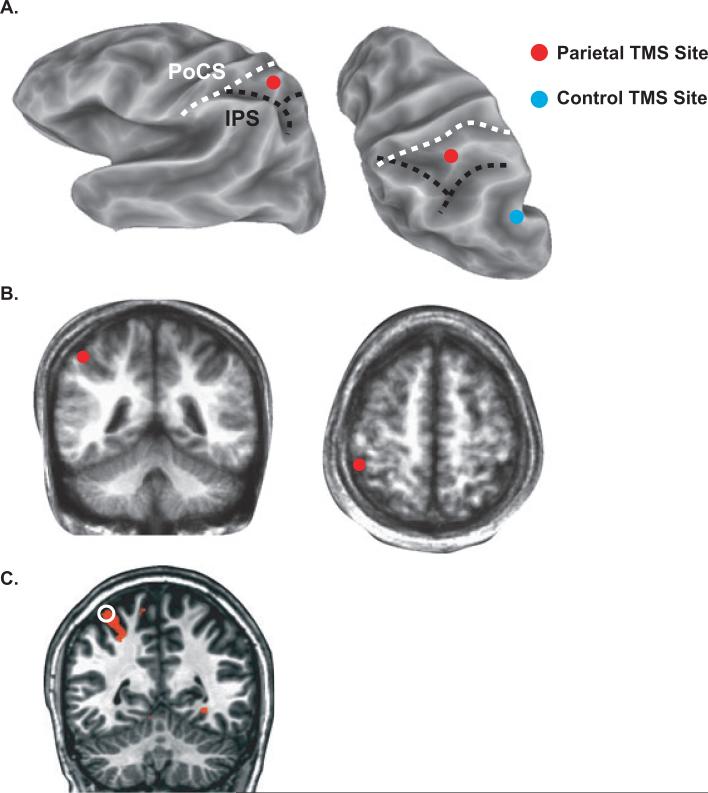
Seated subjects viewed visual stimuli on a liquid crystal display screen placed at eye level 65 cm from the subject. A biphasic TMS unit (Magstim Rapid; Magstim Co., Whitland, UK) with a 70-mm figure-of-eight coil was used to deliver TMS. The coil was positioned using an image-guided neuro-navigation system for frameless stereotaxy (Brainsight, Rogue Research, Montreal, Canada). Neuro-navigation allowed the TMS to be precisely targeted to specific anatomical locations based on high-resolution MRI. Because head position was continuously monitored, and adjustments in coil positioning were made if there was misalignment, we ensured that the same brain location was stimulated throughout each experimental session (Peters et al., 1996). The stimulation site was plotted as the coordinate on the surface of the brain closest to the TMS coil, calculated as the position where a line normal to the surface of the scalp first intersects the brain surface. The hand region of left primary motor cortex (M1) was identified on the high-resolution MRI and targeted using neuro-navigation. The motor threshold intensity was determined for each subject (on average 69% of machine output) and used throughout the session (Ro et al., 2004; Stokes et al., 2005; Balslev et al., 2007). Our previous study identified a candidate area for multisensory integration in posterior parietal cortex (Ro et al., 2004). Thus, we targeted the same area, 3 cm posterior and 2 cm lateral from the hand region of left M1, with the coil flush against the scalp and the coil handle facing backwards at a 45° angle from the mid-sagittal plane. This location was confirmed to be in the PPC [in close proximity to the intraparietal sulcus (IPS) and the post-central sulcus (PoCS)] based upon the subject's individual MRI. Finally, because TMS produces a loud click and scalp sensations under the coil, we also performed TMS of a control site located posterior and ventral to the PPC TMS location.
Experimental design
Trials were presented in two blocks, one in which TMS was delivered to the parietal site and one in which TMS was delivered to the control site (block order was counterbalanced across subjects). Within each block, the finger of tactile stimulation, the visual trial type and the presence or absence of TMS was randomly varied from trial to trial. If TMS was present, a single TMS pulse was delivered at 1.0 s after trial onset (the same time as the mechanical and visual touch). A total of 480 trials were delivered (20 repetitions per condition × two tactile × three visual × TMS/noTMS × PPC TMS/Control TMS). Because the behavioral results for D2 and D5 were similar, they were grouped for analysis.
Analysis
Because single-pulse TMS briefly disrupts neural processing, our main measure of performance was reaction time (RT; Walsh et al., 1999; Pourtois et al., 2001; Cattaneo et al., 2009). RT was defined as the time between the end of the tactile stimulus and the mouse button press by the subjects indicating on which finger they felt the touch. Only the RTs for correct trials were used in the analysis. A small number of trials showed very short or very long RTs; RTs outside of the 250–1150 ms (mean ± 2 SDs) time range were discarded (Collignon et al., 2008). Because there is a trade-off between speed and accuracy, performance was also evaluated using the inverse efficiency (IE) measure, defined as the RT divided by the proportion of correct responses (Townsend & Ashby, 1978; Chambers et al., 2004). An index of multisensory enhancement was calculated as the percentage RT decrease between multisensory and unisensory (No Pointer) trials. The first stage of analysis was a within-subject anova with TMS (parietal, control, and No TMS) and visual–tactile stimulus condition (Congruent, Incongruent, and No Pointer) as factors. Separate anovas were performed with RT, accuracy and IE as dependent measures. The anovas were followed by planned pair-wise within-subject comparisons (t-tests) between conditions.
