Abstract
Serotype K1 Klebsiella pneumoniae is a major cause of liver abscesses and endophthalmitis. This study was designed to identify the role of neutrophils in the development of distant metastatic complications that were caused by serotype K1 K. pneumoniae. An in vitro cellular model was used to assess serum resistance and neutrophil-mediated killing. BALB/c mice were injected with neutrophils containing phagocytosed K. pneumoniae. Serotype K1 K. pneumoniae was significantly more resistant to serum killing, neutrophil-mediated phagocytosis and intra-cellular killing than non-K1 isolates (p<0.01). Electron microscopic examination had similar findings as in the bioassay findings. Intraperitoneal injection of neutrophils containing phagocytosed serotype K1 K. pneumoniae led to abscess formation in multiple sites including the subcutaneous tissue, lung, and liver, whereas no abscess formation was observed in mice injected with non-K1 isolates. The resistance of serotype K1 K. pneumoniae to complement- and neutrophil-mediated intracellular killing results in the dissemination of K. pneumoniae via the bloodstream. Escape from neutrophil intracellular killing may contribute to the dissemination and establishment of distant metastases. Thus, neutrophils play a role as a vehicle for helping K. pneumoniae and contributing to the establishment of liver abscess and distant metastatic complications.
Introduction
Klebsiella pneumoniae is the major pathogen associated with pyogenic liver abscesses [1]–[5]. Most patients with K. pneumoniae liver abscess develop bacteremia and septic metastatic complications including endophthalmitis, meningitis, brain and lung abscesses, and necrotizing fasciitis [5]–[11]. In Europe and North America, the frequencies of K. pneumoniae capsular serotypes K2 and K21 are greater than those of other serotypes [12]. Serotype K1 is the most common serotype of K. pneumoniae isolated from the blood, urine, respiratory tract, and pus (especially from liver abscesses) in Taiwan [13]. However, serotype K1/K2 is the most common cause of liver abscesses [2]. Septic endogenous endophthalmitis, which occurs with a frequency of 3.3–10%, indicates a poor prognosis. Furthermore, 60–93% of patients with liver abscesses and distant metastasis of endophthalmitis were reported to be diabetic [1], [2], [7], [11], [13]–[17].
The association of K. pneumoniae with liver abscesses reflects the substantial pathogenic arsenal of the bacterium (including capsular K antigens, serum resistance, the possession of fimbriae and lipopolysaccharide, and the expression of siderophores) [2], [18]. Less well known, however, is the importance of serotype K1 in the pathogenesis of K. pneumoniae liver abscess with distant metastasis. Bacterial dissemination through the bloodstream is generally believed to promote distant metastasis. However, innate defense mechanisms involving mediators of bacterial cell killing such as polymorphonuclear neutrophils (PMNs) and serum complement are involved in preventing infection and dissemination. Although PMNs are a key anti-infection factor in host defense, they can also cause tissue damage. Previous studies have focused on the molecular mechanisms governing phagocytosis and bacterial killing [19]. The role of these effector functions is not clear when the phagocytosed bacteria are resistant to intracellular killing and when circulating neutrophils are the cause of distant infection. The mechanisms used by K. pneumoniae to colonize, invade, infect, disseminate, and disrupt the host defense are diverse. Each phase of the process involves the interaction of a variety of bacterial and host factors that cause disease. Although neutrophils participate in the first line of defense against bacterial disease, they may no longer play a defensive role when specific bacterial factors are involved. In the present study, we postulated that circulating neutrophils containing serotype K1 K. pneumoniae which are resistant to intracellular killing serve as a vector for disseminating the bacteria to the liver and distant metastatic sites.
Results
Serum bactericidal assay
A total of 37 serotype K1 K. pneumoniae isolates were selected for use in a serum bactericidal assay. Of these, 20 isolates originated from liver abscess. Isolates of serotypes other than K1, 17 isolates, were clinical isolates from sources such as blood, stool and urine. All isolates were incubated with pooled human sera for various time intervals. Serotype K1 K. pneumoniae isolates from liver abscesses were significantly more serum resistant than isolates from non-liver abscesses (p = 0.01). In contrast, the serum resistance of non-K1/K2 strains (one isolate each of K3, K6, K15, K16, K17, K20, K21, K22, K29, K31, K36, K38, and K54, and two isolates each of K55 and K28) from liver abscesses was not significantly different from isolates of the same strains from non-liver abscesses in terms of serum resistance (p = 0.12). Generally, the serum resistance of K1 isolates was higher than that of non-K1/K2 isolates (p = 0.0019) (Table 1).
Table 1. Distribution of serum-sensitive and serum-resistant serotypes of K. pneumoniae selected for this study.
| Serotypes | No. of isolates | p value | |
| Sensitivity (Grade 1–4) | Resistance (Grade 5–6) | ||
| K1 (n = 20) | 4 | 16 | |
| Non-K1/K2 (n = 17)* | 12 | 5 | |
| K1 vs. non-K1/K2 | 0.0019 | ||
*The 17 strains of non-K1/K2 contained one isolate each of K3, K6, K15, K16, K17, K20, K21, K22, K29, K31, K36, K38, and K54 and two isolates each of K55 and K28. The grading system used to assess serum resistance was described by Podschun et al. (1991).
