Abstract
Control of the 3D microenvironment for cultured cells is essential for understanding the complex relationships that biomolecular concentration gradients have on cellular growth, regeneration, and differentiation. This paper reports a microfluidic device for delivering gradients of soluble molecules to cells in an open reservoir without exposing the cells to flow. The cells are cultured on a polyester membrane that shields them from the flow that delivers the gradient. A novel “lid” design is implemented which prevents leakage from around the membrane without requiring sealing agents or adhesives. Once layers are molded, device fabrication can be performed within minutes while at room temperature. Surface gradients were characterized with epifluorescence microscopy; image analysis verified that sharp gradients (∼33 μm wide) can be reproducibly generated. We show that heterogeneous laminar flow patterns of Orange and Green Cell Tracker (CT) applied beneath the membrane can be localized to cells cultured on the other side; concentration profile scans show the extent of CT diffusion parallel to the membrane’s surface to be 10–20 μm. Our device is ideal for conventional cell culture because the cell culture surface is readily accessible to physical manipulation (e.g., micropipette access), the cell culture medium is in direct contact with the incubator atmosphere (i.e., no special protocols for ensuring proper equilibration of gas concentrations are required), and the cells are not subjected to flow-induced shear forces, which are advantageous attributes not commonly found in closed-channel microfluidic designs.
INTRODUCTION
The effects of biomolecular concentration gradients on cellular growth, differentiation, regeneration, and migration have been well studied. Interest in illuminating these phenomena led to the development of first-generation biomolecule gradient platforms including chambers designed by Boyden,1 Zigmond,2 and Dunn,3 as well as 3D gel matrices for diffusible molecules.4, 5 Limitations in spatiotemporal control and a lack of quantitative characterization of these systems have motivated the development of flow-based microfluidic devices that exploit the unique mass-transfer phenomena present at the microscale to more closely reproduce in vivo cellular environments.6, 7, 8, 9, 10 While these microfluidic systems provide powerful methods for controlling a gradient’s shape and function, they suffer from a number of drawbacks: (1) Gradient flow tends to remove cell-secreted molecules essential for cell-cell communication, (2) flow generates potentially damaging shear forces on cellular membranes, and (3) enclosed microfluidic channels are required for many flow-based devices, limiting access to cultured cells. More recently, several new classes of microfluidic devices have emerged that address the limitations of closed-chamber, parallel flow-based devices. Designs with high-resistance microchannels have been developed that significantly reduce shear stress on cells during gradient generation without compromising gradient stability.11, 12, 13, 14 In these systems, fluidic channels only a few microns in height and width, termed “microjets,” are designed which limit the flow velocity “sensed” by the cells. Microjet devices have been used to study bacterial and neutrophil chemotaxis in open11 and closed14 microchambers. Integrating a semipermeable barrier, such as a hydrogel15, 16 or high-resistance membrane,17, 18, 19, 20 between the cell chamber area and the fluid channels has also been explored for producing gradients of negligible flow. Hydrogels are advantageous in that they are inexpensively available and can be selectively formed inside a microfluidic device through gellation of a precursor liquid. Incorporating commercially available polymer membranes into devices for gradient generation has also been investigated.20, 21 The advantages that prefabricated membranes have over hydrogels are that (a) they can be made thin (<10 μm), responding quickly to fast-changing gradients or to gradients of small, rapidly diffusing molecules, and that (b) their surface chemistry and rigidity are more amenable for bonding and integration into devices. Functionalized polymer membranes are used as supports for culturing cells and it was showed by Maharbiz and colleagues20 that cells are exposed to very low-shear fluid gradients when flow is directed parallel to the membrane’s surface (with cells on the other side of the membrane).
The goal of the current study was to develop a microfluidic gradient generating platform capable of exposing cells to fast-changing gradients under shear-free conditions. To avoid shear, we cultured the cells on a transparent polyester membrane and heterogeneous laminar flow patterns were applied below the membrane. The membrane inhibits potentially cell-damaging shear stress while facilitating diffusive transport. In essence, our device consists of a very simple gradient generator interfaced with an open-access surface through a high-resistance membrane; the design is highly amenable for simplified culture, preconditioning, or experimental treatment of cells using external biological analysis tools. An arbitrary channel geometry was chosen for gradient generation, but this configuration can be easily altered to match experimental needs.
