Abstract
Introduction
The epidemiology of oral clefts continually unfolds. Researchers have not reached consensus concerning the significance of maternal smoking, weight gain, diabetes, age, and education and the risk of oral clefts. The purpose of this study was to examine these factors associated with oral clefts in the US population.
Methods
The 2005 US Natality Data File was utilized for this study. Bivariate analyses compared the characteristics of mothers of infants with and without oral clefts. Multivariate analysis calculated adjusted odds ratios for various maternal characteristics overall and for each race/ethnic group.
Results
Significant bivariate associations with oral clefts were found for maternal age, race/ethnicity, education, tobacco use, and pregnancy-associated hypertension. Multivariate models found maternal age (OR=0.98), race/ethnicity (OR=0.36) for non-Hispanic Blacks (OR=0.79 for Hispanics), and tobacco use (OR=1.66) significant after adjustment for covariates. Across all race/ethnic groups maternal age (OR=0.98) and smoking (OR=1.66) were significantly associated with increased risk for oral cleft (OC). Non-Hispanic Blacks and Hispanics were at lower risk for OC regardless of the presence or absence of pregnancy-associated hypertension.
Conclusions
Consistent with previous studies, maternal smoking was found to be associated with an increased risk of oral clefts. This association was significant for non-Hispanic Whites but not for non-Hispanic Blacks and Hispanics. A small inverse association was observed between maternal age, pregnancy-associated hypertension and the risk of oral clefts. This study confirms relationships found in previous studies but cannot establish causality. Further investigations of the risk factors for oral clefts would benefit from the study of gene-environment interactions.
Keywords: Oral Clefts, Maternal Smoking, Maternal Diabetes, Maternal Alcohol Consumption, Disparities, Birth Defects
Introduction
A birth defect is a physical or biochemical abnormality that is present at birth and may be inherited or environmentally induced. These functional or structural anomalies are caused by events preceding birth, whether inherited or acquired. Oral clefts (OC), which include cleft lip and palate either singularly or together, are the fifth most common defect identified at birth in the United States and are generally nonlethal.1–2 Individuals born with oral clefts may, however, be at increased risk for long-term morbidity. This morbidity includes shorter lifespan, increased risk of death for all major causes,3 psychiatric disorders,4 and cancers of the breast and brain in females and lung in males.5
The embryology of oral clefts is fairly well understood although the specific mechanisms have not been definitively elucidated. According to Johnson, craniofacial development can be categorized into five stages which usually occur in the third to eighth week of embryonic development, known as the embryonic period.6 During stage one, the three germ layers (ectoderm, mesoderm, and endoderm) are formed through the process of gastrulation. These germ layers will ultimately form all of the embryo’s tissues and organs such as those found in the mouth and epithelium of the nose (ectoderm), the inner linings of the respiratory and digestive tracts (endoderm), and the circulatory system (mesoderm).7 It is at this point where the actual blueprint for the head region is created. This is immediately followed by the formation of the oro-pharynx and neural tube (stage two). In stage three of development, the craniofacial tissues arise and organ systems such as the primary and secondary palates, pharyngeal arches, eye, ear, and brain are formed in stage four. The fifth and final stage is characterized by the differentiation of neural, muscular, and skeletal tissues.
The embryonic period represents a critical time in development.8 During this period, most of the major organs are formed; therefore, maternal exposures to environmental factors may serve to increase the likelihood of the embryo developing structural abnormalities that include OC. Oral clefts are characterized by incomplete formation of the structures that separate the nasal and oral cavities.
Formation of the lip begins during the sixth week of development with the fusion of the frontonasal and maxillar prominences while palatogenesis usually begins at the eighth week and is characterized by the joining of the palatal shelves (which eventually grow and fuse together to form the secondary palate) to the primary palate. Failure of these tissues to completely fuse results in oral clefting.9
The descriptive epidemiology of oral clefts has identified a number of risk factors. Non-modifiable risk factors include race/ethnicity and genetic polymorphisms. Modifiable factors include diabetes, hypertension, smoking, and alcohol consumption.
The objective of the current study was to evaluate the maternal demographic, medical, and behavioral factors associated with oral clefts in newborns utilizing latest available US national data.
Methods
The data used for this study was derived from the 2005 Natality Data Set (Series 21, Number 17) published by the national Center for Health Statistics, Center for Disease Control and Prevention. Variables on the data file included maternal and paternal demographic factors, maternal morbidity, abnormal conditions of the newborn, and maternal behavioral factors (tobacco and alcohol use).
