Abstract
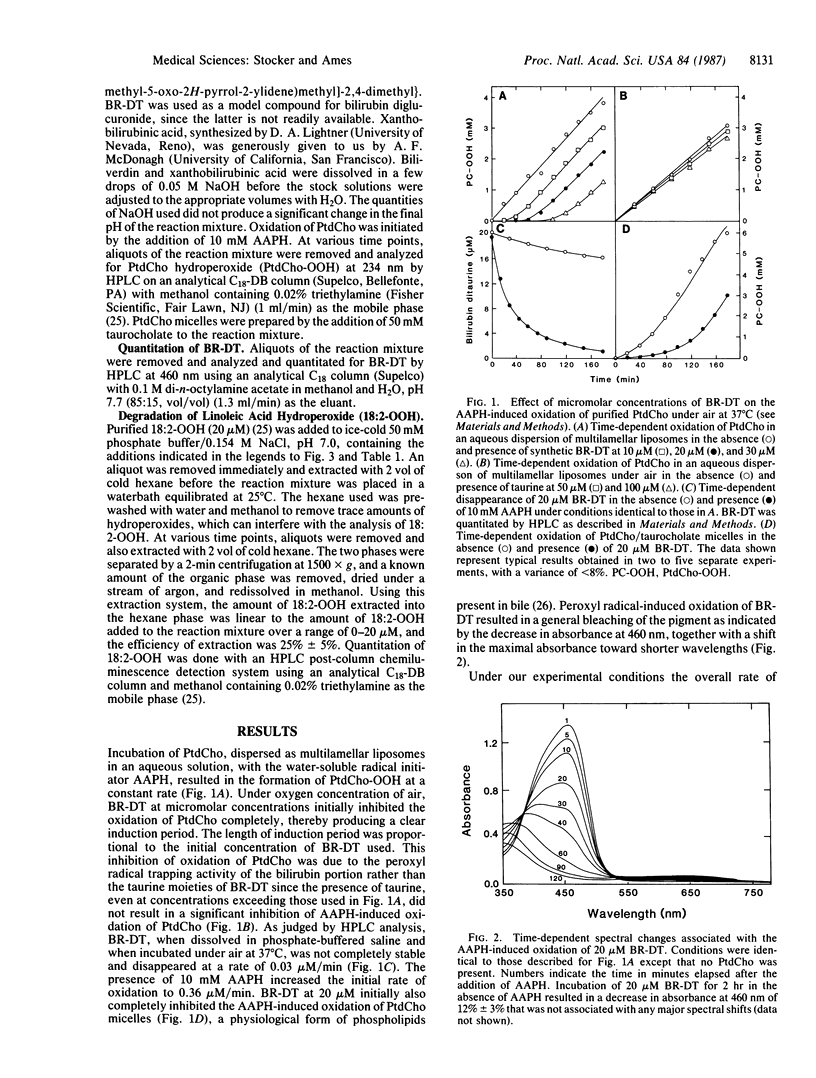
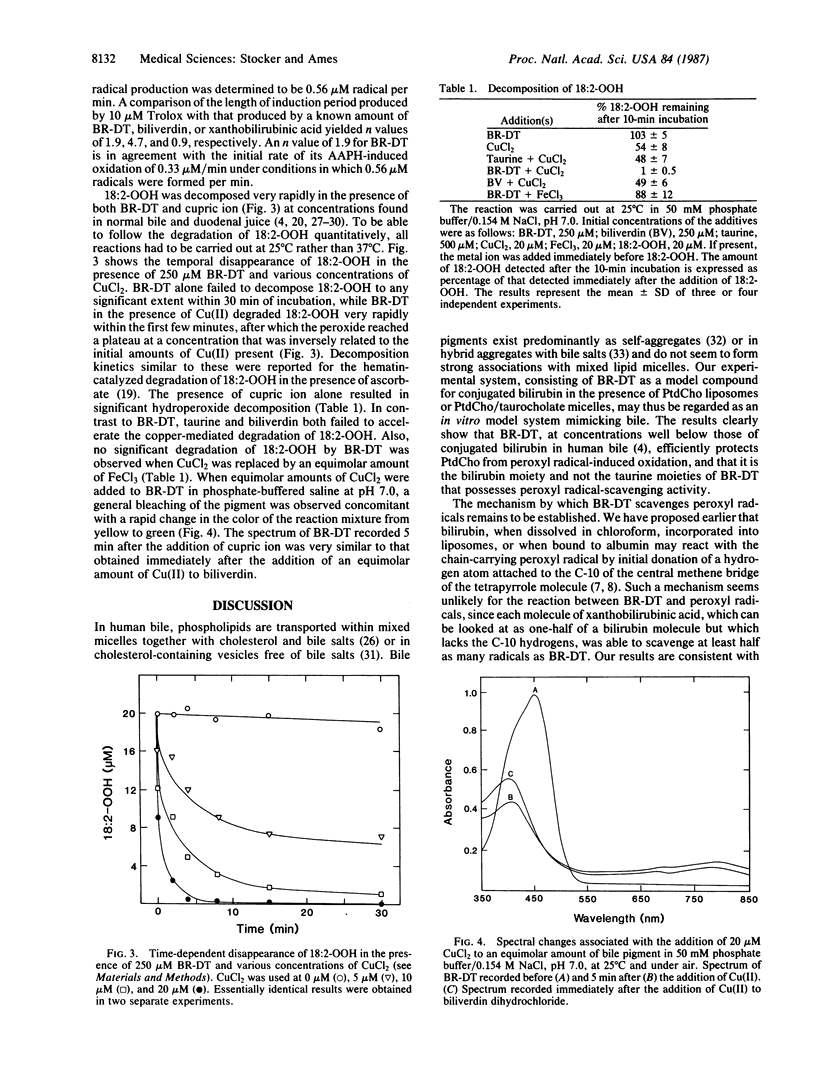
Conjugated bilirubin and copper ions at their physiological concentrations in bile may play an important role in hydroperoxide and other detoxification. Conjugated bilirubin may also be an important chain-breaking antioxidant preventing lipid peroxidation. Bilirubin ditaurine (BR-DT), a water-soluble model compound of conjugated bilirubin, completely prevents the peroxyl radical-induced oxidation of phosphatidylcholine in either multilamellar liposomes or micelles. This antioxidant activity is associated with the bilirubin moiety of BR-DT, since taurine alone is inefficient in scavenging peroxyl radicals. The number of peroxyl radicals trapped per molecule of BR-DT is 1.9, compared to 4.7 trapped per molecule of biliverdin, the water-soluble physiological precursor of bilirubin. Peroxyl radical-induced oxidation of BR-DT results in a spectral shift in maximal absorbance toward shorter wavelengths; biliverdin is not formed as a major oxidation product. BR-DT, but neither taurine nor biliverdin, greatly accelerates the cupric ion-catalyzed decomposition of linoleic acid hydroperoxide. In the presence of ferric ion, BR-DT shows no lipid hydroperoxide-degrading activity. Addition of cupric ion to BR-DT results in formation of a complex with spectral features similar to that of a biliverdin-cupric ion complex, indicating that BR-DT and cupric ion undergo redox reactions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk P. D., Howe R. B., Bloomer J. R., Berlin N. I. Studies of bilirubin kinetics in normal adults. J Clin Invest. 1969 Nov;48(11):2176–2190. doi: 10.1172/JCI106184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliuger A. F., Dudnik L. B., Maiore A. Ia, Mieze I. E. Rol' bilirubina kak prirodnogo antioksidanta v reguliatsii intensivnosti perekisnogo okisleniia lipidov pri ostrom virusnom gepatite. Biull Eksp Biol Med. 1985 Feb;99(2):166–168. [PubMed] [Google Scholar]
- Braganza J. M., Klass H. J., Bell M., Sturniolo G. Evidence of altered copper metabolism in patients with chronic pancreatitis. Clin Sci (Lond) 1981 Mar;60(3):303–310. doi: 10.1042/cs0600303. [DOI] [PubMed] [Google Scholar]