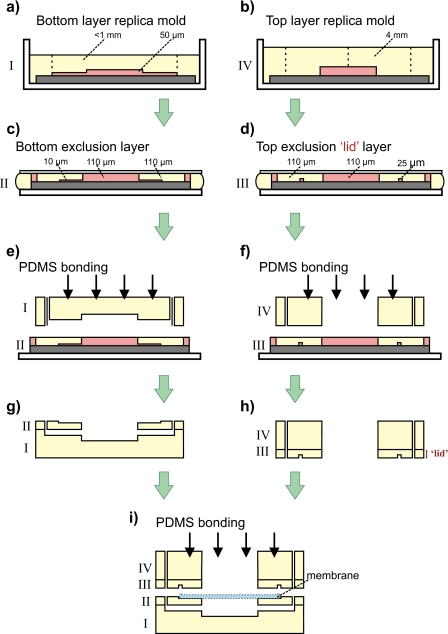
The device is comprised of four stacked poly(dimethylsiloxane) (PDMS) polymer layers, a top layer, two middle layers, and a bottom layer. An aperture in the uppermost layer forms a well for plating cells on the membrane (pore diameter of 0.4 μm) that is positioned below and is sandwiched between the middle layers. Microchannels located in the bottom layer deliver fluid from the device’s source inlets to the main reservoir where molecules freely diffuse through the membrane to cells on the other side. The fabrication of the device is shown schematically in Fig. 1. All of the microfluidic device layers (layers I–IV) are made using well-established replica and exclusion-molding techniques.22, 23 The two middle layers (layers II and III) sandwich the membrane to prevent leakage from the main reservoir. A recess in layer II helps position the membrane and serves as an alignment marker for the application of layer III, the “lid,” which seals the perimeter of the membrane. Additional measures were not required to fortify the seal around the membrane using adhesive agents, a technique commonly used by other groups.24, 25, 26, 27 Because there is no seal around the membrane, a complete device can be fabricated within minutes using plasma bonding (after layer molding). This technique enables faster device fabrication over other microfluidic platforms that use one or more sealing procedures. Often these sealing steps require an additional drying process and many times the seal is incomplete, adversely affecting device performance and∕or fabrication throughput as a result.
Figure 1.
Fabrication schematic of the open-access lid design. [(a) and (b)] Replica molds are cast by pouring 10:1 (prepolymer: curing agent) PDMS over prefabricated features on a silicon wafer. [(c) and (d)] Exclusion molds define the square lid that holds the membrane in place. [(e) and (f)] Both top and bottom replica layers are plasma oxygen bonded to the second and third exclusion layers. (g) Combined bottom half. (h) Combined top half. (i) A CO2 laser cut polyester membrane is aligned inside the excluded ring of layers II and III. Layers III and IV are plasma oxygen bonded to layers I and II to complete device fabrication.
We first characterized the stability of a gradient formed by a Y-configuration of two fluid streams at the bottom channel. The extent of surface-level mixing at the gradient interface over a 4 h time course was quantified using fluorescein dye and Orange G.28 in the two respective fluid streams. Orange G, a nonfluorescent dye, is strongly absorbent at the excitation wavelength (490 nm) and weakly absorbent at the emission wavelength (540 nm) of fluorescein. Bancaud and colleagues first showed that epifluorescence images of a thin optical slice (∼3.5 μm) normal and adjacent to a surface could be acquired due to the exponential decay of excitation light intensity as it passes through the volume of Orange G solution. This method of measuring surface-level concentration profiles at the bottom of the fluid channel (surface closest to objective) allowed us to predict what the diffusion profile of the gradient looked like directly beneath the cells, assuming little material was lost through the membrane to the cells through diffusive transport. Second, we investigated the optical clarity of cultured NIH-3T3 fibroblast cells in the device in comparison with cells cultured on a Transwell support, a common biological tool for cell culture. We show qualitatively that the optical resolution of fibroblasts cultured in our device is comparable to those on a Transwell support. Third, a fluorescent probe uptake assay was performed for the purpose of defining the gradient boundary across the membrane interface. Functional tracer uptake was performed using Orange (CMRA, 550 Da) and Green (CMFDA∕5-chloromethylfluorescein diacetate, 464 Da) Cell Tracker. CMRA is a thiol-reactive molecule that fluoresces when it undergoes esterase-mediated hydrolysis inside a cell, allowing visualization of the cell cytoplasm. We measured a gradient width of 10–20 μm, equivalent to the diameter of one or two fibroblast cells. Finally, we show that the diffusion distances calculated for fluorescein dye and Green Cell Tracker agree well with each other based on a model we propose for membrane pore blockage due to fibroblast spreading.