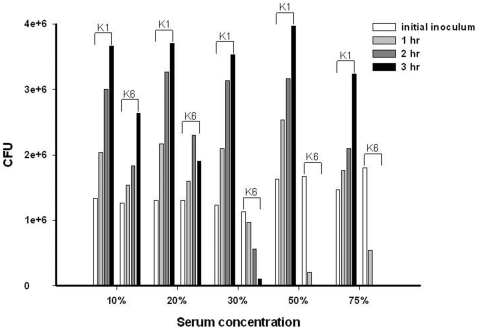
To confirm the strength and specificity of the serum effect, a serum bactericidal assay was performed with different concentrations of serum. Concentrations ranging from 10 to 75% serum were incubated with serotypes K1 and K6 K. pneumoniae (ATCC700603), respectively. Grade 6 level serum resistance was evident for K1 over the serum concentration range 10–75% (Fig. 1). For serotype K6, grade 5 or 6 level serum resistance was evident at serum concentrations at a range of 10–20%, but no serum resistance was evident at a range of 30–75% (Fig. 1). High concentrations of serum inhibited the growth of K6, but not K1.
Figure 1. The effect of serum dilution on the serum killing of K. pneumoniae serotypes K1 and K6.
A high concentration of serum inhibited the growth of K6 but not K1. Each experiment was repeated in triplicate. The bacteria counts are expressed by cell forming unit (CFU).
Viability of serotype K1 and K6 K. pneumoniae following phagocytosis by neutrophils
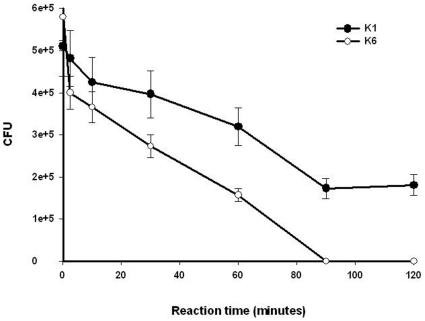
The decline in viable bacterial counts in the neutrophil phagocytosis assay was slower for serotype K1 than serotype K6 (Fig. 2). The viable counts of serotype K1 stabilized and increased modestly after 90 minutes of phagocytosis, whereas the viable counts of serotype K6 declined sharply to below the limit of detection after 90 minutes of phagocytosis (Fig. 2). The difference in the counts of viable intracellular bacteria between phagocytosed K1 and K6 was statistically significant (p<0.01).
Figure 2. The rate at which neutrophils from 11 healthy volunteers killed serotypes K1 and K6 K. pneumoniae.
The percentage of killing is expressed as the mean ± SD and calculated as the total number of bacteria killed divided by the number of viable bacteria in the initial inoculum. K1 vs. K6: p<0.01 (n = 3 for each).
Electron microscopic evaluation of intracellular killing
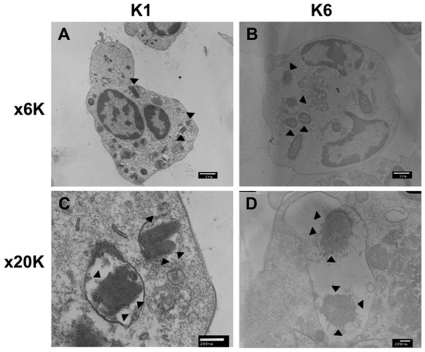
Following phagocytosis, K1 and K6 bacteria were evident within vacuoles. While changes in K1 cell wall morphology were minimal (Fig. 3A), K6 cell walls were completely disrupted, resulting in lysis (Fig. 3B).
Figure 3. Electron microscopic examination of phagocytosed serotypes K1 and K6 K. pneumoniae.
Vacuolated bacteria are evident. The cell wall structure of serotype K1 appears intact, and cell division patterns are visible despite digestion by neutrophils (A & C). In contrast, lysis and the loss of cell wall structure of serotype K6 is seen in the vacuoles of neutrophils (B & D). (Bar = 1 µm, cells magnified ×6K; bar = 200 nm, cells magnified ×20K). Bacteria are pointed by arrow.
Inoculation of neutrophil-phagocytosed serotype K1 and K6 K. pneumoniae
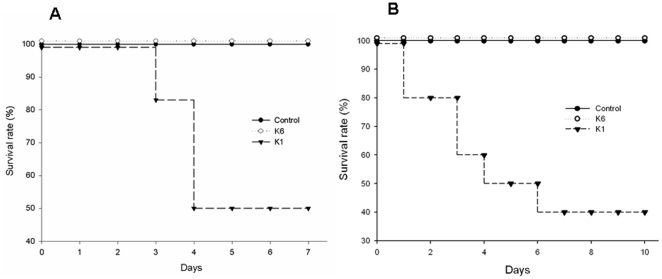
Survival after infection with neutrophil-phagocytosed serotypes K1 and K6 K. pneumoniae was assessed using a murine model. The number of viable bacteria phagocytosed by human neutrophils averaged 4×103 and 5×105 for serotypes K1 and K6, respectively. Mouse mortality was significantly higher (p<0.01) in mice receiving human neutrophils containing phagocytosed K1 than those receiving human neutrophils containing phagocytosed K6 (Fig. 4A). No mortality was observed in mice that received neutrophils containing K6 or bacteria-free neutrophils. The number of viable bacteria phagocytosed by mouse neutrophils averaged 7×103 and 6×105 for serotypes K1 and K6, respectively. Whether the source of the neutrophils containing serotype K1 or K6 K. pneumoniae was mouse or human, the results of their administration were similar (Fig. 4B), indicating that mortality in mice was not caused by the immune incompatibility of human neutrophils.