- Braganza J. M. Pancreatic disease: a casualty of hepatic "detoxification"? Lancet. 1983 Oct 29;2(8357):1000–1003. doi: 10.1016/s0140-6736(83)90983-2. [DOI] [PubMed] [Google Scholar]
- Brodersen R., Bartels P. Enzymatic oxidation of bilirubin. Eur J Biochem. 1969 Oct;10(3):468–473. doi: 10.1111/j.1432-1033.1969.tb00712.x. [DOI] [PubMed] [Google Scholar]
- Brodersen R. Binding of bilirubin to albumin. CRC Crit Rev Clin Lab Sci. 1980 Jan;11(4):305–399. [PubMed] [Google Scholar]
- Brodersen R. Localization of bilirubin pools in the non-jaundiced rat, with a note on bilirubin dynamics in normal human adults and in Gilbert's syndrome. Scand J Clin Lab Invest. 1972 Sep;30(1):95–106. doi: 10.3109/00365517209081097. [DOI] [PubMed] [Google Scholar]
- Coon M. J. Oxygen activation in the metabolism of lipids, drugs and carcinogens. Nutr Rev. 1978 Nov;36(11):319–328. doi: 10.1111/j.1753-4887.1978.tb03697.x. [DOI] [PubMed] [Google Scholar]
- Cuypers H. T., Ter Haar E. M., Jansen P. L. Microsomal conjugation and oxidation of bilirubin. Biochim Biophys Acta. 1983 Jul 29;758(2):135–143. doi: 10.1016/0304-4165(83)90294-5. [DOI] [PubMed] [Google Scholar]
- Fevery J., Van de Vijver M., Michiels R., Heirwegh K. P. Comparison in different species of biliary bilirubin-IX alpha conjugates with the activities of hepatic and renal bilirubin-IX alpha-uridine diphosphate glycosyltransferases. Biochem J. 1977 Jun 15;164(3):737–746. doi: 10.1042/bj1640737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen J., Fedders O. Determination of non-albumin-bound bilirubin in human serum. Scand J Clin Lab Invest. 1970 Nov;26(3):237–241. doi: 10.3109/00365517009046228. [DOI] [PubMed] [Google Scholar]
- Lewis K. O. The nature of the copper complexes in bile and their relationship to the absorption and excretion of copper in normal subjects and in Wilson's disease. Gut. 1973 Mar;14(3):221–232. doi: 10.1136/gut.14.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansbach C. M., 2nd, Rosen G. M., Rahn C. A., Strauss K. E. Detection of free radicals as a consequence of rat intestinal cellular drug metabolism. Biochim Biophys Acta. 1986 Aug 29;888(1):1–9. doi: 10.1016/0167-4889(86)90063-7. [DOI] [PubMed] [Google Scholar]