Results
The Anovas on RT, accuracy and IE showed a significant effect of TMS and of stimulus condition (RT: F2,28 = 5.3, P = 0.02 for TMS and F2,28 = 16.3, P = 0.0002 for stimulus condition; accuracy: F2,28 = 12.7, P = 0.0007 and F2,28 = 17.5, P = 0.0002; IE: F2,28 = 12.8, P = 0.0007 and F2,28 = 13.8, P = 0.0005). To better understand these results, we performed within-subject pair-wise comparisons between conditions.
Behavioral evidence of multisensory integration
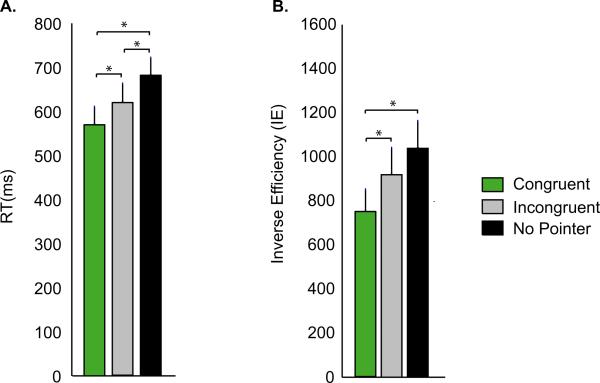
A hallmark of multisensory integration is the improvement of behavioral performance when subjects receive information from more than one sensory modality. In our experiment, multisensory integration was reflected in improved behavioral performance for Congruent Pointer trials (visual + tactile information) compared with No Pointer trials (tactile information alone). As shown in Fig. 2, RT was faster for Visually Congruent than for No Pointer trials [RT of 570 ± 40 ms (mean ± SEM) vs. 680 ± 39 ms, paired t-test, t7 = 6.3, P = 0.0004]. IE also improved (lower IE of 749 ± 99 vs. 1039 ± 123, t7 = 5.1, P = 0.001), demonstrating that the faster RT was not due to decreased accuracy.
Fig. 2.
Behavioral performance during the three trial types. (A) The average RT across subjects during each trial type (error bars show SEM across subjects). Each trial type is identified by a different color, as shown in the legend; only correct trials were included in the analysis. *P < 0.05. (B) The average IE (defined as RT/accuracy) across subjects.
The visual stimulus in Congruent Pointer trials contained both spatial information about the location of the weak touch and temporal information about the precise time of the touch. We examined the behavioral response to Incongruent Pointer trials in which the temporal information about the precise time of the touch was preserved but spatial information about the location of the touch was not. Performance in Incongruent Pointer trials (RT of 618 ± 43 ms and IE of 919 ± 121) was significantly better than No Pointer trials (RT of 680 ± 39 ms, t7 = 3.2, P = 0.02 and IE of 1039 ± 123, t7 = 1.9, P = 0.09) but was significantly worse than Congruent Pointer trials (RT of 570 ± 40 ms, t7 = 4.4, P = 0.003 and IE of 749 ± 99, t7 = 5.8, P = 0.0007). To measure the relative performance improvement in the different conditions, we calculated the percentage decrease in RT for multisensory compared with unisensory trials as an index of multisensory enhancement. The largest degree of multisensory enhancement was observed in congruent trials with a 16.36 ± 2.4% decrease in RT (Fig. 3). A smaller degree of enhancement was observed in incongruent trials, with a 9.15 ± 2.74% decrease in RT. This indicates that both spatial and temporal information from the visual pointer were important for the observed multisensory enhancement.
Fig. 3.
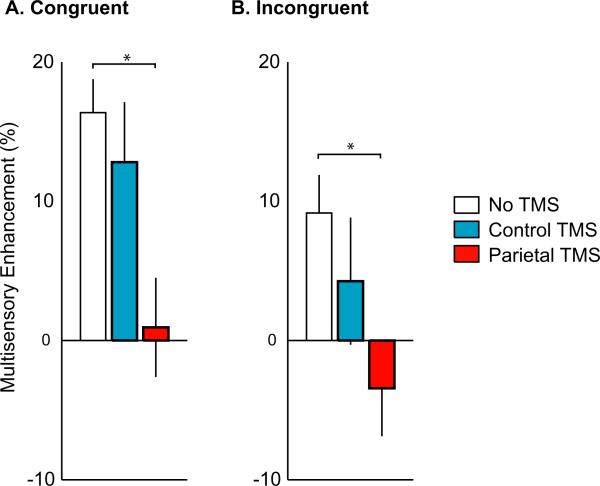
Effects of TMS on multisensory enhancement. The RT decrease between multisensory and unisensory trials was calculated as an index of multisensory enhancement. Each color corresponds to a different TMS condition, as shown in the legend. *P < 0.05. (A) Multisensory enhancement during Congruent Pointer trials. (B) Multisensory enhancement during Incongruent Pointer trials.
Modulating multisensory integration with TMS
Next, the effects of PPC TMS on behavioral multisensory integration were examined. As shown in Fig. 3, PPC TMS eliminated the multisensory enhancement observed in Congruent Pointer trials (0.9 ± 3.6% vs. 16.4 ± 2.4%, t7 = 6.8, P = 0.0002). PPC TMS also eliminated the multisensory enhancement observed in Incongruent Pointer trials (–3.4 ± 3.4% vs. 9.2 ± 2.7%, t7 = 3.7, P = 0.008).
Control TMS
A possible concern with TMS is that nonspecific effects (such as the sound generated by the TMS pulse or scalp muscle stimulation) could interfere with behavioral performance. To control for this possibility, we delivered TMS to a control location. Strong multisensory enhancement in the congruent condition was found for the control TMS site that was not significantly different than that observed in the no TMS condition (12.8 ± 4.3 vs. 16.4 ± 2.4%, t7 = 0.7, P = 0.5); the same was true for incongruent trials (4.3 ± 4.6% vs. 9.2 ± 2.7%, t7 = 1.0, P = 0.4). Because PPC TMS, but not control TMS, eliminated the behavioral advantage of multisensory trials, nonspecific effects of TMS cannot be responsible for the observed disruption of multisensory integration.