Figure 4. Survival rate of BALB/c mice (n = 12 per group) following intraperitoneal injection of neutrophils containing phagocytosed serotypes K1 and K6 K. pneumoniae.
The number of viable intracellular bacteria were 4×103 and 5×105 for serotypes K1 and K6. Survival was observed for 10 days. Each experiment was repeated twice. (A) Human neutrophils. (B) BALB/c neutrophils. (p<0.001, for the serotype K1 group vs. the K6 and control groups in human and mouse experiments.)
Gross and molecular examination of abscess formation after intraperitoneal inoculation of neutrophils
Abscess formation was assessed by gross examination after intraperitoneal (IP) inoculation of serotype K1 K. pneumoniae. All mice died after the inoculation of 4.75×104 colony-forming units (cfu) of phagocytosed serotype K1, whereas no mice died following inoculation with a similar dose of phagocytosed serotype K6 (Table 2). Abscesses formed on the necks (Fig. 5A and 5B) and abdomens of mice that received serotype K1 (range, 2.56×102 to 4.83×103; Fig. 5C and 5D). Abscesses on the neck formed after the administration of approximately 103 cfu, but not following a dose of 102 cfu. The percentage of mice developing abdominal abscesses did not increase with increasing K1 inoculum (102 to 103 cfu; Table 2).
Table 2. Mortality and abscess formation after intraperitoneal (IP) injection of neutrophil-phagocytosed serotype K1 and K6 K. pneumoniae.
| Serotype (no. of mice) | Dose (cfu) | Mortality (%) | Abscess formation | |
| Neck (%) | Abdomen (%) | |||
| K1(10) | 4.75×104 | 10 (100) | 0 | 0 |
| K6 (10) | 0 | 0 | 0 | |
| K1 (119)* | ||||
| Range | 1.42–4.83×103 | 59 (49.58) | 6 (5.04) | 8 (6.72) |
| K1 (50)† | ||||
| Range | 2.56–7.90×102 | 18 (36.00) | 0 | 8 (16.00) |
*10 groups of mice: 8 groups (each n = 10), 1 group (n = 19), and 1 group (n = 20) were injected IP with 103 cfu of neutrophil-phagocytosed serotype K1.
5 groups (each n = 10) were injected IP with 102 cfu of neutrophil-phagocytosed serotype K1.
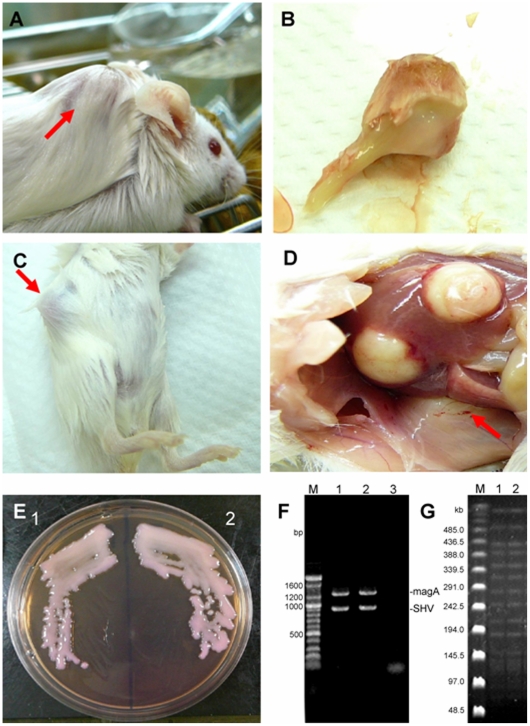
Figure 5. Gross abscess appearance in mice injected with neutrophils containing phagocytosed serotype K1 K. pneumoniae.
A: Neck. B: Surgical removal of the abscess site. C: Abdomen. D: Abscess formation in liver and subcutaneous areas. E: (1) Morphological features of injected serotype K1 K. pneumoniae and (2) culture of pus discharge from the abscess site. F: Detection of the serotype K1 specific magA gene and intrinsic bla SHV gene from (1) the parental strain and (2) bacteria isolated from the abscess site. G: PFGE molecular typing of (1) the parental strain and (2) bacteria isolated from the abscess site. Sites of abscess formation are indicated by red arrows.
The isolate recovered from pus discharge and the inoculated serotype K1 isolate were morphologically identical, and both had magA (Fig. 5E and 5F). Molecular typing by PFGE showed that the strain isolated from the liver was identical to the K1 strain of K. pneumoniae that had been parenterally administered (Fig. 5G).