EXPERIMENTAL PROCEDURES
Chemicals
3 in. silicon wafers were purchased from Silicon Sense, Inc. (Nashua, NH, USA). The SU-8 line of photoresists and developer solution (1-methoxy-2-propyl acetate) were obtained from MicroChem. Corp. (Newton, MA, USA), and fluorosilane solution [(tridecafluoro-1, 1, 2, 2, tetrahydrooctyl)-1 trichlorosilane] from United Chemical Technologies Inc. (Horsham, PA, USA). Dow Corning Sylgard 184 PDMS was purchased from KR Anderson, Inc. (Kent, WA, USA); isopropyl alcohol (propan-2-ol), Orange G (1-phenylazo-2-naphthol-6,8-disulfonic acid disodium salt) (molecular weight of 452.37 g mol), poly-D-lysine (PDL), and fluorescein sodium salt (molecular weight of 376.3 g mol) were from Sigma-Aldrich (St. Louis, MO, USA). Fibronectin, Orange (CMRA), and Green (CMFDA) Cell Tracker were obtained from Invitrogen (Eugene, OR, USA).
Device fabrication
Device features designed using AUTOCAD software (AutoDesk, Inc., San Rafael, CA, USA) were transferred to photomasks via a high resolution plotter (CAD∕Art Services, Inc., Bandon, OR, USA). Multilayer device features were defined on silicon wafers by conventional SU-8 negative-resist photolithography procedures, outlined by the manufacturer.29 The bottom and top layers (I and IV from Fig. 1) were each produced from a single exposure to collimated ultraviolet light through the photomask. The bottom layer [Fig. 1a] contains microchannels (1×1×0.05 mm3) (W×L×H) and the top layer [Fig. 1b] contains alignment markers for device inlets and an open well for membrane access (diameter of 5 mm). Wafer features for middle layers (II and III in Fig. 1) were defined by a two-step photolithography process yielding structures 10 and 110 μm in height for layer II [Fig. 1c], and 25 and 110 μm in height for layer III [Fig. 1d]. A recess (3×3×0.01 mm3) in the second layer functions as a placeholder for the porous membrane and application of the third layer over the second layer fixed the membrane in position, functionally similar to how a lid covers a jar. These layers were formed by applying PDMS to a wafer and compression molding to create an aperture (1.0×1.0×0.11 mm3) from the tallest features enabling fluidic access to the integrated membrane. The first two layers were manually aligned under a stereomicroscope (model SMZ1500, Nikon Instruments, Melville, NY, USA) and covalently bonded via PDMS surface activation with reactive oxygen plasma (18 s, 600 W, model 862, Plasmatic Systems Inc., North Brunswick, NJ, USA) [Fig. 1e]. The process was repeated for layers III and IV [Fig. 1f]. Membranes from Transwell supports were cut and sized directly from packaging (diameter of 24 mm, pore size of 0.4 μm, and tortuosity of 1) (Product No. 3450, Corning, Inc., Corning, New York, USA) with a CO2 laser cutter (24 W, model VLS3.60, Universal Laser Systems, Inc., Scottsdale, Arizona, USA). Cut membranes (3×3 mm2) were positioned in the recess of the second layer [Fig. 1g] and were capped by permanently sealing the device’s top half [Fig. 1h] to the bottom half. This step however, did not prevent leaking between the membrane-PDMS interface or around the membrane border. This issue was resolved by creating a square ring (300×25 μm2) (W×H) in the top exclusion layer (layer III or lid) designed to cover the membrane border and prevent contact between fluid and the membrane except inside the gradient chamber. To maximize optical clarity, a ∼0.5 lb weight was applied on top of the bottom layer during curing to reduce the amount of PDMS polymer in the optical path. When cured, the total thickness of the bottom layer was less than 1 mm. A side-profile schematic of the complete device is shown in Fig. 1i. A stable gradient can be generated within seconds.