Accuracy
In the absence of TMS, accuracy during Congruent Pointer trials was 79.8 ± 4.3%, which was significantly better than PPC TMS (69.1 ± 4.7%, t7 = 4.2, P = 0.004) but not different from control TMS (75.3 ± 5.1%, t7 = 1.9, P = 0.1). Similarily, accuracy during Incongruent Pointer trials was 70.8 ± 4.0% for no TMS which was significantly better than PPC TMS (55.0 ± 4.3%, t7 = 3.3, P = 0.01) but not different from control TMS (59.4 ± 6.2%, t7 = 1.9, P = 0.1). Accuracy during No Pointer trials was greater for no TMS than either of the TMS conditions (69.1 ± 4.5% for no TMS vs. 58.7 ± 3.8% for PPC TMS, t7 = 2.9, P = 0.02 and vs. 60.0 ± 5.5% for control TMS, t7 = 4.4, P = 0.003). These effects cannot be attributed to floor effects (subjects performing at chance, 50%) or ceiling effects (subjects performing perfectly, 100%) because we used a pre-experiment calibration routine that set the perithreshold level of tactile stimulation separately for each finger in each subject. The calibration process was successful, with an average accuracy across all experimental conditions of 66.3 ± 5.3%. In post-experiment debriefings, subjects reported being able to detect the tactile stimulus on most trials even though they were not always able to localize it. This is consistent with known differences in accuracy between somatosensory detection and localization tasks (Seyal et al., 1997).
Details of the behavioral results
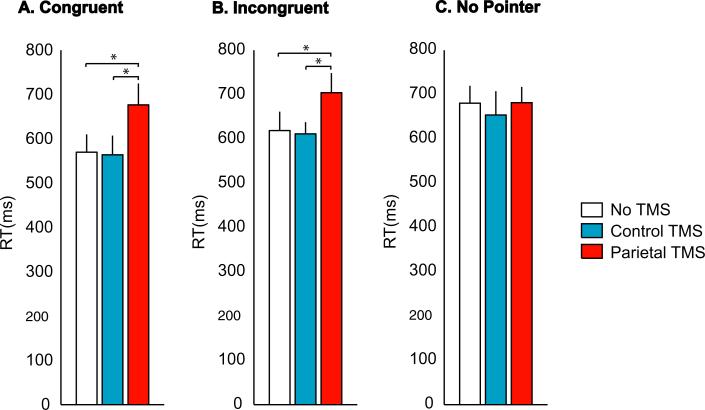
To learn more about the factors underlying the decrease in multisensory integration observed with PPC TMS, we plotted the RT in each stimulus and TMS condition (Fig. 4). During Congruent Pointer trials, performance was significantly worse when PPC TMS was delivered than with No TMS (RT, 677 ± 48 vs. 570 ± 40 ms, t7 = 4.4, P = 0.003; and IE, 1037 ± 133 vs. 749 ± 99, t7 = 4.3, P = 0.004). A similar effect was seen during Incongruent Pointer trials (RT, 704 ± 44 vs. 618 ± 43 ms, t7 = 3.5, P = 0.01; and IE, 1350 ± 144 vs. 919 ± 121, t7 = 3.9, P = 0.006).
Fig. 4.
Behavioral performance during the three trial types and three TMS conditions. (A) The average RT across subjects to Congruent Pointer trials. Each color corresponds to a different TMS condition, as shown in the legend. Only correct trials were included in the analysis. (B) RT to Incongruent Pointer trials during the different TMS conditions. (C) RT to No Pointer trials during the different TMS conditions. *P < 0.05.
This impairment was not due to a nonspecific effect on task performance. During No Pointer trials, subjects performed the same task but TMS of the PPC had no impact on performance. They identified the location of the touch with TMS as rapidly as they did with No TMS (RT, 681 ± 35 vs. 680 ± 39 ms, t7 = 0.05, P = 0.96) and their efficiency was also similar (IE, 1210 ± 123 vs. 1039 ± 123, t7 = 2.1, P = 0.07). Because there was no significant difference in performance between TMS and No TMS trials during No Pointer trials, the effects of TMS are unlikely to be purely due to changes in detection of tactile stimuli.
In the three-way anova the interaction of stimulus condition and TMS was significant (F4,28 = 2.8, P = 0.04), showing that TMS has a differential effect depending on the visual stimulus. This interaction was primarily driven by the increase in RT during Congruent but not during No Pointer trials. Unlike PPC TMS, Control TMS did not change the behavioral measures of response for any of the conditions. During all trial types, subjects identified the location of the touch as rapidly and accurately with control TMS as they did with No TMS (No pointer: RT, 654 ± 54 with control TMS vs. 680 ± 39 ms with No TMS, t7 = 1.0, P = 0.3; IE, 1204 ± 201 with control TMS vs. 1039 ± 123 with No TMS, t7 = 1.8, P = 0.1; Congruent Pointer: RT, 564 ± 43 with control TMS vs. 570 ± 40 ms with No TMS, t7 = 0.2, P = 0.8; and IE, 803 ± 128 with control TMS vs. 749 ± 99 with No TMS, t7 = 1.0, P = 0.4; Incongruent Pointer: RT, 611 ± 27 with control TMS vs. 618 ± 43 ms with No TMS, t7 = 0.3, P = 0.8; and IE, 1087 ± 91 with control TMS vs. 919 ± 121 with No TMS, t7 = 1.7, P = 0.1). Therefore, nonspecific effects of TMS cannot be responsible for the observed disruption of multisensory integration.