- Martin M. T., Jacobs F. A., Brushmiller J. G. Low molecular weight copper-binding ligands in human bile. Proc Soc Exp Biol Med. 1986 Feb;181(2):249–255. doi: 10.3181/00379727-181-42249. [DOI] [PubMed] [Google Scholar]
- Mason K. E. A conspectus of research on copper metabolism and requirements of man. J Nutr. 1979 Nov;109(11):1979–2066. doi: 10.1093/jn/109.11.1979. [DOI] [PubMed] [Google Scholar]
- McCullars G. M., O'Reilly S., Brennan M. Pigment binding of copper in human bile. Clin Chim Acta. 1977 Jan 3;74(1):33–38. doi: 10.1016/0009-8981(77)90384-9. [DOI] [PubMed] [Google Scholar]
- Norman T. R., Walker R. G., Burrows G. D. Renal function related changes in lithium kinetics. Clin Pharmacokinet. 1984 Jul-Aug;9(4):349–353. doi: 10.2165/00003088-198409040-00004. [DOI] [PubMed] [Google Scholar]
- O'Brien P. J. Intracellular mechanisms for the decomposition of a lipid peroxide. I. Decomposition of a lipid peroxide by metal ions, heme compounds, and nucleophiles. Can J Biochem. 1969 May;47(5):485–492. doi: 10.1139/o69-076. [DOI] [PubMed] [Google Scholar]
- Reuben A., Howell K. E., Boyer J. L. Effects of taurocholate on the size of mixed lipid micelles and their associations with pigment and proteins in rat bile. J Lipid Res. 1982 Sep;23(7):1039–1052. [PubMed] [Google Scholar]
- Small D. M., Bourgès M., Dervichian D. G. Ternary and quaternary aqueous systems containing bile salt, lecithin, and cholesterol. Nature. 1966 Aug 20;211(5051):816–818. doi: 10.1038/211816a0. [DOI] [PubMed] [Google Scholar]
- Somjen G. J., Gilat T. A non-micellar mode of cholesterol transport in human bile. FEBS Lett. 1983 Jun 13;156(2):265–268. doi: 10.1016/0014-5793(83)80510-9. [DOI] [PubMed] [Google Scholar]
- Spivak W., Carey M. C. Reverse-phase h.p.l.c. separation, quantification and preparation of bilirubin and its conjugates from native bile. Quantitative analysis of the intact tetrapyrroles based on h.p.l.c. of their ethyl anthranilate azo derivatives. Biochem J. 1985 Feb 1;225(3):787–805. doi: 10.1042/bj2250787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B., Small R. D., Jr The photoperoxidation of unsaturated organic molecules--XV. O21Delta g quenching by bilirubin and biliverdin. Photochem Photobiol. 1976 Jan;23(1):33–36. doi: 10.1111/j.1751-1097.1976.tb06767.x. [DOI] [PubMed] [Google Scholar]