Pathological features at liver, spleen, lung, kidney, and metastatic infection sites
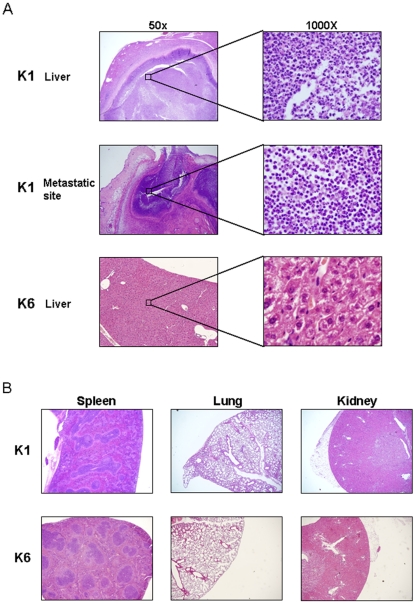
Necrosis was identified in tissue sections from sites of metastatic infection as well as the liver, but not in the lung, spleen, or kidney of mice injected with serotype K1 (Fig. 6A and 6B). Inflammation was not evident in liver, spleen, lung, or kidney samples from mice receiving serotype K6. Numerous PMN and monocytes had infiltrated liver and subcutaneous tissue (a metastatic infection site), indicating severe inflammation. K. pneumoniae were identified in liver and subcutaneous tissues sections (Fig. 6A).
Figure 6. Pathologic features of abscesses due to serotypes K1 and K6 K. pneumoniae in different mouse tissues.
(A). Necrosis can be seen in the liver and at metastatic infection sites in mice injected with K1 K. pneumoniae. No inflammation was seen in the liver of mice injected with serotype K6. (B). Inflammation was not found in the lungs, spleen, or kidneys of mice injected with serotypes K1 and K6.
Discussion
In this study, serotype K1 was found to be more resistant to killing by both serum killing (p<0.01) (Fig. 1) and intracellular killing after neutrophil phagocytosis (p<0.01) (Fig. 2) than non-K1 serotypes in cellular and murine models. Electron microscopy agreed with the finding of the bioassay (Fig. 3). Furthermore, cells that remained viable for extended periods (i.e., phagocytosed K1 cells), but not phagocytosed K6 cells, were linked to lethal septicemia (Fig. 4A and 4B) and abscess formation in mice (Figs. 5, 6).
Clinical infection by serotypes K2, K7, and K21 has been linked to the resistance of the polysaccharide capsule of K. pneumoniae to phagocytosis and serum killing [20]–[22]. Previously, we observed that serotype K1 is the main serotype in K. pneumoniae-mediated liver abscess and is associated with the development of endophthalmitis and meningitis, particularly in diabetic patients [2]. Therefore, it was of interest to understand how K1 virulence factors, particularly the capsule, contribute to the pathogenesis of K. pneumoniae-mediated liver abscess and other complications of infection. Colonization of the gut by K. pneumoniae is believed to be the first step in K. pneumoniae liver abscess development. The intestine communicates with the liver via the hepatic portal system, and a transient bacteremia of bowel flora (such as Escherichia coli) is not uncommon [23], although it remains to be determined how this bacterium passes through the gut epithelial barrier into the bloodstream. Neutrophils and serum complement are key factors in preventing bacterial multiplication leading to further dissemination. However, phagocytosis of K. pneumoniae is also part of the infectious process, as neutrophils carrying phagocytosed K. pneumoniae may circulate in the bloodstream and transport K1 bacteria to distant sites causing abscess formation. The studies presented herein suggest that neutrophils containing phagocytosed K1 were lysed by the immune system shortly after their injection into mice, releasing free K1 bacteria that then disseminated through the bloodstream. However, we previously showed that injection of as few as <10 cfu of free K1 could induce septic shock, leading to multiorgan failure and death in all mice within four days [24]. The chronic development of abscesses at the liver or other sites was not observed. In the present model using neutrophils containing phagocytosed K1 bacteria, at least ≥102 cfu were required for the development of liver and neck abscesses. Abscess formation became grossly apparent around 18–21 days, indicating a chronic infection. If neutrophils containing phagocytosed K1 were rapidly lysed after injection, they should have caused mortality even sooner than free K1 bacteria because the inoculum released from neutrophils contained a greater number of bacteria. Thus, immigrating neutrophils play a role in killing bacteria trapped in the liver [25].
Bacteremia frequently occurs in K. pneumoniae liver abscesses with septic endophthalmitis via portal entry and perhaps via the bloodstream [1], [5], [7], [11]. This study demonstrated the ability of bacteria to escape serum lysis and neutrophil killing during their passage through the circulatory system. Our results indicate that serum resistance and intracellular survival within neutrophils contribute to the pathogenesis of this disease. Previous studies have shown that bacterial pathogens such as Mycobacterium tuberculosis and Salmonella typhimurium persist for years within phagocytic cells such as macrophages and neutrophils, and within lung granulomas and mesenteric lymph nodes of humans [26], [27]. These pathogens will sometimes reactivate, causing an acute infection. During instances of typhoid fever, Salmonella-infected phagocytes gain access to the lymphatic system and bloodstream, facilitating bacterial spread to the liver. Whether Kelbsiella-infected neutrophils gain access through a similar means, leading to liver abscess and distant metastasis, remains to be shown. Further studies are needed to confirm this hypothesis.