Infants included in this study were: born to US citizens in the continental United States, and its territories and possessions; singleton births; and births where the race of the mother was indicated. The study group included those infants with an isolated oral cleft. The comparison group was comprised of a 1 to 1 random sample of babies born with no notation of the presence of any birth defect. Figure 1 shows a diagram of this process.
Fig 1.
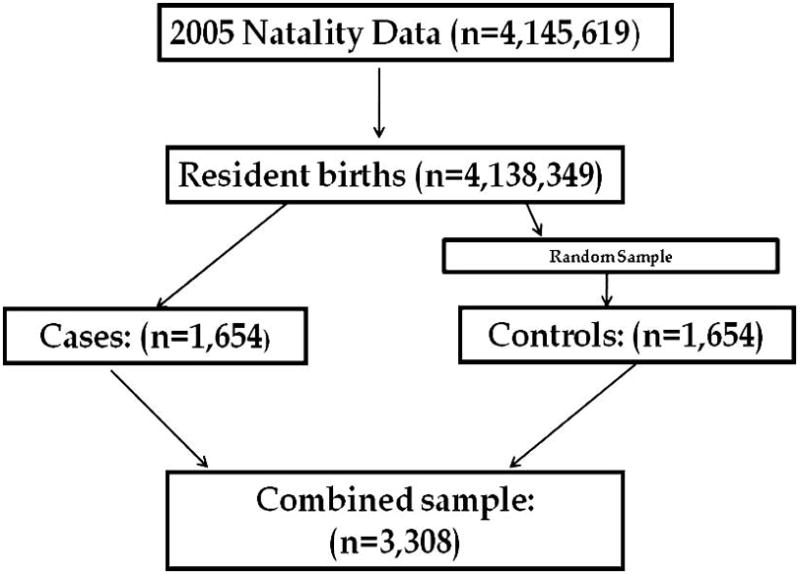
Subject selection process
Several variables were recoded. Maternal race/ethnicity was derived from the maternal race and the Hispanic ethnicity variables. Consequently, the race ethnicity groups were non-Hispanic Whites, non-Hispanic Blacks, Asian/Pacific Islander, Native American/Alaska Native, and Hispanic. The Asian/Pacific Islander and Native American/Alaska Native groups were found to be medically, demographically, and behaviorally indistinct from the non-Hispanic Whites and were combined with this race/ethnicity group. Maternal education was recoded as less than high school, high school graduate, some college, and college graduate. Maternal medical and behavioral factors were coded as 0/1 dummy variables with 0 indicating absence of the factor and 1 indicating presence. Maternal smoking was classified as nonsmokers, light (<10 cigarettes per day), moderate (11–20 cigarettes per day) and heavy (≥21 cigarettes per day).
Statistical analysis was conducted utilizing Stata version 10. To describe the OC and non-OC groups, frequency distributions were calculated. Contingency tables were constructed to calculate the unadjusted odds of OC and to identify potential confounding factors. Multivariate logistic regression was employed to calculate odds ratios adjusted for relevant covariates. The risk of OC was compared in three models, maternal behaviors and morbidity (alcohol, tobacco, hypertension, and diabetes), demographics (race/ethnicity, maternal age, and education) and a comprehensive model comprising significant factors from the previous two models. A P value <.05 was considered statistically significant.
Results
Oral clefts were identified in 3,236 infants in the 2005 birth cohort. Of these, 1,654 were isolated (non-syndromic). Table 1 compares the characteristics of mothers of infants with OC to those without a birth defect. The mean age was similar for the two groups as was the prevalence of alcohol use, maternal diabetes, and maternal hypertension. The groups differed in the race/ethnicity of the mothers, and prevalence of pregnancy-associated hypertension. The race/ethnicity distribution of the two groups was significantly different with non-Hispanic Whites overrepresented in the OC group compared to non-Hispanic Blacks and Hispanics. Tobacco use was significantly higher in the OC mothers than the non-OC mothers (18.4% vs 10.4% respectively). The prevalence of pregnancy-associated hypertension was significantly higher in the OC group.
Table 1.
