Abstract
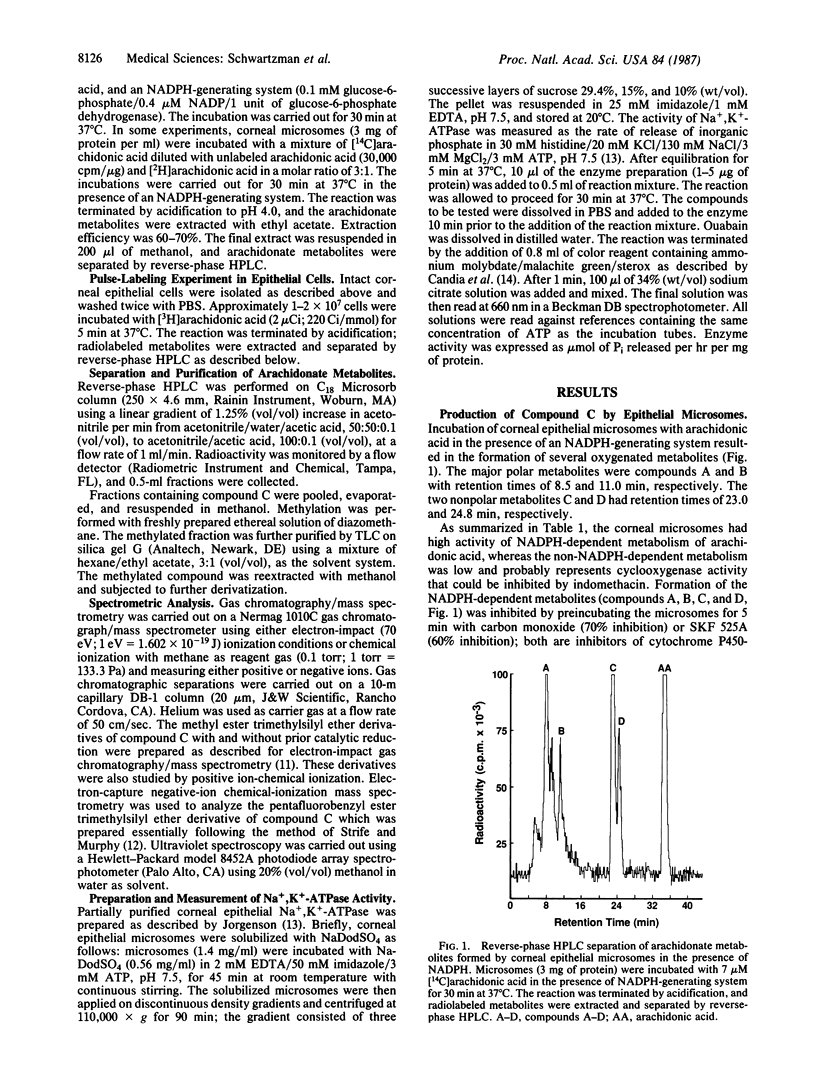
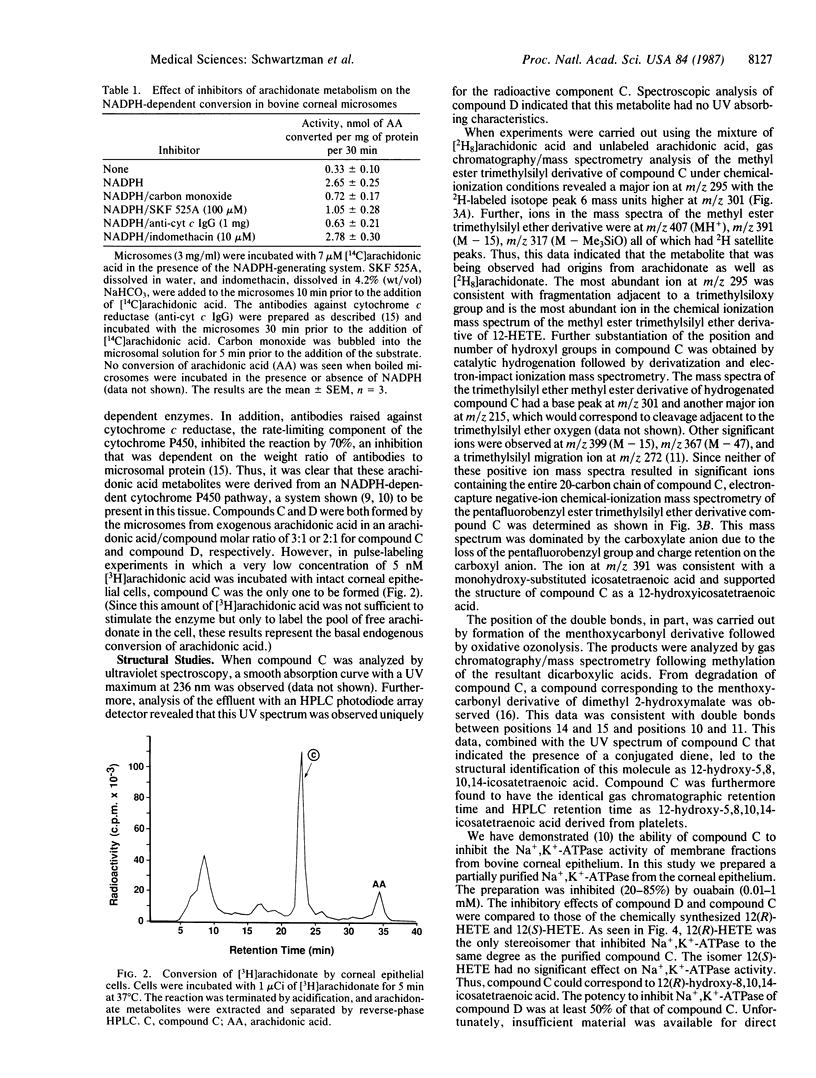
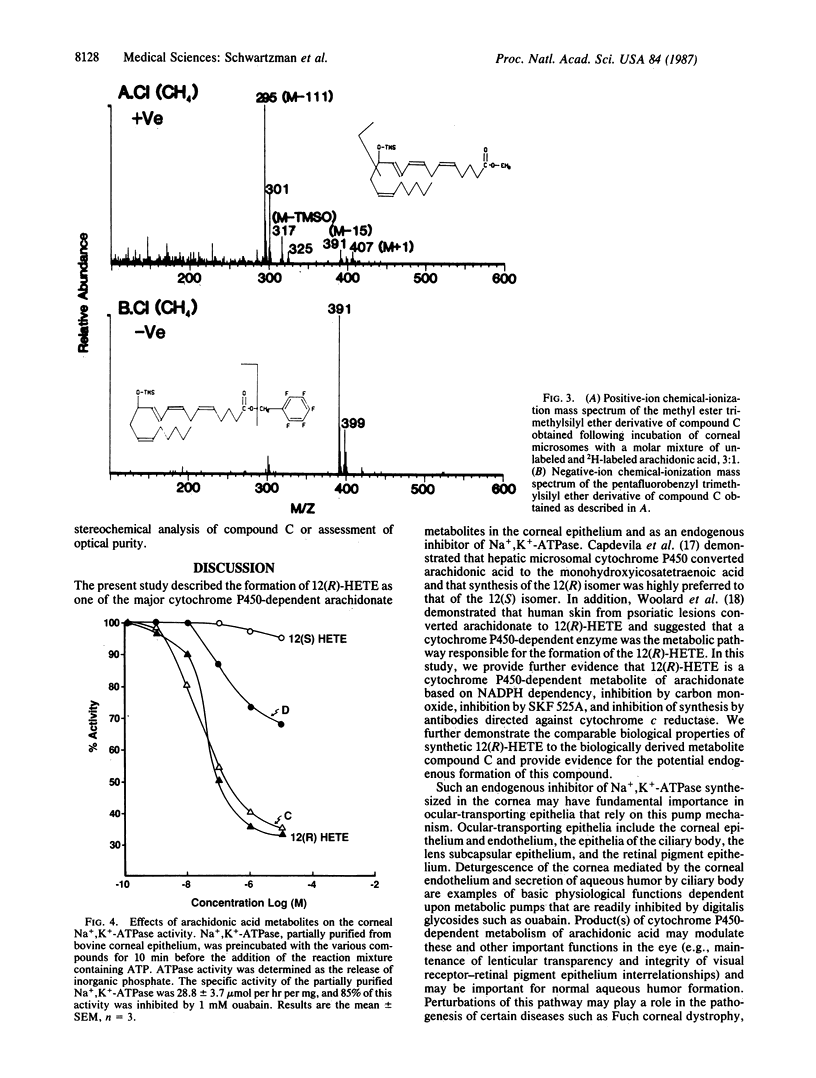
When corneal microsomes were incubated with arachidonic acid in the presence of an NADPH-generating system, four polar metabolites (compounds A-D) were formed. Synthesis of these metabolites could be inhibited by carbon monoxide, SKF 525A, and anti-cytochrome c reductase antibodies. One of the metabolites, compound C, was found to inhibit partially purified Na+,K+-ATPase from the corneal epithelium in a dose-dependent manner with an ID50 of approximately 50 nM. After compound C was purified by TLC and HPLC, it was found to have a UV absorption spectrum with a maximum absorbance at 236 nm suggesting the presence of a conjugated diene. Mass spectrometric analysis using positive- and negative-ionization modes was carried out on derivatized compound C that had been synthesized from a mixture of specifically labeled ([5,6,8,9,11,12,14,15-2H8]arachidonic acid) and unlabeled arachidonic acid. Abundant fragment ions were consistent with compound C being a monooxygenated derivative of arachidonic acid with a hydroxyl substituent at carbon-12 of the icosanoid backbone; all deuterium atoms from [2H8]arachidonate were retained in the structure. Oxidative ozonolysis yielded products indicating double bonds between carbons at positions 10 and 11 and positions 14 and 15 of the 20-carbon chain. Compound C was, therefore, characterized as a 