Abstract
Background
During its developmental cycle within the sand fly vector, Leishmania must survive an early proteolytic attack, escape the peritrophic matrix, and then adhere to the midgut epithelia in order to prevent excretion with remnants of the blood meal. These three steps are critical for the establishment of an infection within the vector and are linked to interactions controlling species-specific vector competence. PpChit1 is a midgut-specific chitinase from Phlebotomus papatasi presumably involved in maturation and degradation of the peritrophic matrix. Sand fly midgut chitinases, such as PpChit1, whether acting independently or in a synergistic manner with Leishmania-secreted chitinase, possibly play a role in the Leishmania escape from the endoperitrophic space. Thus, we predicted that silencing of sand fly chitinase will lead to reduction or elimination of Leishmania within the gut of the sand fly vector.
Methodology/Principal Findings
We used injection of dsRNA to induce knock down of PpChit1 transcripts (dsPpChit1) and assessed the effect on protein levels post blood meal (PBM) and on Leishmania major development within P. papatasi. Injection of dsPpChit1 led to a significant reduction of PpChit1 transcripts from 24 hours to 96 hours PBM. More importantly, dsPpChit1 led to a significant reduction in protein levels and in the number of Le. major present in the midgut of infected P. papatasi following a infective blood meal.
Conclusion/Significance
Our data supports targeting PpChit1 as a potential transmission blocking vaccine candidate against leishmaniasis.
Author Summary
For a successful development within the midgut of the sand fly vector, Leishmania must overcome several barriers which are imposed by the vector. The ability to overcome these barriers has been associated with species specificity, and interference with the sand fly vector-parasite balance can change the outcome of the infection in the vector. Recently, our group has carried out a transcriptome assessment of the sand fly Phlebotomus papatasi midgut, uncovering many transcripts possibly associated with the barrier to Leishmania development. In order to validate the role of such genes, we have developed a dedicated RNA interference (RNAi) platform to assess whether RNAi targeting such genes can reduce Leishmania major development. PpChit1 is a midgut-specific chitinase presumably involved in the maturation/degradation of the peritrophic matrix in the gut of the sand fly after a blood meal. Our results show that knockdown of PpChit1 via RNAi led to a significant reduction of Le. major within the gut, supporting the potential use of PpChit1 as a target for transmission blocking strategies against sand fly-transmitted leishmaniasis.
Introduction
Emerging and reemerging vector-borne diseases pose significant threats to human and animal health [1]. The emergence of insecticide resistance as well as the lack of other efficient insecticidal tools to control disease vectors imply that new methodologies need to be developed in order to reduce vector-borne disease transmission [1]. For this, the study of vector-pathogen interaction pinpointing factors underlying vector competence can reveal new molecular targets to be disrupted, preventing pathogen transmission [2], [3].
In sand flies, midgut molecules are known or believed to be involved in defining a species ability to transmit Leishmania in nature. For a successful development within the midgut of the sand fly vector, Leishmania must overcome several barriers that include an early proteolytic attack [4], [5], [6], [7], [8], the need to escape the peritrophic matrix (PM) [8], [9], [10], [11], [12], and attachment to the midgut epithelia to prevent excretion with the remnants of the blood meal [13], [14], [15], [16].
Attachment to midgut epithelia has long been associated with the type of lipophosphoglycan (LPG) present on the surface of Leishmania, and is associated with defining sand fly-Leishmania pairs in nature [15], [16], [17]. For Leishmania major V1 strain, with LPG displaying highly decorated side chains with prominent galactose residues, we demonstrated that PpGalec, a P. papatasi galactose-binding protein, is the docking site for Le. major on the midgut epithelium of Phlebotomus papatasi [14]. Recently, LPG-independent midgut binding has been associated with the degree of glycosylation detected on proteins expressed by midgut epithelial cells [18].
For events leading up to the midgut binding, such as early parasite survival during the proteolytic attack and escape of the endoperitrophic space, some investigators suggested that midgut proteases, such as trypsins and chymotrypsins, also are responsible for defining vector-Leishmania specificity [4], [5], [6], [7]. Such proteases were shown to be specially harmful to transitional stages amastigotes [8].
A role of the PM on sand fly vector competence was suggested through comparisons of Leishmania development in different sand fly species displaying different PM degradation rates [9], [10], [11]. Studies later revealed a dual role for the sand fly PM in parasite development; protecting Leishmania from digestive enzymes in the beginning of blood digestion, yet becoming a barrier to parasite escape when mature [8]. Recent data also indicate that an anterior PM plug located at the junction between the anterior and posterior midgut acts as a barrier to Leishmania migration towards the stomodeal valve [12].
Regarding Leishmania escape from the PM, it was firstly proposed to be solely accomplished by a parasite chitinase [19]. Further work demonstrated that a Le. mexicana chitinase-overexpressing strain had an accelerated escape from the PM in Lutzomyia longipalpis [20]. However, since the characterization of a blood induced chitinolytic system in the sand fly midgut [21], it became apparent that the parasite must take advantage of the sand fly peak chitinolytic activity within midgut, approximately 40–48 hours after a blood meal, for their escape [12], [21].