Comparison of controls (non-OC) and oral cleft infants
| Controls (n=1,654) | Oral clefts (n=1,654) | P value | |
|---|---|---|---|
| Male | 51.1% | 55.7% | .001 |
| Maternal mean age | 27.7±6.2 | 27.2±5.9 | .01 |
| Maternal race/ethnicity | .00 | ||
| NH-White | 55.1% | 67.5% | |
| NH- Black | 14.0% | 6.2% | |
| Hispanic/Latino | 23.5% | 18.8% | |
| Maternal education (years) | 13.0±3.0 | 12.8±2.9 | .03 |
| Maternal tobacco use | 10.4% | 18.4% | .00 |
| Maternal alcohol use | .7% | .9% | NS |
| Maternal diabetes | 3.5% | 4.4% | NS |
| Maternal chronic hypertension | .7% | 1.2% | NS |
| Pregnancy-associated hypertension | 3.2% | 4.6% | .05 |
Table 2 shows the results of multivariate analyses on the risk factors for oral clefts. The analysis reports odds ratios adjusted for maternal age, race/ethnicity, pregnancy-associated hypertension, and tobacco use. Pregnancy-associated hypertension, while significant in the bivariate model was not significantly associated with an OC in the multivariate model. Maternal education did not achieve the requisite level of significance in the multivariate model and was not kept in the regression model. After adjustment, maternal age was significantly associated with the risk of a child with an OC (OR=.98 95% CI .97, .99) for all race/ethnic groups. Non-Hispanic Black mothers (OR=.36 95% CI .28, .47) and Hispanic mothers (OR=0.79 95% CI .63, .98) were less likely than White mothers to have a child with an OC. Cigarette smokers were also at significantly greater risk for giving birth to a child with an OC when compared to non-smokers for all race/ethnic groups combined.
Table 2.
Multivariate analysis of oral clefts*
| Risk factor | Odds ratio | 95% Confidence interval |
|---|---|---|
| Maternal age | .98 | .97, .99 |
| Maternal education | .97 | .94, 1.01 |
| Non-Hispanic Black† | .36 | .28, .47 |
| Hispanic/Latino† | .79 | .63, .98 |
| Pregnancy associated hypertension† | 1.40 | .95, 2.06 |
| Maternal smoking† | 1.66 | 1.32, 2.09 |
Analysis adjusted for maternal age, maternal education, race/ethnicity, pregnancy-associated hypertension, tobacco smoking.
Reference groups are non-Hispanic Whites, no pregnancy-associated hypertension, and non-smokers.
Table 3 provides data addressing the question of whether the significant predictors of OC were equal for non-Hispanic Whites, non-Hispanic Blacks, and Hispanics. Increasing maternal age was consistently associated with a significantly lower risk for all racial/ethnic groups (OR=.98 95% CI .97, .99). Non-Hispanic Blacks with pregnancy-associated hypertension were at lower risk for giving birth to a child with an OC (OR=.09 95% CI .02, .42) and Hispanics with pregnancy-associated hypertension were also at lower risk (OR=.79 95% CI .63, .98) compared to non-Hispanic Whites. Compared to normotensive non-Hispanic Whites, normotensive non-Hispanic Blacks were at lower risk (OR=.38 95% CI .29, .50) as were normotensive Hispanics (OR=.79 95% CI .63, .98). Tobacco smoking conferred an increase in OC risk across all racial/ethnic groups (OR=1.66 95% CI 1.32, 2.09).
Table 3.
Risk factors for Oral Clefts by Ethnic Group*
| Risk Factor | Non-Hispanic White | Non-Hispanic Black | Hispanic/Latino |
|---|---|---|---|
| Maternal age | .98 (.97, .99) | ||
| Pregnancy-associated hypertension | 1 | .09 (.02, .42) | .79 (.63, .98) |
| Normotensive | 1 | .38 (.29, .50) | .79 (.63, .98) |
| Cigarette smoking | 1.66 (1.32, 2.09) |
Adjusted for maternal age, pregnancy-associated hypertension, and tobacco smoking.
Discussion
The current study evaluated the relationship between maternal alcohol use, chronic diabetes, maternal age, pregnancy-related hypertension, maternal smoking, with isolated oral clefts in a national cohort of over 4 million births. The results are in agreement with the majority of studies which examined the relationship between maternal smoking and oral clefts.10–15
These data are in agreement with prior studies that found increased risk for male infants,16 and no association between OC and maternal alcohol consumption.17 The data are at variance with recently published data on the risk of maternal diabetes for OC.18 Maternal age in this analyses was found to be slightly protective (OR=.98) for oral clefts and is contradictory to the findings of previous work,19 but consistent with others.20 Maternal smoking was a consistent risk factor for OC across all race/ethnic groups. The risk for OC among the race/ethnic groups with pregnancy-associated hypertension was not equivalent. It is unknown as to why this exists, however, these differences could be associated with genetic polymorphisms.