Time-lapse microscopy and cell culture setup
Gradients at the surface of the fluid channels were visualized inside a microfluidic device by recording the signal intensity of a mixture of fluorescein sodium salt (1 mM) and Orange G (45 mM). Orange G is a nonfluorescent dye that absorbs strongly at its excitation wavelength (490 nm) and weakly at the emission wavelength (540 nm) of fluorescein. Measurements were recorded using an inverted fluorescence microscope (model TE2000U, Nikon Instruments, Inc., Melville, NY, USA) and an Orca-HR high resolution digital charged-coupled device (CCD) camera (Hamamatsu Photonics, Hamamatsu City, Japan). For cell culture experiments, the polyester membrane and walls of the PDMS well were sterilized under an ultraviolet transmitting lamp for 15 min (25 W, model TFL-40, Ultra-Violet Products, CA, USA) before the membrane was coated with PDL (50 μg∕ml). After 4 h at room temperature, excess PDL was flushed several times with Dulbecco’s phosphate buffered saline (D-PBS) (Invitrogen, Carlsbad, CA, USA) and the membrane was coated overnight with fibronectin (10 mM) at 37 °C. Fibroblasts from the NIH-3T3 line were densely seeded (600 000 cells∕ml) on the membrane and given 3–4 h for ample attachment and spreading to take place. Cells were placed in an incubator at 37 °C and 5% CO2 atmosphere during seeding. For control experiments, Transwell supports were similarly coated using the same concentrations of PDL and fibronectin. The cell culture well of the microfluidic device facilitated the membrane coating and cell seeding protocols by enabling pipette access. Prior to imaging, fluorescent probe solutions of Orange and Green Cell Tracker were diluted with D-PBS to 10 μM. Postimage processing was performed using METAMORPH (Molecular Devices Co., Downingtown, PA, USA). Flow rates were acquired by measuring the total volume of filtered de-ionized water passing through two devices operating in parallel in 1 h intervals over a 3 h period. In total, six measurements were taken.
RESULTS AND DISCUSSION
Stable gradients can be generated for a 376 Da molecule
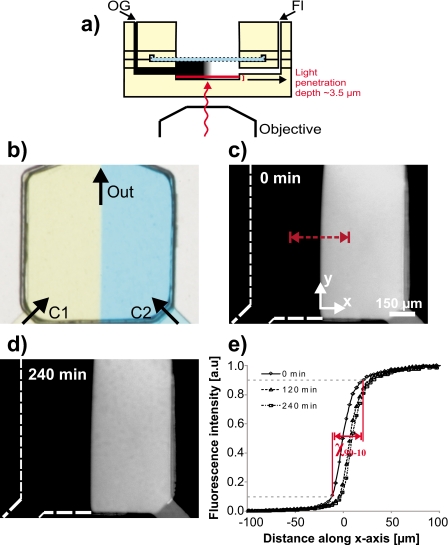
A side profile schematic of device operation is given in Fig. 2a, showing the optical path of visible light. Two fluid streams converge in a typical Y-shape configuration, creating a boundary for molecular diffusion [Fig. 2b]. Surface-level gradient stability of the fluidic channels can be quantified by acquiring time-lapse images of a diffusible fluorescent dye coupled with a diluent (Orange G). Soluble fluorescein sodium salt (1 mM) mixed with Orange G (45 mM) was introduced in one inlet and pure Orange G (45 mM) in the other prior to pressurization (∼0.5 psi) with compressed air. Since Orange G absorbs strongly at the excitation wavelength of fluorescein, a thin (∼3.5 μm) optical slice of surface-level fluorescence intensity was easily visualized [Fig. 2c]. Time-lapse images were acquired at 30 min intervals over a 4 h period. Figure 2d shows a fluorescence micrograph (20×) of the gradient boundary taken at t=4 h after the micrograph in Fig. 2c, highlighting the stability of the gradient over long term. By incorporating a high-resistance membrane under the cell culture surface, as first demonstrated by Maharbiz and colleagues,20 we are able to flow fluid at a high rate (average device flow rate=20.9±1.4 μl∕min, Re=0.8), thereby creating a sharp gradient boundary without subjecting cells to potentially damaging forces. We note that in traditional microfluidic channels containing cells, generating steep gradient boundaries is often not feasible due to the high shear stress imparted on cell membranes from flow.
Figure 2.