Serotypes K1 and K2 are associated with complicated endophthalmitis, particularly in cases of diabetes mellitus, which occurs in 93% of afflicted patients [2]. A Diabetes-related reduction in the efficiency of phagocytosis, chemotaxis, and intracellular killing has been reported [28]–[32]. Although diabetes mellitus contributes to the pathogenesis of K. pneumoniae-induced liver abscess complicated with endophthalmitis [1], [9], [10], metastatic infection sites do not arise in all cases of liver abscess due to serotype K1/K2 bacteria. Whether septic endophthalmitis in patients without diabetes mellitus is due to differences in the virulence of serotype K1 bacteria remain to be elucidated. In the present in vivo experiments, the survival of serotype K1 K. pneumoniae within neutrophils and escape from serum-mediated killing contributed to the development of septic infections. One intriguing finding is that 12–16% of mice developed metastatic infections. These results are quite similar to the previous surveillance of liver abscesses in which approximately 10% of patients developed distant metastatic foci such as endophthalmitis or meningitis [2]. Metastatic infections in the neck were only found after the injection of a relatively high (1.42–4.83×103 cfu), but not a low (2.56–7.90×102 cfu) dose of phagocytosed K1. Furthermore, increasing the number of cells containing phagocytosed K1 bacteria increased mortality but not the percentage of mice developing distant metastases. These results reflect the contribution made by increasing numbers of phagocytosed K1 bacteria in both dissemination of the bacteria to distant sites and the severity of infection. In conclusion, neutrophils containing phagocytosed serotype K1 bacteria may contribute to the establishment of liver abscesses and distant metastasis in disease caused by hepatovirulent K. pneumoniae.
Materials and Methods
Bacterial strains
Thirty-seven strains of serotype K1 and non-K1/K2 K. pneumoniae were isolated from patients with liver abscesses complicated with endophthalmitis and from patients with non-liver abscesses. The study focused on serotype K1 because serotypes K1/K2 are linked to the development of liver abscess [2]. The capsular serotype was confirmed by a capsular swelling technique and countercurrent immunoelectrophoresis [13]. All strains were kept frozen at –80°C in beads containing a cryopreservative. Suspensions were established by an overnight incubation at 37°C in brain heart infusion broth prior to experiments. The concentration and viability of the bacteria were determined by quantitative plate counting using established techniques.
Mice
Pathogen-free, 6–8 week-old, male BALB/c mice weighing 20–25 g were obtained from the National Laboratory Animal Center (Taipei, Taiwan). The protocols for all experiments involving mice were approved by the Committee on Institutional Animal Care and Use, Tri-Service General Hospital and National Defense Medical Hospital (IACUC-05-166).
Preparation of normal human and BALB/c sera
Normal human serum was obtained from ten healthy volunteers, who had provided informed written consent. Sixty milliliters of freshly drawn heparin-free blood was clotted at room temperature. BALB/c serum was prepared following heart puncture after inhalation of carbon monoxide. The clotted blood was centrifuged at 1000× g for 20 min at 20°C. The sera were pooled, and aliquots were stored at –70°C until use.
Isolation of human and BALB/c mouse neutrophils
Isolation of neutrophils from healthy volunteers met ethically approved guidelines [33], [34]. Healthy volunteers were screened after giving informed written consent [34]. Neutrophils from BALB/c mice were isolated from the peritoneum as previously described [33]. The cell concentration was adjusted to 1×107 cells/mL. The viability of the isolated neutrophils exceeded 95%, as determined by Trypan blue exclusion. All procedures involving animals and humans were approved by the Tri-Service General Hospital Institutional Review Board and the Committee on Institutional Animal Care, Use, and Ethics, National Defense Medical Center (TSGHIRB 095-05-0054 and IACUC-05-166).
In vitro serum bactericidal assay
Serum bactericidal activity was measured as described previously with slight modifications [22], [35]. Bacteria grown in nutrient broth were collected during the early logarithmic phase. The viable bacterial concentration was adjusted to 1×106 cfu/mL. Twenty-five microliters of bacteria was added to 75 µL of pooled human sera in a 10×75 mm Falcon polypropylene tube (BD Biosciences, Franklin Lakes, New Jersey). Tubes were agitated for 0, 60, 120, or 180 min. To determine the number of viable bacteria after exposure to serum, an aliquot of each bacterial suspension was removed at the designated time point, diluted 10-fold by the addition of Mueller-Hinton broth, plated on Mueller-Hinton agar, and assayed as described below. Results are expressed as percentage of the inoculum, and responses in terms of viable counts were graded from 1 to 6 as described previously [22], [35]. Grade 1 represented viable counts that were <10% of the inoculum after 1 and 2 h, and <0.1% after 3 h. Grade 2 represented viable counts that were 10–100% of the inoculum after 1 h and <10% after 3 h. Grade 3 represented viable counts that exceeded those of the inoculum after 1 h, but were <100% after 2 and 3 h. Grade 4 represented viable counts that were >100% of the inoculum after both 1 and 2 h, but <100% after 3 h. Grade 5 represented viable counts that were >100% of the inoculum after 1, 2, and 3 h but that decreased during the third hour. Finally, grade 6 represented viable counts that exceeded those of the inoculum after 1, 2, and 3 h and that increased throughout this time period. Each strain was tested at least three times. A strain was considered serum resistant or serum sensitive if the grading was the same in all experiments. Each isolate was classified as highly sensitive (grades 1 or 2), intermediately sensitive (grades 3 or 4), or resistant (grades 5 or 6).