PpChit1 is presumably involved in PM maturation/degradation in P. papatasi [8]. Based on the fact that Leishmania must escape the PM, and that this escape may be aided by the vector's own chitinase, we predicted that PpChit1 knock down (via RNAi) would interfere with Le. major development. Our data indicates that dsRNA-mediated silencing of PpChit1 transcripts leads to a reduction in the parasite load within the midgut of P. papatasi, pointing to the role of this molecule in P. papatasi vector competence and its potential for the development of a transmission-blocking vaccine.
Methods
Ethics statement
The use of animals during this study was reviewed and approved by the Kansas State University Institutional Animal Care and Use Committee (KSU-IACUC).
Sand fly rearing, dissection, and infection with Le. major
P. papatasi (Israeli strain -PPIS) was reared in the Department of Entomology, Kansas State University, according to conditions described [21]. For all experiments, three-to-five day old female sand flies were used. Blood feeding was performed through a chicken skin membrane attached to a feeding device. Prior to sand fly feeding, fresh mouse blood was heat inactivated for 30min at 56°C and supplemented with 50 µl/ml of Pen/Strep solution (MP Biomedicals, Solon, OH, USA) as well as 1 mM ATP (MP Biomedicals). Sixteen to twenty four hours after blood feeding, fully engorged females were separated from partially engorged and non-blood fed by anesthetizing flies with CO2 and examining the midgut distension under a stereoscope microscope. Only fully fed individuals were maintained for further analyses.
Fully engorged sand fly midguts were individually dissected on RNAse free (cleaned with ELIMINase, Fisher Scientific, Pittsburgh, PA, USA) glass slides, transferred to 50 µl of 1× PBS buffer (RNase free, pH 7.4; Fisher Scientific), and thoroughly homogenized using a hand held tissue homogenizer and RNAse-free pestle. Half the homogenate volume (25 µl) was transferred to 350 µl of RLT buffer (supplemented with 1% β-mercaptoethanol) provided by the RNA extraction kit (RNAeasy mini kit, Qiagen, Valencia, CA, USA) and stored at −80°C for quantitative real-time PCR assays. The remaining 25 µl of midgut homogenate was used in Western blot assays, as described below.
Infections of sand flies with Le. major amastigotes V1 strain were done by addition of 5×106 parasites/ml into the blood meal. Le. major amastigotes were harvested from BALB/c mouse footpads lesions formed roughly 30 days after inoculation with 5×105 parasites from late phase culture according to [22].
dsRNA synthesis and injection
dsRNA for PpChit1 were synthesized using the primers PpChit1/T7i_2–F (5′–TAATACGACTCACTATAGGGAGAATGAAGATATCATTGTGTGC-3′) and PpChit1/T7i_2–R (5′– TAATACGACTCACTTAGGGAGATCAGCATTGGACCAGGAAGG-3′), which contain the complete T7 promoter and amplify the full length sequence encoding the mature PpChit1. PCR reactions were performed with 0.5pmoles of each primer along with 1 µl of cDNA (synthesized from midgut dissected at 72 h post-blood meal, PBM), and 10 µl of GoTaq PCR master mix (Promega, Madison, WI, USA). The 20 µl PCR reactions were carried according to the conditions: 10 cycles of 95°C for 1 min, 55°C for 1 min, and 72°C for 1 min and 15 sec, followed by 35 cycles 95°C for 1 min, 65°C for 1 min, and 72°C for 1 min and 15 sec. The reaction products were purified and concentrated using the YM-100 filters (Millipore, Billerica, MA, USA), and 1 µg DNA was used for dsRNA synthesis using the Megascript RNAi kit (Ambion, Austin, TX, USA). dsRNA synthesis reactions were performed for four hours at 37°C, and the products were further purified following manufacturer's recommendations. Thereafter, dsRNAs were suspended in ultra-pure water and further purified and concentrated to approximately 3.5 mg/ml or 4.5 mg/ml using the YM-100 filters (Millipore). The positive control provided by the Megascript RNAi kit (Ambion; used in Real-Time PCR and Western blot assays) or a dsRNA specific to a green fluorescence protein gene (dsGFP [23]; for parasite counting assays) was used as controls for dsRNA injection assays.
For dsRNA injections, individual females were anesthetized with CO2, kept on a cold dish, and injected intra-thoraxically with either 23 nl (3.5 mg/ml, 80.5 ng) or 32 nl (4.5 mg/ml, 144 ng) of dsRNA using Nanoject II microinjector (Broomall, PA, USA). Immediately following injection, flies were transferred to a 500 ml plastic container, provided with 30% sugar embedded cotton, and maintained inside a high humidity chamber (85–95% humidity at 25°C). Flies were allowed to recover for 48 hours and blood fed on an uninfected blood meal through a chicken membrane, as described above.
RNA isolation and cDNA synthesis
Total RNA was isolated from individual midguts dissected as described above. RNA extraction was carried out using the RNAeasy mini kit (Qiagen) following manufacturer's instructions. Following extraction, the Turbo DNA-free kit (Ambion) was used to eliminate DNA contamination. After quantification, 25 ng total RNA was used for cDNA synthesis using 200 units of SuperScript III Reverse Transcriptase (200 u/µl), 2.5 µM Oligo (dT)20 primer, and 0.5 µM dNTPs (10 mM). These reagents were incubated at 65°C for 5 minutes (min) and kept in ice for at least 1 min. This step was followed by addition of a mix containing 4 µl 5× SuperScript III Reverse Transcriptase First-Strand Buffer, 5 mM DTT (0.1 M), 20 Units of RNaseOUT to the reaction. The mixture was incubated for one hour at 50°C and stored at −20°C. All the reagents for cDNA synthesis were purchase from Invitrogen (Carlsbad, CA, USA).