In summary, the observed increases in RT during multisensory stimulation are best explained by an impairment in multisensory integration caused by PPC TMS, and cannot be explained by a uniform decrease in tactile sensitivity or a nonspecific effect of TMS.
Location of multisensory integration
TMS of posterior parietal cortex, but not of a control site, eliminated multisensory integration of viewed touches. To learn more about the precise location of the stimulated region, we created cortical surface models of each subject's brain and plotted the location of the stimulation sites. Figure 5A shows the cortical surface of a single subject labeled with the parietal and control TMS sites. The PPC TMS site for this subject was between the PoCS and the IPS, while the control site was posterior and inferior to both sulci. Individual subject brains were normalized to an atlas brain, allowing computation of the average coordinates of the PPC TMS site (x = –48 ± 3, y = 43 ± 3 and z = 53 ± 3 mm) between the PoCS and the IPS in the superior parietal lobule. The average coordinates of the control TMS site (x = –48 ± 4, y = 67 ± 4 and z = 35 ± 6 mm) were in the left angular gyrus. The average Euclidean distance between the parietal and control stimulation sites was 34 ± 3 mm.
Fig. 5.
The anatomical location of TMS as measured with neuro-navigation. (A) A partially inflated cortical surface model of one subject's left hemisphere is shown from a lateral view (left) and superior–posterior view (right). The fundus of the intraparietal sulcus (IPS) is shown with a black dashed line. The fundus of the postcentral sulcus (PoCS) is shown with a dashed white line. The red circle shows the location of posterior parietal cortex (PPC) TMS, the blue circle shows the location of control TMS. (B) The average location of PPC TMS (red circles) plotted on an average anatomical volume created by averaging each subject's anatomical MRI in standard space, shown in coronal (left) and axial (right) sections. (C) Location of TMS in a single subject (white open circle) with blood oxygenation level-dependent (BOLD) fMRI activation to viewed and felt touches (orange color).
One subject also participated in a functional MRI (fMRI) study of responses to viewed touches. For this subject, a conjunction analysis was used to identify brain areas responsive to both visual and tactile stimulation. As shown in Fig. 5C, the PPC TMS site that interfered with multisensory integration was directly adjacent to areas that were active during the perception of visual and tactile touches (primarily in the banks of the PoCS).
Discussion
In these experiments, viewing a spatially congruent touch enhanced subjects’ ability to localize real touches. These behavioral improvements were eliminated by TMS of the PPC. Both findings can be accounted for by a multisensory map of space in the PPC that receives both somatosensory and visual inputs and is centered on the viewed hand (‘peri-personal’ or ‘peri-hand’ space). In non-human primates, single neurons in the IPS respond maximally when presented with visual and somatosensory stimuli that are both spatially and temporally congruent (Cavada & Goldman-Rakic, 1989; Wise et al., 1997; Duhamel et al., 1998; Rizzolatti et al., 1998; Avillac et al., 2007; Breveglieri et al., 2008). In our experiment, the Incongruent Pointer condition contained information about the moment that the touch occurred because the visual stimulus was temporally congruent, although spatially incongruent, with the tactile stimulus. Therefore, multisensory PPC neurons might be expected to show a greater response in this condition than in the No Pointer condition in which there was no information about the exact location or timing of the touch. Correspondingly, subjects were better able to detect touch in the Incongruent Pointer than in the No Pointer condition. In the Congruent Pointer condition, the visual and somatosensory stimuli were both temporally and spatially congruent. Therefore, multisensory PPC neurons should show an even greater response than in the Incongruent Pointer condition, corresponding to subjects’ improved performance in the Congruent Pointer condition compared to the Incongruent Pointer condition. The baseline visual stimulus presented in the No Pointer condition consisted of a static image of a hand which itself modulates touch detection differently than a static image of a shape or no visual stimulus (Tipper et al., 1998; Igarashi et al., 2008). This can also be understood in reference to a peri-hand map of space in the PPC. Simply viewing a hand or a hand-held manipulable object leads to increased activity in the PPC (Beauchamp et al., 2002, 2003; Frey et al., 2005) which could boost the ability to detect the small incremental activity resulting from a weak tactile touch.
Compelling behavioral experiments show an interaction between the visual and tactile spatial maps of peri-hand space: for instance, the use of a tool extends the peri-hand space (Farne et al., 2007). Neuroimaging experiments are consistent with the idea that this map of peri-hand space is located in the PPC (Sereno & Huang, 2006; Makin et al., 2007). Single-pulse TMS of PPC interferes with visually-induced enhanced percepts of touch (Ro et al., 2004) and repetitive TMS of the PPC interferes with visual–tactile remapping in subjects with crossed hands (Bolognini & Maravita, 2007). In our study, we used neuro-navigation to target cortex in the PPC between the IPS and the PoCS; this was the region of the brain closest to the TMS coil, and hence the region that experienced the largest induced electromagnetic fields. While we did not determine the precise extent of the disrupted cortex, combined studies of motor cortex using TMS and positron emission tomography suggest that TMS preferentially activates neurons on the banks of sulci, where the cortex is perpendicular to the induced current (Fox et al., 2004, 2006). Therefore, the bulk of the activity induced by TMS in our experiment was likely to reside within the PPC in the banks of the IPS and the PoCS, consistent with the fMRI activity we observed for one subject in the banks of the PoCS. Our experiments demonstrate that disrupting PPC abolishes multisensory enhancement, suggesting a causal link between activity in the peri-hand map of space and multisensory enhancement.