12-hydroxyicosatetraenoic acid. However, only 12(R) isomer was found to be an inhibitor of the Na+,K+-ATPase from the corneal epithelium, suggesting that the biologically active compound C was 12(R)-hydroxy-5,8,10,14-icosatetraenoic acid. Such an inhibitor of Na+,K+-ATPase synthesized in the cornea may have an important role in regulating ocular transparency and aqueous human secretion.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Candia O. A., Lanzetta P. A., Alvarez L. J., Gaines W. Inhibition of ionic transport and ATPase activities by serotonin analogues in the isolated toad lens. Biochim Biophys Acta. 1980 Nov 4;602(2):389–400. doi: 10.1016/0005-2736(80)90319-3. [DOI] [PubMed] [Google Scholar]
- Candia O. A., Reinach P. S. Thermodynamic analysis of active sodium and potassium transport in the frog corneal epithelium. Am J Physiol. 1982 Jun;242(6):F690–F698. doi: 10.1152/ajprenal.1982.242.6.F690. [DOI] [PubMed] [Google Scholar]
- Capdevila J., Marnett L. J., Chacos N., Prough R. A., Estabrook R. W. Cytochrome P-450-dependent oxygenation of arachidonic acid to hydroxyicosatetraenoic acids. Proc Natl Acad Sci U S A. 1982 Feb;79(3):767–770. doi: 10.1073/pnas.79.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capdevila J., Yadagiri P., Manna S., Falck J. R. Absolute configuration of the hydroxyeicosatetraenoic acids (HETEs) formed during catalytic oxygenation of arachidonic acid by microsomal cytochrome P-450. Biochem Biophys Res Commun. 1986 Dec 30;141(3):1007–1011. doi: 10.1016/s0006-291x(86)80144-9. [DOI] [PubMed] [Google Scholar]