The epidemiology of oral clefts is not completely understood although it has been extensively studied. Several researchers have postulated that exogenous exposures represent a small portion of the etiologic fraction. Recent evidence suggests that changes in the DNA, whether inherited or not (epigenetic) may play a major role in determining if clefting will occur.21 It is believed that inheritance related clefting (race and sex) is a result of three to fourteen genes acting multiplicatively,22 while epigenetic DNA modification results from non-inherited (modifiable) factors such as maternal smoking, and pregnancy-associated hypertension which could potentially affect whether the genes that direct the proper formation of the lip and palate are properly expressed.
The limitations of the purely epidemiologic approach to this study are clearly illustrated in the analysis of this phenomenon and serves to demonstrate the need for further study of gene-environment interactions such as those between genes associated with development (tumor growth factor (TGFA, TGFB3) and homeobox containing genes (MSX1)); or genes associated with detoxification (Cytochrome P450 enzymes (CYP1A1 and CYP2E1), microsomal epoxide hydrolases (EPHX), and glutathione-S-transferases (GST) and smoking.23–26 In addition, research focused on the influence of smoking on specific DNA methylation genes such as betaine-homocysteine methyltransferases (BHMT and BHMT2) would serve to help define the exact mechanism(s) involved in smoking-related oral clefts.27
The implications for addressing health disparities are several. First, a disparity has been documented that identifies non-Hispanic Whites at greater risk for OC than either non-Hispanic Blacks or Hispanics. The lack of consistent race/ethnicity-specific associations points to the need for focused approaches to the control of tobacco use during and before pregnancy. The need for continued research on gene-environment interactions is critical to the elucidation of the epidemiology of OC.
Conclusion
The current study found maternal smoking and pregnancy-associated hypertension to be associated with the risk of OC for non-Hispanic White women. No risk factors studied were associated with an increased risk for OC among non-Hispanic Black or Hispanic women. Increasing age conferred minimal risk reduction for all race/ethnic groups. These findings illustrate the limitations of purely epidemiologic analysis of this phenomenon and the need for genetic studies to further elucidate the gene-environment interaction mechanisms.
Acknowledgments
This research was supported in part by the Pharmaceutical Research Center NIH/NCRR grant G12 RR0 3020
References
- 1.Murray J. Invited editorial: Face facts: Gene, environment, and clefts. Am J Hum Genet. 1995;57:227–232. [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell LE, Beaty TH, Lidral AC, et al. Guidelines for the design and analysis of studies on nonsyndromic cleft lip and cleft palate in humans: summary report from a Workshop of the International Consortium for Oral Clefts Genetics. Cleft Palate Craniofac J. 2002;39(1):93–100. doi: 10.1597/1545-1569_2002_039_0093_gftdaa_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 3.Christensen K, Juel K, Hersking AM, Murray JC. Long term follow up study of survival associated with cleft lip and palate at birth. BMJ. 2004;328(7453):1405–1407. doi: 10.1136/bmj.38106.559120.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christensen K, Mortensen PB. Facial clefting and psychiatric diseases: a follow-up of the Danish 1936–1987 Facial Cleft cohort. Cleft Palate Craniofac J. 2002;39(4):392–396. doi: 10.1597/1545-1569_2002_039_0392_fcapda_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 5.Bille C, Winther JF, Bautz A, Murray JC, Olsen J, Christensen K. Cancer risk in persons with oral cleft–a population-based study of 8,093 cases. Am J Epidemiol. 2005;161(11):1047–1055. doi: 10.1093/aje/kwi132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnston MC, Bronsky PT. Prenatal craniofacial development: new insights on normal and abnormal mechanisms. Crit Rev Oral Biol Med. 1995;6(4):368–422. doi: 10.1177/10454411950060040601. [DOI] [PubMed] [Google Scholar]
- 7.Sadler TW, Langman J, Ecker PM. Langman’s Essential Medical Embryology. 9. Baltimore: Lippincott Williams & Wilkins; 2004. [Google Scholar]