Quantitative real-time PCR analyses
Real-Time PCR reactions were performed using BioRad SYBR green and BioRad iCycler (BioRad, Hercules, CA, USA). The reactions were carried out in duplicate using 0.5 µl cDNA, 6pmoles of each primer (10 µM), 10 µl of 2× SYBR green, and 8.3 µl of Ultra Pure DNase/RNase-Free Water (Invitrogen). The primers used for chitinase amplification were PpChit_137F (5′ - ATGATCTGCATGGTTCTTGG - 3′) and PpChit_137R (5′ - GGAGCTCCATTTCGAATCC - 3′) while the S3 primers (Pp40S_S3_136F: 5′ - GGACAGAAATCATCATCATG – 3′ and Pp40S_S3_136R: 5′ – CCTTTTCAGCGTACAGCTC – 3′) were used for amplifications of the housekeeping control gene (encoding the protein S3 of ribosomal subunit 40S). The reaction cycle of 94°C for 1 min, 57°C for 1 min, and 72°C for 30 sec was repeated 40 times, and the amplification profiles were assessed using the BioRad iCycler software (BioRad).
PpChit1 anti-sera and Western blot analyses
Polyclonal anti-PpChit1 sera were obtained by injecting three month old female BALB/c mice subcutaneously into the ears. Mice were injected three times in two weeks intervals with approximately 10 µg of purified VR2001 plasmid [24] encoding the mature chitinase protein [21] per mouse ear. Blood was collected from the submandibular vein (“cheek bleed”) of injected animals and antibody levels accessed via Easy-Titer IgG Assay Kit (Pierce, Rockford, IL, USA). Sera were maintained at −20°C until used. For Western blots, seven midgut extracts from flies injected with dsPpChit1 and dsControl were pooled together in RNasefree microcentrifuge tubes containing 1 µl of complete protease inhibitor (Thermo Scientific, Rockford, IL, USA) and concentrated using the YM-10 filters (Millipore). Total protein concentration in midgut extracts was quantified using BCA Protein Assay Kit (Thermo Scientific). Similar proteins amounts (5 µg per lane) from midguts of dsPpChit1 and dsControl injected sand flies were fractionated on 10% Bis-Tris NuPAGE gels (Invitrogen). Proteins were transferred to a nitrocellulose filter (Whatman, Dassel, Germany), incubated with PpChit1 antisera (1:100 dilution) overnight at 4°C, washed three times in TBS-T (1× TBS buffer with 0.1% tween-20) for 15 minutes each time. Blot was incubated with anti-mouse conjugated to alkaline phosphatase (1:10,000 in TBS-T) antibodies (Promega) for one hour at room temperature and washed in TBS-T as indicated above. The protein bands (56 kDa, [21]) were visualized using the Western Blue substrate for Alkaline Phosphatase (Promega). Alternatively, Western blot was incubated with anti-mouse-Horseradish Peroxidase secondary antibody (1:10,000) and detected with SuperSignal West Pico Chemiluminescence Substrate (Thermo Scientific) in chemiluminescence assays. Densitometry analysis was performed using the TotalLab TL100 software (Nonlinear Dynamics, Durham, NC, USA).
P. papatasi dissection and parasite counting
In order to assess the PpChit1 knockdown effects on Le. major development, 80.5 ng of dsRNA was injected intra-thoraxically into P. papatasi, and flies were fed on an infected blood meal as described above. Midguts from fully engorged-only flies were dissected at 48 h and 120 h after the infective blood meal and homogenized in 30 µl of PBS buffer (pH 7.4). Parasites were counted with a hemocytometer. Two independent experiments were carried out for each time point.
Statistical analysis
Mann-Whitney U test was performed to compare expression profiles as well as parasite numbers between sand flies injected with either dsRNA targeting PpChit1 transcripts (dsPpChit1) or the dsRNA control (dsControl) injected flies. D'Agostino & Pearson omnibus normality test was performed to assess whether parasite numbers followed a normal distribution. The Chi-square test (or Fisher's exact test) was performed in order to assess whether dsPpChit1-injected flies exhibit altered Le. major load compared to the dsControl-injected flies. Parasite infection load in flies dissected at 48 h post infection was scored according to parasite numbers in the sand fly midgut as no parasite, light infection (1–1,000 parasites), moderate infection (1,001–10,000), or heavy infection (>10,000), in accordance to [25]. For flies dissected at 120 h PBM parasite loads were categorized in two groups: zero or light infections (0–1,000 parasites) was arranged as one group, and moderate infection (>1,000 parasites) as another. Differences were considered statistically significant at p<0.05, and tests were carried out using GraphPad Prism v. 5.01 software (GraphPad Software, Inc).