While a model of a multisensory peri-hand map of space in the PPC offers a complete account for the observed results, we also considered other explanations for the results, especially attention. The different visual stimuli in our different experimental conditions could differentially reorient visuospatial attention, a process known to involve the PPC (Corbetta et al., 2008). Visuospatial attention enhances behavioral performance, and disrupting the PPC with TMS could interfere with the allocation of attention and impair behavioral performance (Rushworth et al., 2001; Rushworth & Taylor, 2006). Simply viewing a static image of a hand, relative to a simple shape or no visual stimulus, induces visual–tactile interactions (Tipper et al., 1998; Igarashi et al., 2008). In the attentional account, this occurs because of the allocation of visuospatial attention to the location of the hand. However, in the present experiment, TMS of the PPC did not impair performance in the No Pointer baseline (even though performance was above chance, allowing for performance decreases). Hence, the TMS results from the No Pointer condition argue against a purely attentional account.
It might be expected that the moving visual pointer would attract visuospatial attention to the finger touched by the visual pointer, similar to the classic cueing experiments of Posner (Posner et al., 1980). Posner found that an invalid cue (analogous to our Incongruent Pointer condition) incurred a substantial cost, with performance much worse than in the neutral cue condition (analogous to No Pointer). This was not observed in our experiments; instead we observed that performance in the Incongruent Pointer condition was significantly better than in the No Pointer condition. An alternative account would hypothesize a different distribution of attention, with attention directed equally to the fingertips in both moving pointer conditions. While this hypothesis could produce a behavioral enhancement in the moving pointer conditions relative to No Pointer, it does not explain why Congruent Pointer performance was better than Incongruent Pointer performance. Evidence from other studies also does not support attention as the sole explanation for multisensory enhancements. Visual enhancement of tactile grating orientation discrimination is abolished by TMS of primary somatosensory cortex (S1), but not secondary somatosensory cortex (S2) (Fiorio & Haggard, 2005), opposite to the pattern that would be predicted by an attentional account, as S2 but not S1 is strongly modulated by attention (Hsiao et al., 2002; Romo et al., 2002).
Previous TMS studies measuring effects on somatosensory reception, have used electrical current applied to the skin to directly stimulate afferent nerve fibers. In contrast, the present study used mechanical tactile stimulation, which is slower than electrical stimulation at inducing cortical activity for two reasons: the relatively long delay between stimulus onset and peak deflection of the stimulator (75 ms in our study) and the delay induced by signal transduction in the skin mechanoreceptors. In the present study, TMS was delivered at mechanical stimulus onset, roughly equivalent to delivering TMS 50–100 ms before electrical stimulus onset. Therefore, our results are consistent with those of Ro et al. (2004), who found that TMS delivered 50 ms before an electrical tactile stimulus interfered with visual–tactile interactions, and of Seyal et al. (1997) who demonstrated that TMS delivered at a range of latencies between 500 ms before electrical stimulus onset and 200 ms after stimulus onset interfered with stimulus localization, the task used in the present study.
TMS significantly reduces neuronal responses to sensory stimuli (Allen et al., 2007). Intracranial recordings show that human PPC contains a multisensory zone in which visual inputs arrive ~75 ms after stimulus onset (Molholm et al., 2006; Moran et al., 2008). Disrupting neural activity in the PPC during this time might be expected to interfere with multisensory integration. Electroencephalography and magnetoencephalography studies suggest that multisensory integration may be subserved by neuronal oscillations (Bauer et al., 2009; Kanayama et al., 2009). Phase resetting, in which a sensory stimulus causes ongoing oscillations across different areas to become phase-locked, may be particularly important for integration (Senkowski et al., 2008). Because TMS interferes with spectral coherence and phase locking between brain locations (Pasley et al., 2009), it may be particularly effective at disrupting multisensory integration. Sensitivity to synchrony in different frequency bands, and between brain regions that represent the same region of space, could also help explain the temporal and spatial congruence that are one of the defining features of multisensory integration. Additional experiments examining the effects of TMS on phase locking and resetting will provide deeper insights into the neuronal mechanisms underlying multisensory integration.
Acknowledgements
This research was supported by NSF 642532 to M.S.B and NSF 847607 to T.R. S.P. was supported by NIH T32HD049350. Partial funding for purchase of the MRI scanner was provided by NIH S10 RR19186. We thank Nafi Yasar for assistance with TMS and Vips Patel for assistance with MR data collection.