- Hamberg M. Steric analysis of hydroperoxides formed by lipoxygenase oxygenation of linoleic acid. Anal Biochem. 1971 Oct;43(2):515–526. doi: 10.1016/0003-2697(71)90282-x. [DOI] [PubMed] [Google Scholar]
- Jorgensen P. L. Isolation of (Na+ plus K+)-ATPase. Methods Enzymol. 1974;32:277–290. [PubMed] [Google Scholar]
- Kinoshita J. H. Mechanisms initiating cataract formation. Proctor Lecture. Invest Ophthalmol. 1974 Oct;13(10):713–724. [PubMed] [Google Scholar]
- Klyce S. D., Crosson C. E. Transport processes across the rabbit corneal epithelium: a review. Curr Eye Res. 1985 Apr;4(4):323–331. doi: 10.3109/02713688509025145. [DOI] [PubMed] [Google Scholar]
- Oliw E. H., Guengerich F. P., Oates J. A. Oxygenation of arachidonic acid by hepatic monooxygenases. Isolation and metabolism of four epoxide intermediates. J Biol Chem. 1982 Apr 10;257(7):3771–3781. [PubMed] [Google Scholar]
- Oliw E. H., Oates J. A. Rabbit renal cortical microsomes metabolize arachidonic acid to trihydroxyeicosatrienoic acids. Prostaglandins. 1981 Dec;22(6):863–871. doi: 10.1016/0090-6980(81)90017-4. [DOI] [PubMed] [Google Scholar]
- Schwartzman M. L., Abraham N. G., Carroll M. A., Levere R. D., McGiff J. C. Regulation of arachidonic acid metabolism by cytochrome P-450 in rabbit kidney. Biochem J. 1986 Aug 15;238(1):283–290. doi: 10.1042/bj2380283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzman M. L., Abraham N. G., Masferrer J., Dunn M. W., McGiff J. C. Cytochrome P450 dependent metabolism of arachidonic acid in bovine corneal epithelium. Biochem Biophys Res Commun. 