In vitro neutrophil bactericidal assay
A neutrophil bactericidal assay was performed as described previously, with slight modifications [36], [37]. Serum was thawed immediately prior to use and was kept on ice until assayed. Neutrophils (1×106), 10% v/v pooled normal human serum, and viable K. pneumoniae (4×107) were added to 10×75 mm polypropylene tubes (BD Biosciences). The capped tubes were incubated in a shaking water bath at 37°C with continuous agitation for 0, 2.5, 10, 30, 60, 90, or 120 min. Samples were removed at each timepoint and placed in an ice bath. Prior to counting bacteria that survived neutrophil phagocytosis, gentamicin (100 µg/ml) was used to kill extracellular and adherent bacteria and to ensure that no extracellular bacteria were counted as intracellular bacteria. Supernatants of culture medium were removed after incubation with gentamicin for 30 minutes and spread onto an agar plate to confirm that no viable bacteria were present before counting. After confirming that no viable extracellular bacteria or adherent bacteria were present, neutrophils were lysed with a hypotonic (sterilized double distilled water) solution for 5 min. Complete lysis of neutrophils was confirmed by flow cytometry. Adjusted zero was applied in the first tube, followed by the immediate addition of gentamicin and a 30 minute-incubation. Bacterial viability was determined as described above, and the reduction in viable numbers was used to determine the number of bacteria killed. Results are expressed as the percentage of bacteria killed over time: 100%× (1- [number of viable bacteria after adding neutrophils/number of viable bacteria before adding neutrophils]).
Evaluation of intracellular killing by electron microscopy
Purified neutrophils were mixed with opsonized bacteria for 30 min under the conditions described above for the neutrophil killing assay. Extracellular undigested bacteria and adherent bacteria were killed by gentamicin (100 µg/ml, 37°C, 30 min). Cells were fixed as previously detailed [37], dehydrated in a graded ethanol series, and embedded in Eponate-12 (Ted Pella, Redding, California). Ultra-thin sections were stained with uranyl acetate and lead citrate (Electron Microscopy Sciences, Fort Washington, Pennsylvania) and examined using a JEOL 1230 transmission electron microscope (JEOL, Tokyo, Japan) as described previously [38].
Survival of K. pneumoniae after neutrophil-mediated phagocytosis
To confirm whether K. pneumoniae can survive after phagocytosis and contribute to infection, purified neutrophils (1×106 cells/mL) from normal healthy subjects or BALB/c mice were incubated with opsonized and viable K1 or K6 K. pneumoniae (4×107 cells/mL), respectively, for 30 minutes and then treated with gentamicin (100 µg/ml, 37°C, 30 min). The number of phagocytosed bacteria was determined by hypotonic lysis and the plating of serially diluted preparations. Neutrophils (1×105 cells/mL) containing viable intracellular bacteria were injected intraperitoneally (IP) into BALB/c mice. An equivalent number of similarly injected neutrophils without intracellular bacteria served as controls. Mortality was assessed for up to 10 days. Each experiment was repeated twice.
Murine sepsis model
To replicate murine IP-derived sepsis experimentally, BALB/c mice were injected with varying numbers of viable forms of K1 or K6 serotypes of K. pneumoniae. The mice were scored for their gross appearance every 24 h for 21 days, after which they were sacrificed. The liver, lungs, and spleen were removed aseptically, fixed with 10% (v/v) formalin and embedded in paraffin. Histological sections were stained using hematoxylin and eosin stain (H&E stain) and observed by light microscopy.
Pulsed-field gel electrophoresis (PFGE) analysis
Total DNA was prepared and PFGE was performed as described previously [39]. The restriction enzyme XbaI (New England Biolabs, Beverly, MA, USA) was used at the manufacturer's suggested temperature. Restriction fragments were separated by PFGE in 1% agarose gel (Bio-Rad, Hercules, CA, USA) in 0.5×TBE buffer (45 mM Tris, 45 mM boric acid, 1.0 mM EDTA, pH 8.0) for 22 h at 200 V at a temperature of 14°C, with ramp times of 2 to 40 s using the Bio-Rad CHEF MAPPER apparatus (Bio-Rad Laboratories, Richmond, CA, USA). Gels were then stained with ethidium bromide and photographed under ultraviolet light. The resulting genomic DNA profiles, or “fingerprints”, were interpreted according to established guidelines [40].