Abbreviations
- D2
index finger
- D5
little finger
- fMRI
functional MRI
- IE
inverse efficiency
- IPS
intraparietal sulcus
- MP-RAGE
magnetization-prepared 180° radio-frequency pulses and rapid gradient-echo
- MRI
magnetic resonance imaging
- PoCS
post-central sulcus
- PPC
posterior parietal cortex
- RT
reaction time
- TMS
transcranial magnetic stimulation
References
- Allen EA, Pasley BN, Duong T, Freeman RD. Transcranial magnetic stimulation elicits coupled neural and hemodynamic consequences. Science. 2007;317:1918–1921. doi: 10.1126/science.1146426. [DOI] [PubMed] [Google Scholar]
- Amedi A, Malach R, Hendler T, Peled S, Zohary E. Visuo-haptic object-related activation in the ventral visual pathway. Nat. Neurosci. 2001;4:324–330. doi: 10.1038/85201. [DOI] [PubMed] [Google Scholar]
- Argall BD, Saad ZS, Beauchamp MS. Simplified intersubject averaging on the cortical surface using SUMA. Hum. Brain Mapp. 2006;27:14–27. doi: 10.1002/hbm.20158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avillac M, Ben Hamed S, Duhamel JR. Multisensory integration in the ventral intraparietal area of the macaque monkey. J. Neurosci. 2007;27:1922–1932. doi: 10.1523/JNEUROSCI.2646-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balslev D, Braet W, McAllister C, Miall RC. Inter-individual variability in optimal current direction for transcranial magnetic stimulation of the motor cortex. J. Neurosci. Methods. 2007;162:309–313. doi: 10.1016/j.jneumeth.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Bauer M, Oostenveld R, Fries P. Tactile stimulation accelerates behavioral responses to visual stimuli through enhancement of occipital gamma-band activity. Vision Res. 2009;49:931–942. doi: 10.1016/j.visres.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Lee KE, Haxby JV, Martin A. Parallel visual motion processing streams for manipulable objects and human movements. Neuron. 2002;34:149–159. doi: 10.1016/s0896-6273(02)00642-6. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Lee KE, Haxby JV, Martin A. fMRI responses to video and point-light displays of moving humans and manipulable objects. J. Cogn. Neurosci. 2003;15:991–1001. doi: 10.1162/089892903770007380. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Yasar NE, Kishan N, Ro T. Human MST but not MT responds to tactile stimulation. J. Neurosci. 2007;27:8261–8267. doi: 10.1523/JNEUROSCI.0754-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MS, Yasar NE, Frye RE, Ro T. Touch, sound and vision in human superior temporal sulcus. Neuroimage. 2008;41:1011–1020. doi: 10.1016/j.neuroimage.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MS, Laconte S, Yasar N. Distributed representation of single touches in somatosensory and visual cortex. Hum. Brain Mapp. 2009;30:3163–3171. doi: 10.1002/hbm.20735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Bristow D, Bird G, Frith C, Ward J. Somatosensory activations during the observation of touch and a case of vision-touch synaesthesia. Brain. 2005;128:1571–1583. doi: 10.1093/brain/awh500. [DOI] [PubMed] [Google Scholar]
- Bolognini N, Maravita A. Proprioceptive alignment of visual and somatosensory maps in the posterior parietal cortex. Curr. Biol. 2007;17:1890–1895. doi: 10.1016/j.cub.2007.09.057. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Schlack A, Shah NJ, Zafiris O, Kubischik M, Hoffmann K, Zilles K, Fink GR. Polymodal motion processing in posterior parietal and premotor cortex: a human fMRI study strongly implies equivalencies between humans and monkeys. Neuron. 2001;29:287–296. doi: 10.1016/s0896-6273(01)00198-2. [DOI] [PubMed] [Google Scholar]
- Breveglieri R, Galletti C, Monaco S, Fattori P. Visual, somatosensory, and bimodal activities in the macaque parietal area PEc. Cereb. Cortex. 2008;18:806–816. doi: 10.1093/cercor/bhm127. [DOI] [PubMed] [Google Scholar]
- Brisben AJ, Hsiao SS, Johnson KO. Detection of vibration transmitted through an object grasped in the hand. J. Neurophysiol. 1999;81:1548–1558. doi: 10.1152/jn.1999.81.4.1548. [DOI] [PubMed] [Google Scholar]
- Cattaneo Z, Rota F, Walsh V, Vecchi T, Silvanto J. TMS-adaptation reveals abstract letter selectivity in the left posterior parietal cortex. Cereb. Cortex. 2009;19:2321–2325. doi: 10.1093/cercor/bhn249. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J. Comp. Neurol. 1989;287:393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Stokes MG, Mattingley JB. Modality-specific control of strategic spatial attention in parietal cortex. Neuron. 2004;44:925–930. doi: 10.1016/j.neuron.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Collignon O, Girard S, Gosselin F, Roy S, Saint-Amour D, Lassonde M, Lepore F. Audio-visual integration of emotion expression. Brain Res. 2008;1242:126–135. doi: 10.1016/j.brainres.2008.04.023. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Culham JC, Valyear KF. Human parietal cortex in action. Curr. Opin. Neurobiol. 2006;16:205–212. doi: 10.1016/j.conb.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Duhamel JR, Colby CL, Goldberg ME. Ventral intraparietal area of the macaque: congruent visual and somatic response properties. J. Neurophysiol. 1998;79:126–136. doi: 10.1152/jn.1998.79.1.126. [DOI] [PubMed] [Google Scholar]
- Farne A, Serino A, Ladavas E. Dynamic size-change of peri-hand space following tool-use: determinants and spatial characteristics revealed through cross-modal extinction. Cortex. 2007;43:436–443. doi: 10.1016/s0010-9452(08)70468-4. [DOI] [PubMed] [Google Scholar]