Results
dsPpChit1 effects on mRNA levels
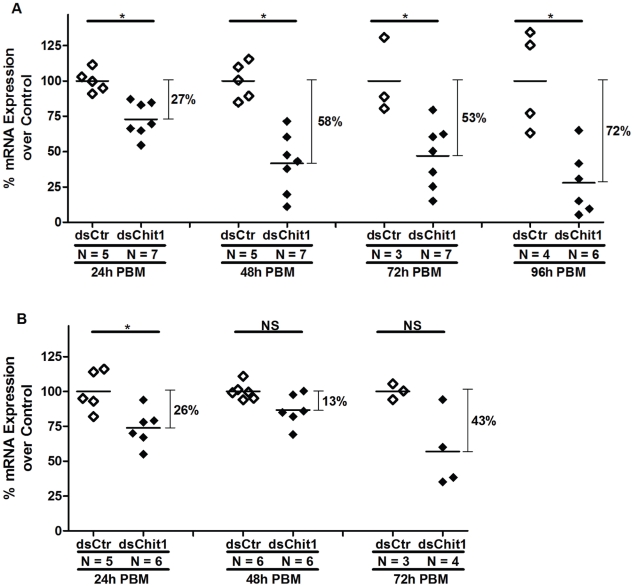
Injection of 80.5 ng of dsRNA into the sand fly thorax targeting the midgut expressed PpChit1 gene led to a significant decrease in PpChit1 mRNA levels in comparison with the control dsRNA-injected flies (Figure 1). Reduction of PpChit1 expression after a blood meal varied over time. Twenty four hours after blood meal (and 72 h after injection of dsPpChit1), a 27% reduction of PpChit1 transcripts was detected (Figure 1A). At 48 h PBM (previously shown to be the maximum activity for PpChit1 [21]) and at 72 h PBM, reductions of 58% and 53% on average of the PpChit1 expression were observed (Figure 1A). Finally, at 96 h PBM (120 h after dsRNA injection), when no chitinolytic activity was detected [21], the reduction in PpChit1 expression was 72%.
Figure 1. dsRNA effect on PpChit1 RNA levels.
Real-Time PCR comparing the mRNA level of PpChit1 between flies injected with 80.5 ng (A) or 144 ng (B) of dsPpChit1 (dsChit1) or dsControl (dsCtr) double-strand RNAs. Significant PpChit1 transcript reduction was exhibited by dsPpChit1 injected flies at 24 h (A and B), 48 h, 72 h, and 96 h PBM (A). PpChit1 mRNA levels were normalized with the S3 housekeeping gene. Results are presented as a percent of PpChit1 expression levels in dsPpChit1 injected flies over the mean of PpChit1 expression levels in dsControl injected flies (considered as 100%) for each time point. The variance in PpChit1 expression in dsControl injected flies is also shown. Each dot represents PpChit1 RNA levels in a single fly. Horizontal bars indicate mean expression level. *: Statistically significant at p<0.05.
On the other hand, injection of 144 ng of dsPpChit1 into P. papatasi thorax displayed a weaker reduction in PpChit1 expression levels than injection of 80.5 ng (Figure 1B). Although similar expression reduction at 24 h PBM was exhibited (26%, Figure 1B), expression differences between dsPpChit1 and dsControl injected flies at 48 h and 72 h PBM were lower (13% and 43%, respectively) than detected at the same time points when 80.5 ng of dsRNA was injected (Figure 1B). These differences could be occurring due to a still obscure feedback loop for transcription activation upon knock down, as proposed elsewhere [26].
dsPpChit1 effects on protein levels
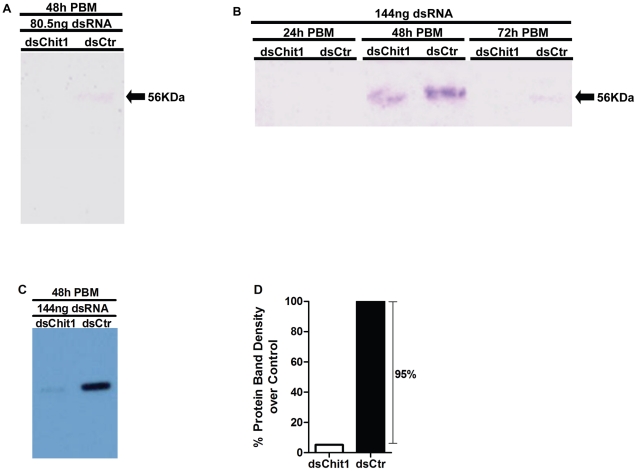
Silencing of the PpChit1 message RNA produced a concomitant reduction in the amount of PpChit1 protein as determined by Western blots (Figure 2). Similar to the Real-Time PCR data, reduction in PpChit1 protein levels in dsPpChit1 injected flies was detected at 48 h and 72 h PBM (Figures 2A–C) when either 80.5 ng or 144 ng of dsRNA was injected. No PpChit1 expression was detected at 24 h PBM (Figure 2B). Likewise, densitometry analysis of blot developed using a chemiluminescence method displayed 95% reduction in PpChit1 protein levels at 48 h PBM when 144 ng dsPpChit1 (Figure 2C and D). Interestingly, the corresponding time point only led to 13% reduction of PpChit1 mRNA levels, as shown in Figure 1B.
Figure 2. dsRNA effect on PpChit1 protein levels.