Statistical analyses
Multivariate analyses were performed to compare differences in the adjusted serum resistance rates and neutrophil killing rates between K1 and non-K1/K2 serotypes. If significant results were found, these variables were examined by univariate analyses. Data are expressed as least squares mean (LSMEAN) ± standard error of the mean (SEM). Statistical analyses of survival curves were conducted using the log rank test. All statistical tests were two-sided, and p-values <0.05 were considered to be statistically significant.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by grants from National Science Council (NSC93-2314-B-016-018, NSC94-2314-B-016-004, NSC95-2314-B-016-001, NSC96-2314-B-016-006-MY1&2), Tri-Service General Hospital (DOD96-22 & 96-32) and from National Health Research Institutes, Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Chang FY, Chou MY. Comparison of pyogenic liver abscesses caused by Klebsiella pneumoniae and non-K. pneumoniae pathogens. J Formos Med Assoc. 1995;94:232–237. [PubMed] [Google Scholar]
- 2.Fung CP, Chang FY, Lee SC, Hu BS, Kuo BI, et al. A global emerging disease of Klebsiella pneumoniae liver abscess: is serotype K1 an important factor for complicated endophthalmitis? Gut. 2002;50:420–424. doi: 10.1136/gut.50.3.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lederman ER, Crum NF. Pyogenic liver abscess with a focus on Klebsiella pneumoniae as a primary pathogen: an emerging disease with unique clinical characteristics. Am J Gastroenterol. 2005;100:322–331. doi: 10.1111/j.1572-0241.2005.40310.x. [DOI] [PubMed] [Google Scholar]
- 4.Rahimian J, Wilson T, Oram V, Holzman RS. Pyogenic liver abscess: recent trends in etiology and mortality. Clin Infect Dis. 2004;39:1654–1659. doi: 10.1086/425616. [DOI] [PubMed] [Google Scholar]
- 5.Tsay RW, Siu LK, Fung CP, Chang FY. Characteristics of bacteremia between community-acquired and nosocomial Klebsiella pneumoniae infection: risk factor for mortality and the impact of capsular serotypes as a herald for community-acquired infection. Arch Intern Med. 2002;162:1021–1027. doi: 10.1001/archinte.162.9.1021. [DOI] [PubMed] [Google Scholar]
- 6.Casanova C, Lorente JA, Carrillo F, Perez-Rodriguez E, Nunez N. Klebsiella pneumoniae liver abscess associated with septic endophthalmitis. Arch Intern Med. 1989;149:1467. [PubMed] [Google Scholar]
- 7.Cheng DL, Liu YC, Yen MY, Liu CY, Wang RS. Septic metastatic lesions of pyogenic liver abscess. Their association with Klebsiella pneumoniae bacteremia in diabetic patients. Arch Intern Med. 1991;151:1557–1559. [PubMed] [Google Scholar]
- 8.Dylewski JS, Dylewski I. Necrotizing fasciitis with Klebsiella liver abscess. Clin Infect Dis. 1998;27:1561–1562. doi: 10.1086/517760. [DOI] [PubMed] [Google Scholar]
- 9.Higashi T, Makino Y, Katsurada K. [A case of gas-containing liver abscess with multiple metastatic lesions]. Kansenshogaku Zasshi. 1995;69:1017–1020. doi: 10.11150/kansenshogakuzasshi1970.69.1017. [DOI] [PubMed] [Google Scholar]
- 10.Ho PL, Tang WM, Yuen KY. Klebsiella pneumoniae necrotizing fasciitis associated with diabetes and liver cirrhosis. Clin Infect Dis. 2000;30:989–990. doi: 10.1086/313791. [DOI] [PubMed] [Google Scholar]
- 11.Liu YC, Cheng DL, Lin CL. Klebsiella pneumoniae liver abscess associated with septic endophthalmitis. Arch Intern Med. 1986;146:1913–1916. [PubMed] [Google Scholar]
- 12.Cryz SJ, Jr, Mortimer PM, Mansfield V, Germanier R. Seroepidemiology of Klebsiella bacteremic isolates and implications for vaccine development. J Clin Microbiol. 1986;23:687–690. doi: 10.1128/jcm.23.4.687-690.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fung CP, Hu BS, Chang FY, Lee SC, Kuo BI, et al. A 5-year study of the seroepidemiology of Klebsiella pneumoniae: high prevalence of capsular serotype K1 in Taiwan and implication for vaccine efficacy. J Infect Dis. 2000;181:2075–2079. doi: 10.1086/315488. [DOI] [PubMed] [Google Scholar]
- 14.Chou FF, Sheen-Chen SM, Chen YS, Chen MC. Single and multiple pyogenic liver abscesses: clinical course, etiology, and results of treatment. World J Surg. 1997;21:384–388; discussion 388–389. doi: 10.1007/pl00012258. [DOI] [PubMed] [Google Scholar]
- 15.Saccente M. Klebsiella pneumoniae liver abscess, endophthalmitis, and meningitis in a man with newly recognized diabetes mellitus. Clin Infect Dis. 1999;29:1570–1571. doi: 10.1086/313539. [DOI] [PubMed] [Google Scholar]
- 16.Yarng SS, Hsieh CL, Chen TL. Vitrectomy for endogenous Klebsiella pneumoniae endophthalmitis with massive subretinal abscess. Ophthalmic Surg Lasers. 1997;28:147–150. [PubMed] [Google Scholar]
- 17.Yoon YH, Lee SU, Sohn JH, Lee SE. Result of early vitrectomy for endogenous Klebsiella pneumoniae endophthalmitis. Retina. 2003;23:366–370. doi: 10.1097/00006982-200306000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998;11:589–603. doi: 10.1128/cmr.11.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sawyer DW, Donowitz GR, Mandell GL. Polymorphonuclear neutrophils: an effective antimicrobial force. Rev Infect Dis. 1989;11(Suppl 7):S1532–1544. doi: 10.1093/clinids/11.supplement_7.s1532. [DOI] [PubMed] [Google Scholar]