- Filimon F, Nelson JD, Huang RS, Sereno MI. Multiple parietal reach regions in humans: cortical representations for visual and proprioceptive feedback during on-line reaching. J. Neurosci. 2009;29:2961–2971. doi: 10.1523/JNEUROSCI.3211-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorio M, Haggard P. Viewing the body prepares the brain for touch: effects of TMS over somatosensory cortex. Eur. J. Neurosci. 2005;22:773–777. doi: 10.1111/j.1460-9568.2005.04267.x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fox PT, Narayana S, Tandon N, Sandoval H, Fox SP, Kochunov P, Lancaster JL. Column-based model of electric field excitation of cerebral cortex. Hum. Brain Mapp. 2004;22:1–14. doi: 10.1002/hbm.20006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Narayana S, Tandon N, Fox SP, Sandoval H, Kochunov P, Capaday C, Lancaster JL. Intensity modulation of TMS-induced cortical excitation: primary motor cortex. Hum. Brain Mapp. 2006;27:478–487. doi: 10.1002/hbm.20192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey SH, Vinton D, Norlund R, Grafton ST. Cortical topography of human anterior intraparietal cortex active during visually guided grasping. Brain Res. Cogn. Brain Res. 2005;23:397–405. doi: 10.1016/j.cogbrainres.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Haggard P, Christakou A, Serino A. Viewing the body modulates tactile receptive fields. Exp. Brain Res. 2007;180:187–193. doi: 10.1007/s00221-007-0971-7. [DOI] [PubMed] [Google Scholar]
- Hsiao SS, Lane J, Fitzgerald P. Representation of orientation in the somatosensory system. Behav. Brain Res. 2002;135:93–103. doi: 10.1016/s0166-4328(02)00160-2. [DOI] [PubMed] [Google Scholar]
- Igarashi Y, Kimura Y, Spence C, Ichihara S. The selective effect of the image of a hand on visuotactile interactions as assessed by performance on the crossmodal congruency task. Exp. Brain Res. 2008;184:31–38. doi: 10.1007/s00221-007-1076-z. [DOI] [PubMed] [Google Scholar]
- James TW, Humphrey GK, Gati JS, Servos P, Menon RS, Goodale MA. Haptic study of three-dimensional objects activates extrastriate visual areas. Neuropsychologia. 2002;40:1706–1714. doi: 10.1016/s0028-3932(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Johnson RM, Burton PC, Ro T. Visually induced feelings of touch. Brain Res. 2006;1073–1074:398–406. doi: 10.1016/j.brainres.2005.12.025. [DOI] [PubMed] [Google Scholar]
- Kanayama N, Sato A, Ohira H. The role of gamma band oscillations and synchrony on rubber hand illusion and crossmodal integration. Brain Cogn. 2009;69:19–29. doi: 10.1016/j.bandc.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Kennett S, Taylor-Clarke M, Haggard P. Noninformative vision improves the spatial resolution of touch in humans. Curr. Biol. 2001;11:1188–1191. doi: 10.1016/s0960-9822(01)00327-x. [DOI] [PubMed] [Google Scholar]
- Keysers C, Wicker B, Gazzola V, Anton JL, Fogassi L, Gallese V. A touching sight: SII/PV activation during the observation and experience of touch. Neuron. 2004;42:335–346. doi: 10.1016/s0896-6273(04)00156-4. [DOI] [PubMed] [Google Scholar]
- Ladavas E, di Pellegrino G, Farne A, Zeloni G. Neuropsycho-logical evidence of an integrated visuotactile representation of peripersonal space in humans. J. Cogn. Neurosci. 1998;10:581–589. doi: 10.1162/089892998562988. [DOI] [PubMed] [Google Scholar]
- Makin TR, Holmes NP, Zohary E. Is that near my hand? Multisensory representation of peripersonal space in human intraparietal sulcus. J. Neurosci. 2007;27:731–740. doi: 10.1523/JNEUROSCI.3653-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maravita A, Spence C, Driver J. Multisensory integration and the body schema: close to hand and within reach. Curr. Biol. 2003;13:R531–R539. doi: 10.1016/s0960-9822(03)00449-4. [DOI] [PubMed] [Google Scholar]
- Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, Zilles K, Woods R, Paus T, Simpson G, Pike B, Holmes C, Collins L, Thompson P, MacDonald D, Iacoboni M, Schormann T, Amunts K, Palomero-Gallagher N, Geyer S, Parsons L, Narr K, Kabani N, Le Goualher G, Boomsma D, Cannon T, Kawashima R, Mazoyer B. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001;356:1293–1322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molholm S, Sehatpour P, Mehta AD, Shpaner M, Gomez-Ramirez M, Ortigue S, Dyke JP, Schwartz TH, Foxe JJ. Audio-visual multisensory integration in superior parietal lobule revealed by human intracranial recordings. J. Neurophysiol. 2006;96:721–729. doi: 10.1152/jn.00285.2006. [DOI] [PubMed] [Google Scholar]
- Moran RJ, Molholm S, Reilly RB, Foxe JJ. Changes in effective connectivity of human superior parietal lobule under multisensory and unisensory stimulation. Eur. J. Neurosci. 2008;27:2303–2312. doi: 10.1111/j.1460-9568.2008.06187.x. [DOI] [PubMed] [Google Scholar]
- Nakashita S, Saito DN, Kochiyama T, Honda M, Tanabe HC, Sadato N. Tactile-visual integration in the posterior parietal cortex: a functional magnetic resonance imaging study. Brain Res. Bull. 2008;75:513–525. doi: 10.1016/j.brainresbull.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Pasley BN, Allen EA, Freeman RD. State-dependent variability of neuronal responses to transcranial magnetic stimulation of the visual cortex. Neuron. 2009;62:291–303. doi: 10.1016/j.neuron.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Pellegrino G, Ladavas E, Farne A. Seeing where your hands are [letter]. Nature. 1997;388:730. doi: 10.1038/41921. [DOI] [PubMed] [Google Scholar]