(A). Western blot assay pointing to PpChit1 knock down in dsPpChit1 injected flies (80.5 ng dsRNA) at 48 h PBM. (B). Midgut extracts from flies injected with 144 ng dsPpChit1 (dsChit1) displayed weaker bands (56 kDa) than dsControl (dsCtr) injected flies at 48 h and 72 h PBM. A–B, Colorimetric development. (C). Western blot assay depicting strong PpChit1 expression reduction in flies injected with 144 ng dsPpChit1 (dsChit1) compared with dsCtr injected ones at 48 h PBM (Chemiluminescence development). (D). Densitometry analysis of PpChit1 protein bands obtained in the chemiluminescence assay revealing 95% reduction in PpChit1 expression between dsPpChit1 and dsControl injected flies.
dsPpChit1 effects on Le. major development within P. papatasi midgut
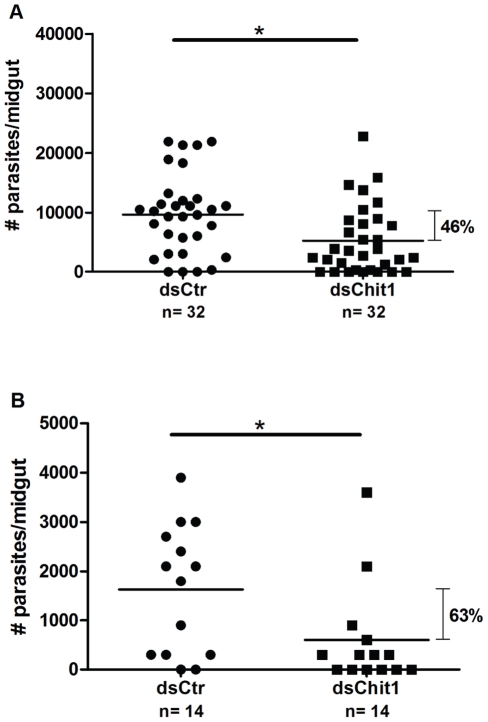
As injection of either 80.5 ng or 144 ng of dsRNA targeting PpChit1 transcripts are capable of significantly reducing PpChit1 expression levels in the midgut of P. papatasi (Figure 1 and 2), we assessed the effects of injecting 80.5 ng of the dsRNA on Le. major development within the injected flies. Following the injection of the PpChit1 dsRNA, flies were provided an infective blood meal, and dissected at different time points after feeding. Our results demonstrate that dsPpChit1-targeted knock-down resulted in significant reductions in parasite load within the sand fly midgut as the numbers of Le. major were reduced by 46% (or 1.85 fold) at 48 h post infection (Figure 3A) and by 63% (or 2.70 fold) at 120 h PBM post infection (Figure 3B).
Figure 3. dsRNA effect on Le. major development.
Intra-thoracic injections of dsPpChit1 (80.5 ng) reduce Le. major load in P. papatasi midgut. (A). At 48 h PBM, Le. major density was reduced on average 46% in dsPpChit1 (dsChit1) injected compared with dsControl (dsCtr) injected. (B). Le. major parasites per midgut were further reduced at 120 h PBM in dsPpChit1 injected flies, reaching on average 63% reduction over the dsControl injected ones. Each dot represents parasite number in a single P. papatasi midgut. Horizontal bars display mean parasite numbers. n: Number of flies analyzed. *: Statistically significant at p<0.05. Graphs represent one similar result of two independent experiments.
The injection of dsPpChit1 also affected the range of parasite loads. An analysis of the range of parasite load at 48 h and 120 h post infection points to a normal distribution of parasite numbers in the dsControl-injected flies (48 h PBM, p = 0.51, and 120 h PBM, p = 0.26, D'Agostino & Pearson omnibus normality test), whereas for dsPpChit1-injected flies this distribution was significantly affected (48 h PBM, p = 0.004, and 120 h PBM, p<0.0001, D'Agostino & Pearson omnibus normality test).
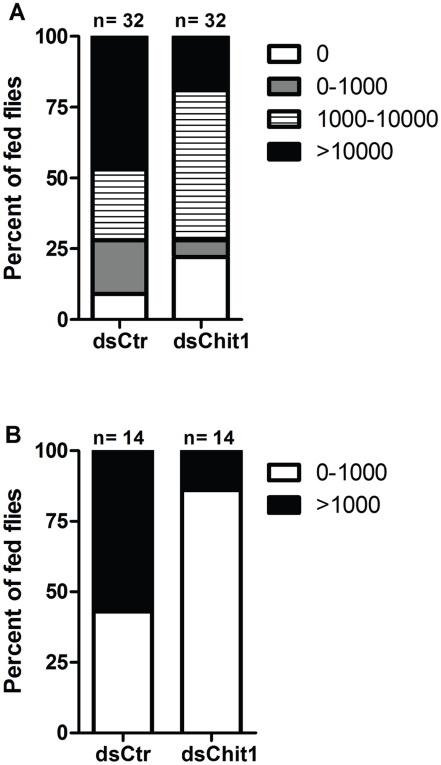
Changes in P. papatasi infection levels following silencing of PpChit1 were further confirmed by comparing infection prevalence. For instance, injection of dsPpChit1 reduced the prevalence of heavy infection from 47% (dsControl-injected) to 19%, and of light infection from 19% (dsControl-injected) to 6% at 48 h post blood feeding (Figure 4A). Likewise, moderate infections levels were reduced from 57% (dsControl-injected) to 14% at 120 h post infection (Figure 4B).