- Stocker R., Glazer A. N., Ames B. N. Antioxidant activity of albumin-bound bilirubin. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5918–5922. doi: 10.1073/pnas.84.16.5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker R., Yamamoto Y., McDonagh A. F., Glazer A. N., Ames B. N. Bilirubin is an antioxidant of possible physiological importance. Science. 1987 Feb 27;235(4792):1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- Tazuma S., Holzbach R. T. Transport of conjugated bilirubin and other organic anions in bile: relation to biliary lipid structures. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2052–2056. doi: 10.1073/pnas.84.7.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela A., Fernandez V., Videla L. A. Hepatic and biliary levels of glutathione and lipid peroxides following iron overload in the rat: effect of simultaneous ethanol administration. Toxicol Appl Pharmacol. 1983 Aug;70(1):87–95. doi: 10.1016/0041-008x(83)90181-3. [DOI] [PubMed] [Google Scholar]
- Wayner D. D., Burton G. W., Ingold K. U., Locke S. Quantitative measurement of the total, peroxyl radical-trapping antioxidant capability of human blood plasma by controlled peroxidation. The important contribution made by plasma proteins. FEBS Lett. 1985 Jul 22;187(1):33–37. doi: 10.1016/0014-5793(85)81208-4. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Brodsky M. H., Baker J. C., Ames B. N. Detection and characterization of lipid hydroperoxides at picomole levels by high-performance liquid chromatography. Anal Biochem. 1987 Jan;160(1):7–13. doi: 10.1016/0003-2697(87)90606-3. [DOI] [PubMed] [Google Scholar]
- van Berge Henegouwen G. P., Tangedahl T. N., Hofmann A. F., Northfield T. C., LaRusso N. F., McCall J. T. Biliary secretion of copper in healthy man. Quantitation by an intestinal perfusion technique. Gastroenterology. 1977 Jun;72(6):1228–1231. [PubMed] [Google Scholar]