- Peters T, Davey B, Munger P, Comeau R, Evans A, Olivier A. Three-dimensional multimodal image-guidance for neurosurgery. IEEE Trans. Med. Imaging. 1996;15:121–128. doi: 10.1109/42.491414. [DOI] [PubMed] [Google Scholar]
- Posner MI, Snyder CR, Davidson BJ. Attention and the detection of signals. J. Exp. Psychol. 1980;109:160–174. [PubMed] [Google Scholar]
- Pourtois G, Vandermeeren Y, Olivier E, de Gelder B. Event-related TMS over the right posterior parietal cortex induces ipsilateral visuo-spatial interference. Neuroreport. 2001;12:2369–2374. doi: 10.1097/00001756-200108080-00017. [DOI] [PubMed] [Google Scholar]
- Ramos-Estebanez C, Merabet LB, Machii K, Fregni F, Thut G, Wagner TA, Romei V, Amedi A, Pascual-Leone A. Visual phosphene perception modulated by subthreshold crossmodal sensory stimulation. J. Neurosci. 2007;27:4178–4181. doi: 10.1523/JNEUROSCI.5468-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G, Matelli M. The organization of the cortical motor system: new concepts. Electroencephalogr. Clin. Neurophysiol. 1998;106:283–296. doi: 10.1016/s0013-4694(98)00022-4. [DOI] [PubMed] [Google Scholar]
- Ro T, Wallace R, Hagedorn J, Farne A, Pienkos E. Visual enhancing of tactile perception in the posterior parietal cortex. J. Cogn. Neurosci. 2004;16:24–30. doi: 10.1162/089892904322755520. [DOI] [PubMed] [Google Scholar]
- Romo R, Hernandez A, Zainos A, Lemus L, Brody CD. Neuronal correlates of decision-making in secondary somatosensory cortex. Nat. Neurosci. 2002;5:1217–1225. doi: 10.1038/nn950. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Taylor PC. TMS in the parietal cortex: updating representations for attention and action. Neuropsychologia. 2006;44:2700–2716. doi: 10.1016/j.neuropsychologia.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Paus T, Sipila PK. Attention systems and the organization of the human parietal cortex. J. Neurosci. 2001;21:5262–5271. doi: 10.1523/JNEUROSCI.21-14-05262.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito DN, Okada T, Morita Y, Yonekura Y, Sadato N. Tactile-visual cross-modal shape matching: a functional MRI study. Brain Res. Cogn. Brain Res. 2003;17:14–25. doi: 10.1016/s0926-6410(03)00076-4. [DOI] [PubMed] [Google Scholar]
- Sathian K, Zangaladze A, Hoffman JM, Grafton ST. Feeling with the mind's eye. Neuroreport. 1997;8:3877–3881. doi: 10.1097/00001756-199712220-00008. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Heinze HJ, Rotte M. Seeing the hand being touched modulates the primary somatosensory cortex. Neuroreport. 2005;16:1101–1105. doi: 10.1097/00001756-200507130-00014. [DOI] [PubMed] [Google Scholar]
- Senkowski D, Schneider TR, Foxe JJ, Engel AK. Crossmodal binding through neural coherence: implications for multisensory processing. Trends Neurosci. 2008;31:401–409. doi: 10.1016/j.tins.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Huang RS. A human parietal face area contains aligned head-centered visual and tactile maps. Nat. Neurosci. 2006;9:1337–1343. doi: 10.1038/nn1777. [DOI] [PubMed] [Google Scholar]
- Seyal M, Siddiqui I, Hundal NS. Suppression of spatial localization of a cutaneous stimulus following transcranial magnetic pulse stimulation of the sensorimotor cortex. Electroencephalogr. Clin. Neurophysiol. 1997;105:24–28. doi: 10.1016/s0924-980x(96)96090-7. [DOI] [PubMed] [Google Scholar]
- Stein BE, Meredith MA. The Merging of the Senses. MIT Press; Cambridge, MA, USA: 1993. [Google Scholar]
- Stokes MG, Chambers CD, Gould IC, Henderson TR, Janko NE, Allen NB, Mattingley JB. Simple metric for scaling motor threshold based on scalp-cortex distance: application to studies using transcranial magnetic stimulation. J. Neurophysiol. 2005;94:4520–4527. doi: 10.1152/jn.00067.2005. [DOI] [PubMed] [Google Scholar]
- Tipper S, Lloyd D, Shorland B, Dancer C, Howard L, McGlone F. Vision influences tactile perception without proprioceptive orienting. Neuroreport. 1998;9:1741–1744. doi: 10.1097/00001756-199806010-00013. [DOI] [PubMed] [Google Scholar]
- Townsend JT, Ashby FG. Methods of modeling capacity in simple processing systems. In: Castellan NJ, Rastle F, editors. Cognitive Theory VIII. Erlbaum; Hillsdale, NJ: 1978. pp. 199–239. [Google Scholar]
- Valyear KF, Cavina-Pratesi C, Stiglick AJ, Culham JC. Does tool-related fMRI activity within the intraparietal sulcus reflect the plan to grasp? Neuroimage. 2007;36(Suppl. 2):T94–T108. doi: 10.1016/j.neuroimage.2007.03.031. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. Surface-based approaches to spatial localization and registration in primate cerebral cortex. Neuroimage. 2004;23(Suppl. 1):S97–S107. doi: 10.1016/j.neuroimage.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Gillebert CR. Parcellation of parietal cortex: convergence between lesion-symptom mapping and mapping of the intact functioning brain. Behav. Brain Res. 2009;199:171–182. doi: 10.1016/j.bbr.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Walsh V, Ellison A, Ashbridge E, Cowey A. The role of the parietal cortex in visual attention–hemispheric asymmetries and the effects of learning: a magnetic stimulation study. Neuropsychologia. 1999;37:245–251. doi: 10.1016/s0028-3932(98)00099-2. [DOI] [PubMed] [Google Scholar]
- Wise SP, Boussaoud D, Johnson PB, Caminiti R. Premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Annu. Rev. Neurosci. 1997;20:25–42. doi: 10.1146/annurev.neuro.20.1.25. [DOI] [PubMed] [Google Scholar]