1985 Oct 15;132(1):343–351. doi: 10.1016/0006-291x(85)91028-9. [DOI] [PubMed] [Google Scholar]
- Schwartzman M. L., Abraham N. G., Masferrer J., Dunn M. W., McGiff J. C. Cytochrome P450-dependent arachidonate metabolism in corneal epithelium: formation of biologically active compounds. Adv Prostaglandin Thromboxane Leukot Res. 1987;17A:78–83. [PubMed] [Google Scholar]
- Schwartzman M. L., Pagano P. J., McGiff J. C., Abraham N. G. Immunochemical studies on the contribution of NADPH cytochrome P-450 reductase to the cytochrome P-450-dependent metabolism of arachidonic acid. Arch Biochem Biophys. 1987 Feb 1;252(2):635–645. doi: 10.1016/0003-9861(87)90069-5. [DOI] [PubMed] [Google Scholar]
- VanRollins M., Murphy R. C. Autooxidation of docosahexaenoic acid: analysis of ten isomers of hydroxydocosahexaenoate. J Lipid Res. 1984 May;25(5):507–517. [PubMed] [Google Scholar]
- Woollard P. M. Stereochemical difference between 12-hydroxy-5,8,10,14-eicosatetraenoic acid in platelets and psoriatic lesions. Biochem Biophys Res Commun. 1986 Apr 14;136(1):169–176. doi: 10.1016/0006-291x(86)90891-0. [DOI] [PubMed] [Google Scholar]
- Zadunaisky J. A. Active transport of chloride in frog cornea. Am J Physiol. 1966 Aug;211(2):506–512. doi: 10.1152/ajplegacy.1966.211.2.506. [DOI] [PubMed] [Google Scholar]