- 8.Merritt L. Part 1: Understanding the embryology and genetics of cleft lip and palate. Adv Neonatal Care. 2005;5(2):64–71. doi: 10.1016/j.adnc.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Jugessur A, Murray JC. Orofacial clefting: recent insights into a complex trait. Curr Opin Genet Dev. 2005;15(3):270–278. doi: 10.1016/j.gde.2005.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bille C, Olsen J, Vach W, Knudsen VK, et al. Oral clefts and life style factors–a case-cohort study based on prospective Danish data. Eur J Epidemiol. 2007;22(3):173–181. doi: 10.1007/s10654-006-9099-5. [DOI] [PubMed] [Google Scholar]
- 11.Honein MA, Paulozzi LJ, Watkins ML. Maternal smoking and birth defects: validity of birth certificate data for effect estimation. Public Health Rep. 2001;116(4):327–335. doi: 10.1016/S0033-3549(04)50054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Little J, Cardy A, Munger RG. Tobacco smoking and oral clefts: a meta-analysis. Bull World Health Organ. 2004;82(3):213–218. [PMC free article] [PubMed] [Google Scholar]
- 13.Lorente C, Cordier S, Bergeret A, et al. Maternal occupational risk factors for oral clefts. Occupational Exposure and Congenital Malformation Working Group. Scand J Work Environ Health. 2000;26(2):137–145. doi: 10.5271/sjweh.523. [DOI] [PubMed] [Google Scholar]
- 14.Shi M, Christensen K, Weinberg CR, et al. Orofacial cleft risk is increased with maternal smoking and specific detoxification-gene variants. Am J Hum Genet. 2007;80(1):76–90. doi: 10.1086/510518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wyszynski DF, Wu T. Use of US birth certificate data to estimate the risk of maternal cigarette smoking for oral clefting. Cleft Palate Craniofac J. 2002;39(2):188–192. doi: 10.1597/1545-1569_2002_039_0188_uousbc_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 16.Natsume N, Kawai T, Ogi N, Yoshida W. Maternal risk factors in cleft lip and palate: case control study. Br J Oral Maxillofac Surg. 2000;38(1):23–25. doi: 10.1054/bjom.1999.0133. [DOI] [PubMed] [Google Scholar]
- 17.Meyer KA, Werler MM, Hayes C, Mitchell AA. Low maternal alcohol consumption during pregnancy and oral clefts in offspring: the Slone Birth Defects Study. Birth Defects Res A Clin Mol Teratol. 2003;67(7):509–514. doi: 10.1002/bdra.10057. [DOI] [PubMed] [Google Scholar]
- 18.Correa A, Gilboa SM, Besser LM, et al. Diabetes mellitus and birth defects. Am J Obstet Gynecol. 2008;199(3):237, e1–9. doi: 10.1016/j.ajog.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bille C, Skytthe A, Vach W, et al. Parent’s age and the risk of oral clefts. Epidemiology. 2005;16(3):311–316. doi: 10.1097/01.ede.0000158745.84019.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vieira AR, Orioli IM, Murray JC. Maternal age and oral clefts: a reappraisal. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94(5):530–535. doi: 10.1067/moe.2002.128875. [DOI] [PubMed] [Google Scholar]
- 21.Kuriyama M, Yoshimoto UA, Ichinose S, et al. DNA methylation changes during cleft palate formation induced by retinoic acid in mice. Cleft Palate Craniofac J. 2008;45(5):545–551. doi: 10.1597/07-134.1. [DOI] [PubMed] [Google Scholar]
- 22.Schliekelman P, Slatkin M. Multiplex Relative Risk and Estimation of the Number of Loci Underlying an Inherited Disease. Am J Hum Genet. 2002;71(6):1369–1385. doi: 10.1086/344779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chevrier C, Bahuam M, Perret C, et al. Genetic susceptibilities in the association between maternal exposure to tobacco smoke and the risk of nonsyndromic oral cleft. Am J Med Genet A. 2008;146(A):2396–2406. doi: 10.1002/ajmg.a.32505. [DOI] [PubMed] [Google Scholar]
- 24.Hartsfield JK, Jr, Hickman TA, Everett ET, Shaw GM, Lammer EJ, Finnell RA. Analysis of the EPHX1 113 polymorphism and GSTM1 homozygous null polymorphism and oral clefting associated with maternal smoking. Am J Med Genet A. 2001;102(1):21–24. doi: 10.1002/1096-8628(20010722)102:1<21::aid-ajmg1409>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 25.Ramirez D, Lammer EJ, Iovannisci DM, Laurent C, Finnell RH, Shaw GM. Maternal smoking during early pregnancy, GSTP1 and EPHX1 variants, and risk of isolated orofacial clefts. Cleft Palate Craniofac J. 2007;44(4):366–373. doi: 10.1597/06-011.1. [DOI] [PubMed] [Google Scholar]
- 26.Shaw GM, Wasserman CR, Lammer EJ, et al. Orofacial clefts, parental cigarette smoking, and transforming growth factor-alpha gene variants. Am J Hum Genet. 1996;58(3):551–561. [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu H, Curry S, Wen S, et al. Are the betaine-homocysteine methyltransferase (BHMT and BHMT2) genes risk factors for spina bifida and orofacial clefts? Am J Med Genet A. 2005;135(3):274–277. doi: 10.1002/ajmg.a.30739. [DOI] [PubMed] [Google Scholar]