- 20.Agapova OV, Bondarenko VM, Polikarpov NA, Lykova EA, Pokhil SN, et al. [The pathogenicity enzymes of clinical strains of Klebsiella pneumoniae]. Zh Mikrobiol Epidemiol Immunobiol. 1999:5–8. [PubMed] [Google Scholar]
- 21.Highsmith AK, Jarvis WR. Klebsiella pneumoniae: selected virulence factors that contribute to pathogenicity. Infect Control. 1985;6:75–77. doi: 10.1017/s0195941700062640. [DOI] [PubMed] [Google Scholar]
- 22.Podschun R, Ullmann U. Klebsiella capsular type K7 in relation to toxicity, susceptibility to phagocytosis and resistance to serum. J Med Microbiol. 1992;36:250–254. doi: 10.1099/00222615-36-4-250. [DOI] [PubMed] [Google Scholar]
- 23.Gauger EJ, Leatham MP, Mercado-Lubo R, Laux DC, Conway T, et al. Role of motility and the flhDC Operon in Escherichia coli MG1655 colonization of the mouse intestine. Infect Immun. 2007;75:3315–3324. doi: 10.1128/IAI.00052-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeh KM, Kurup A, Siu LK, Koh YL, Fung CP, et al. Capsular serotype K1 or K2, rather than magA and rmpA, is a major virulence determinant for Klebsiella pneumoniae liver abscess in Singapore and Taiwan. J Clin Microbiol. 2007;45:466–471. doi: 10.1128/JCM.01150-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gregory SH, Sagnimeni AJ, Wing EJ. Bacteria in the bloodstream are trapped in the liver and killed by immigrating neutrophils. J Immunol. 1996;157:2514–2520. [PubMed] [Google Scholar]
- 26.Monack DM, Bouley DM, Falkow S. Salmonella typhimurium persists within macrophages in the mesenteric lymph nodes of chronically infected Nramp1+/+ mice and can be reactivated by IFNgamma neutralization. J Exp Med. 2004;199:231–241. doi: 10.1084/jem.20031319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhen M, Eriksson S, Clements M, Bergstrom S, Normark SJ. The basis of persistent bacterial infections. Trends Microbiol. 2003;11:80–86. doi: 10.1016/s0966-842x(02)00038-0. [DOI] [PubMed] [Google Scholar]
- 28.Brayton RG, Stokes PE, Schwartz MS, Louria DB. Effect of alcohol and various diseases on leukocyte mobilization, phagocytosis and intracellular bacterial killing. N Engl J Med. 1970;282:123–128. doi: 10.1056/NEJM197001152820303. [DOI] [PubMed] [Google Scholar]
- 29.Han SH. Review of hepatic abscess from Klebsiella pneumoniae. An association with diabetes mellitus and septic endophthalmitis. West J Med. 1995;162:220–224. [PMC free article] [PubMed] [Google Scholar]
- 30.Lin JC, Siu LK, Fung CP, Tsou HH, Wang JJ, et al. Impaired phagocytosis of capsular serotypes K1 or K2 Klebsiella pneumoniae in type 2 diabetes mellitus patients with poor glycemic control. J Clin Endocrinol Metab. 2006;91:3084–3087. doi: 10.1210/jc.2005-2749. [DOI] [PubMed] [Google Scholar]
- 31.Molenaar DM, Palumbo PJ, Wilson WR, Ritts RE., Jr Leukocyte chemotaxis in diabetic patients and their nondiabetic first-degree relatives. Diabetes. 1976;25:880–883. [PubMed] [Google Scholar]
- 32.Mowat A, Baum J. Chemotaxis of polymorphonuclear leukocytes from patients with diabetes mellitus. N Engl J Med. 1971;284:621–627. doi: 10.1056/NEJM197103252841201. [DOI] [PubMed] [Google Scholar]
- 33.Itou T, Collins LV, Thoren FB, Dahlgren C, Karlsson A. Changes in activation states of murine polymorphonuclear leukocytes (PMN) during inflammation: a comparison of bone marrow and peritoneal exudate PMN. Clin Vaccine Immunol. 2006;13:575–583. doi: 10.1128/CVI.13.5.575-583.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin JC, Chang FY, Fung CP, Xu JZ, Cheng HP, et al. High prevalence of phagocytic-resistant capsular serotypes of Klebsiella pneumoniae in liver abscess. Microbes Infect. 2004;6:1191–1198. doi: 10.1016/j.micinf.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Podschun R, Teske E, Ullmann U. Serum resistance properties of Klebsiella pneumoniae and K. oxytoca isolated from different sources. Zentralbl Hyg Umweltmed. 1991;192:279–285. [PubMed] [Google Scholar]
- 36.Dugger KO, Galgiani JN. Neutrophil killing of single microorganisms as measured by a new method. Diagn Microbiol Infect Dis. 1989;12:199–203. doi: 10.1016/0732-8893(89)90015-1. [DOI] [PubMed] [Google Scholar]
- 37.Nielsen SL, Black FT. Extracellular and intracellular killing in neutrophil granulocytes of Staphylococcus aureus with rifampicin in combination with dicloxacillin or fusidic acid. J Antimicrob Chemother. 1999;43:407–410. doi: 10.1093/jac/43.3.407. [DOI] [PubMed] [Google Scholar]
- 38.Wang JJ, Lu Y, Ratner L. Particle assembly and Vpr expression in human immunodeficiency virus type 1-infected cells demonstrated by immunoelectron microscopy. J Gen Virol. 1994;75(Pt 10):2607–2614. doi: 10.1099/0022-1317-75-10-2607. [DOI] [PubMed] [Google Scholar]
- 39.D'Agata EM, Gerrits MM, Tang YW, Samore M, Kusters JG. Comparison of pulsed-field gel electrophoresis and amplified fragment-length polymorphism for epidemiological investigations of common nosocomial pathogens. Infect Control Hosp Epidemiol. 2001;22:550–554. doi: 10.1086/501950. [DOI] [PubMed] [Google Scholar]
- 40.Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]