Figure 4. Effect of dsRNA injection on Le. major infection level in P. papatasi.
Parasite load was categorized according to the number of Le. major per midgut. (A) Percentage of sand flies injected with either dsCtr or dsChit1 exhibiting no infection (0 parasites), as well as light (1–1,000 parasites), moderate (1,000–10,000 parasites), or heavy (>10,000 parasites) infection at 48 h PBM. Differences are statistically significant (Chi-square, p = 0.01). (B) Percentage of sand flies injected with either dsCtr or dsChit1 exhibiting either no parasites or light infection (0–1000 parasites), or moderate infection (>1,000 parasites) at 120 h PBM. Differences are statistically significant (Fisher's exact test, p = 0.04). n: Number of flies dissected.
Discussion
After a blood meal, sand flies synthesize a PM type 1 that is fully developed at approximately 36–40 h PBM [27]. In addition to compartmentalizing the blood meal and protecting the epithelia, the sand fly PM serves an additional dual role regarding Leishmania infection: as a barrier to these parasites but also as protection against proteolytic attack on transitional-stage amastigotes [8], [20], [28], [29], [30]. In order to successfully complete its cycle within the sand fly, Leishmania nectomonads must escape from endoperitrophic space, through the PM, to prevent being passed together with remnants of the digested blood meal [8].
We have previously characterized a functional, blood-induced chitinolytic system, in the midgut of P. papatasi and L. longipalpis sand flies [21], [31]. We also demonstrated that polyclonal antibodies to PpChit1 inhibit the midgut chitinolytic activity in vitro, and this effect also was shown across different sand fly species [21]. PpChit1 is presumably involved in the maturation and degradation of P. papatasi PM (as is its ortholog in L. longipalpis, LlChit1) [21], [31], and addition of allosamidin, a chitinase inhibitor to the infective blood meal of this sand fly led to entrapment of Le. major within the peritrophic space [8]. Although allosamidin may have also inhibited chitinase secreted by Leishmania, taken together, these data suggested that PpChit1 also can be involved with Leishmania escape from the endoperitrophic space.
To address whether silencing of PpChit1 transcripts via RNAi-induced pathway would affect Le. major development within its natural vector, P. papatasi, we synthesized a dsRNA specifically targeting PpChit1.
Injection of dsRNA targeting specific transcripts has now been widely applied in disease vectors and proven an invaluable tool for the understanding of underlying events in pathogen-vector relationships [32], [33], [34]. In sand flies, gene silencing with dsRNA was first applied to L. longipalpis cell culture [35], inducing a non-specific antiviral response. Recently, dsRNA injection of adult sand flies led to a specific reduction of Xanthine dehydrogenase expression [36], and to an effect on Le. mexicana development when a midgut trypsin produced by L. longipalpis was silenced [30].
The midgut chitinase PpChit1 is only expressed following a blood meal [21]. Thus, following injection of dsPpChit1 double-stranded RNA, sand flies were blood fed and midguts dissected at different intervals after feeding. Specific silencing of PpChit1 transcripts was detected by quantitative real-time PCR analyses (Figure 1), with concomitant knock down of PpChit1 protein levels assessed by Western blots (Figure 2).
Based on the presumptive role of PpChit1 in the maturation and degradation of the PM1, we expected that silencing of this gene would lead to entrapment of Leishmania within the endoperitrophic space. Our results are consistent with this hypothesis, as Le. major load was reduced 120 h PBM in midguts of dsPpChit1 injected P. papatasi (Figures 3 and 4) suggesting that PpChit1 is indeed involved in PM1 degradation. Moreover, reduction of the Le. major load at 48 h PBM in dsChit1 compared to control-injected flies might have been caused by at least two scenarios: 1) a reduction in nutrient availability in the endoperitrophic space as the PM may be less permeable to proteolytic enzymes, or in the contrary, 2) to inability of parasites to escape leading to longer exposure to digestive enzymes inside the peritrophic space. Regardless of the mechanism, it still remains to be determined.
Future studies will assess whether this is a feasible approach in preventing transmission from an infected animal to a naïve host. Moreover, the results support the targeting of PpChit1 as a mean to interfere with Leishmania development within the sand fly – a candidate transmission-blocking vaccine.
Acknowledgments
We are grateful to Drs. Carolina Barillas-Mury, Jesus Valenzuela, Shaden Kamhawi, and Ryan Jochim (LMVR/NIH) for suggestions and encouragement, and to Dr. Yoonseong Park (K-State) for kindly providing a GFP clone. We also would like to acknowledge the reviewers for their constructive criticism of the original version of the manuscript.
Footnotes
The authors have declared that no competing interests exist.
The project described was supported by Award Number R01AI074691 from the National Institute of Allergy and Infectious Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hill CA, Kafatos FC, Stansfield SK, Collins FH. Arthropod-borne diseases: vector control in the genomics era. Nat Rev Microbiol. 2005;3:262–268. doi: 10.1038/nrmicro1101. [DOI] [PubMed] [Google Scholar]
- 2.Coutinho-Abreu IV, Ramalho-Ortigao M. Transmission blocking vaccines to control insect-borne diseases: a review. Mem Inst Oswaldo Cruz. 2010;105:1–12. doi: 10.1590/s0074-02762010000100001. [DOI] [PubMed] [Google Scholar]
- 3.Coutinho-Abreu IV, Zhu KY, Ramalho-Ortigao M. Transgenesis and paratransgenesis to control insect-borne diseases: current status and future challenges. Parasitol Int. 2009;59:1–8. doi: 10.1016/j.parint.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borovsky D, Schlein Y. Trypsin and chymotrypsin-like enzymes of the sandfly Phlebotomus papatasi infected with Leishmania and their possible role in vector competence. Med Vet Entomol. 1987;1:235–242. doi: 10.1111/j.1365-2915.1987.tb00349.x. [DOI] [PubMed] [Google Scholar]
- 5.Dillon RJ, Lane RP. Bloodmeal digestion in the midgut of Phlebotomus papatasi and Phlebotomus langeroni. Med Vet Entomol. 1993;7:225–232. doi: 10.1111/j.1365-2915.1993.tb00681.x. [DOI] [PubMed] [Google Scholar]
- 6.Dillon RJ, Lane RP. Influence of Leishmania infection on blood-meal digestion in the sandflies Phlebotomus papatasi and P. langeroni. Parasitol Res. 1993;79:492–496. doi: 10.1007/BF00931590. [DOI] [PubMed] [Google Scholar]
- 7.Schlein Y, Jacobson RL. Resistance of Phlebotomus papatasi to infection with Leishmania donovani is modulated by components of the infective bloodmeal. Parasitology. 1998;117(Pt 5):467–473. doi: 10.1017/s0031182098003321. [DOI] [PubMed] [Google Scholar]
- 8.Pimenta PF, Modi GB, Pereira ST, Shahabuddin M, Sacks DL. A novel role for the peritrophic matrix in protecting Leishmania from the hydrolytic activities of the sand fly midgut. Parasitology. 1997;115(Pt 4):359–369. doi: 10.1017/s0031182097001510. [DOI] [PubMed] [Google Scholar]
- 9.Lawyer PG, Ngumbi PM, Anjili CO, Odongo SO, Mebrahtu YB, et al. Development of Leishmania major in Phlebotomus duboscqi and Sergentomyia schwetzi (Diptera: Psychodidae). Am J Trop Med Hyg. 1990;43:31–43. doi: 10.4269/ajtmh.1990.43.31. [DOI] [PubMed] [Google Scholar]
- 10.Walters LL, Irons KP, Modi GB, Tesh RB. Refractory barriers in the sand fly Phlebotomus papatasi (Diptera: Psychodidae) to infection with Leishmania panamensis. Am J Trop Med Hyg. 1992;46:211–228. doi: 10.4269/ajtmh.1992.46.211. [DOI] [PubMed] [Google Scholar]
- 11.Feng LC. The role of the peritrophic membrane in Leishmania and Trypanosome infections in sand flies. Peking Natural History Bulletin. 1950;19:327–334. [Google Scholar]
- 12.Sadlova J, Volf P. Peritrophic matrix of Phlebotomus duboscqi and its kinetics during Leishmania major development. Cell Tissue Res. 2009;337:313–325. doi: 10.1007/s00441-009-0802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamhawi S. Phlebotomine sand flies and Leishmania parasites: friends or foes? Trends Parasitol. 2006;22:439–445. doi: 10.1016/j.pt.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Kamhawi S, Ramalho-Ortigao M, Pham VM, Kumar S, Lawyer PG, et al. A role for insect galectins in parasite survival. Cell. 2004;119:329–341. doi: 10.1016/j.cell.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Pimenta PF, Saraiva EM, Rowton E, Modi GB, Garraway LA, et al. Evidence that the vectorial competence of phlebotomine sand flies for different species of Leishmania is controlled by structural polymorphisms in the surface lipophosphoglycan. Proc Natl Acad Sci U S A. 1994;91:9155–9159. doi: 10.1073/pnas.91.19.9155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pimenta PF, Turco SJ, McConville MJ, Lawyer PG, Perkins PV, et al. Stage-specific adhesion of Leishmania promastigotes to the sandfly midgut. Science. 1992;256:1812–1815. doi: 10.1126/science.1615326. [DOI] [PubMed] [Google Scholar]
- 17.Sacks DL, Saraiva EM, Rowton E, Turco SJ, Pimenta PF. The role of the lipophosphoglycan of Leishmania in vector competence. Parasitology. 1994;108(Suppl):S55–62. doi: 10.1017/s0031182000075727. [DOI] [PubMed] [Google Scholar]
- 18.Myskova J, Svobodova M, Beverley SM, Volf P. A lipophosphoglycan-independent development of Leishmania in permissive sand flies. Microbes Infect. 2007;9:317–324. doi: 10.1016/j.micinf.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schlein Y, Jacobson RL, Shlomai J. Chitinase secreted by Leishmania functions in the sandfly vector. Proc Biol Sci. 1991;245:121–126. doi: 10.1098/rspb.1991.0097. [DOI] [PubMed] [Google Scholar]
- 20.Rogers ME, Hajmova M, Joshi MB, Sadlova J, Dwyer DM, et al. Leishmania chitinase facilitates colonization of sand fly vectors and enhances transmission to mice. Cell Microbiol. 2008;10:1363–1372. doi: 10.1111/j.1462-5822.2008.01132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramalho-Ortigao JM, Kamhawi S, Joshi MB, Reynoso D, Lawyer PG, et al. Characterization of a blood activated chitinolytic system in the midgut of the sand fly vectors Lutzomyia longipalpis and Phlebotomus papatasi. Insect Mol Biol. 2005;14:703–712. doi: 10.1111/j.1365-2583.2005.00601.x. [DOI] [PubMed] [Google Scholar]
- 22.Sacks DL. Experimental Leishmania infection. In: Coligan J, Kruisbeek A, Margulies D, Shevach E, Strober W, editors. Current protocols in immunology. New York: John Wiley & Sons; 2003. pp. 19.12.11–19.12.20. [Google Scholar]
- 23.Arakane Y, Li B, Muthukrishnan S, Beeman RW, Kramer KJ, et al. Functional analysis of four neuropeptides, EH, ETH, CCAP and bursicon, and their receptors in adult ecdysis behavior of the red flour beetle, Tribolium castaneum. Mech Dev. 2008;125:984–995. doi: 10.1016/j.mod.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Oliveira F, Kamhawi S, Seitz AE, Pham VM, Guigal PM, et al. From transcriptome to immunome: identification of DTH inducing proteins from a Phlebotomus ariasi salivary gland cDNA library. Vaccine. 2006;24:374–390. doi: 10.1016/j.vaccine.2005.07.085. [DOI] [PubMed] [Google Scholar]
- 25.Svarovska A, Ant TH, Seblova V, Jecna L, Beverley SM, et al. Leishmania major glycosylation mutants require phosphoglycans (lpg2-) but not lipophosphoglycan (lpg1-) for survival in permissive sand fly vectors. PLoS Negl Trop Dis. 4:e580. doi: 10.1371/journal.pntd.0000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belles X. Beyond Drosophila: RNAi in vivo and functional genomics in insects. Annu Rev Entomol. 2010;55:111–128. doi: 10.1146/annurev-ento-112408-085301. [DOI] [PubMed] [Google Scholar]
- 27.Secundino NF, Eger-Mangrich I, Braga EM, Santoro MM, Pimenta PF. Lutzomyia longipalpis peritrophic matrix: formation, structure, and chemical composition. J Med Entomol. 2005;42:928–938. doi: 10.1093/jmedent/42.6.928. [DOI] [PubMed] [Google Scholar]
- 28.Ramalho-Ortigao JM, Kamhawi S, Rowton ED, Ribeiro JM, Valenzuela JG. Cloning and characterization of trypsin- and chymotrypsin-like proteases from the midgut of the sand fly vector Phlebotomus papatasi. Insect Biochem Mol Biol. 2003;33:163–171. doi: 10.1016/s0965-1748(02)00187-x. [DOI] [PubMed] [Google Scholar]
- 29.Telleria EL, Pitaluga AN, Ortigao-Farias JR, de Araujo AP, Ramalho-Ortigao JM, et al. Constitutive and blood meal-induced trypsin genes in Lutzomyia longipalpis. Arch Insect Biochem Physiol. 2007;66:53–63. doi: 10.1002/arch.20198. [DOI] [PubMed] [Google Scholar]
- 30.Sant'anna MR, Diaz-Albiter H, Mubaraki M, Dillon RJ, Bates PA. Inhibition of trypsin expression in Lutzomyia longipalpis using RNAi enhances the survival of Leishmania. Parasit Vectors. 2009;2:62. doi: 10.1186/1756-3305-2-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramalho-Ortigao JM, Traub-Cseko YM. Molecular characterization of Llchit1, a midgut chitinase cDNA from the leishmaniasis vector Lutzomyia longipalpis. Insect Biochem Mol Biol. 2003;33:279–287. doi: 10.1016/s0965-1748(02)00209-6. [DOI] [PubMed] [Google Scholar]
- 32.Molina-Cruz A, DeJong RJ, Charles B, Gupta L, Kumar S, et al. Reactive oxygen species modulate Anopheles gambiae immunity against bacteria and Plasmodium. J Biol Chem. 2008;283:3217–3223. doi: 10.1074/jbc.M705873200. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez-Lazaro M, Dinglasan RR, Hernandez-Hernandez Fde L, Rodriguez MH, Laclaustra M, et al. Anopheles gambiae Croquemort SCRBQ2, expression profile in the mosquito and its potential interaction with the malaria parasite Plasmodium berghei. Insect Biochem Mol Biol. 2009;39:395–402. doi: 10.1016/j.ibmb.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinto SB, Lombardo F, Koutsos AC, Waterhouse RM, McKay K, et al. Discovery of Plasmodium modulators by genome-wide analysis of circulating hemocytes in Anopheles gambiae. Proc Natl Acad Sci U S A. 2009;106:21270–21275. doi: 10.1073/pnas.0909463106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pitaluga AN, Mason PW, Traub-Cseko YM. Non-specific antiviral response detected in RNA-treated cultured cells of the sandfly, Lutzomyia longipalpis. Dev Comp Immunol. 2008;32:191–197. doi: 10.1016/j.dci.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 36.Sant'Anna MR, Alexander B, Bates PA, Dillon RJ. Gene silencing in phlebotomine sand flies: Xanthine dehydrogenase knock down by dsRNA microinjections. Insect Biochem Mol Biol. 2008;38:652–660. doi: 10.1016/j.ibmb.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]