Gradient stability quantified with epifluorescence microscopy. (a) Side profile schematic showing light penetration depth during fluorescence microscopy imaging. Orange G, when added to the system, absorbs all visible light further than 3.5 μm from the channel floor. (b) 20× magnification of the gradient chamber showing gradient formation from confronting streams of blue and yellow dyes. Concentrations C1 and C2 were used to form the gradient interface. The volumetric flow rate was measured to be 20.9±1.4 μl∕min at an inlet driving pressure of 0.5 psi. [(c) and (d)] Time-lapse fluorescence micrographs used to quantify stability and sharpness of a fluorescein-Orange G gradient. Images were taken every 10 min over a 4h period. (e) Fluorescence intensity graphed as a function of distance along the x-axis in (c). The χ90-10 distance calculated was 33 μm. Fluorescence images were corrected for background signal and uneven illumination.
We performed concentration profile scans across the gradient interface [dotted line in Fig. 2c] to measure the steepness and stability of the gradient [Fig. 2e]. Gradient steepness was quantified by calculating the χ90-10 distance, a value corresponding to the distance the gradient falls between 90% and 10% maximum fluorescence intensity. Using fluorescein (376 Da) in our device, the χ90-10 distance was measured to be 33 μm at 0.5 psi. This distance is comparable to or lower than the smallest reported χ90-10 distances in flow-resistive devices employing gradient flow with molecules of a similar size to fluorescein.7, 8, 20, 30 Additionally, by either increasing or decreasing fluid driving pressures at one or both inlets, the steepness of the gradient can be manipulated to meet experimental objectives. Gradient stability was quantified by calculating the distance the fluorescence boundary at 50% maximal intensity shifted along the x-axis during the 4 h time lapse. This shift was reported to be 10.9 μm, or approximately the width of a single fibroblast, demonstrating that generation of a stable gradient can be maintained with cellular precision over several hours. Gradient shifting occurred primarily between 0 and 120 min (the gradient shifted less than 2 μm between 120 and 240 min), and could result from a perturbation to the system, possibly from a small difference in inlet pressure (e.g., caused by the nucleation of a small bubble in the tubing).
We note that convective transport through the pores of the membrane can be an important issue with membrane systems; the transmembrane resistance can be smaller than the flow channel resistance. The flow rate through the membrane pores can be quantified using Darcy’s law (Q=−(κA∕μL)ΔP), which describes fluid flow through a porous medium. The membrane’s free surface area (A=5×10−8 m2) and solution viscosity (μ=0.001 kg m−1 s−1) are known with precision. The transport rate Q is expressed as the ratio of the membrane’s permeability factor (κ=10−12 m2)31 to the total diffusion distance through the membrane (L=10−5 m) times the pressure drop (ΔP=0.14 psi) incurred by the hydrodynamic resistance of the device. Using conservative values, the estimated flow rate through the membrane was at most 12 μl∕s. During operation however, the gradient chamber above the membrane took approximately 90 min to fill 20 μl, which sets the rate of fluid transport through the membrane closer to 3–4 μl∕s.
Visualizing fluorescently labeled cells
A salient feature of our device is that the open reservoir design facilitates free access to cell cultures, and provides gas∕pH equilibrium and humidity stability (similar to what has been optimized for standard cell culture incubators) without subjecting the cells to flow-induced shear forces, a prevailing drawback in systems with closed channels. With our device, manipulating the cellular environment with solutions containing small molecules, growth factors, or fluorescent markers, without flow, is relatively simple and it allows direct access to single cells for experimentation (e.g., intracellular injection and patch-clamp recording). Furthermore, we have been able to maintain the viability and growth of cells cultured on the membrane for more than a week simply by exchanging nutrient media in the open reservoir using a handheld pipettor.
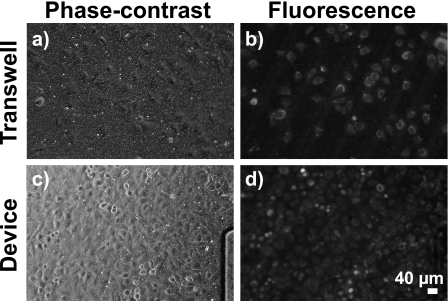
To demonstrate the utility of our device for cell culture, 3T3 fibroblasts were plated onto a fibronectin-coated membrane inside the reservoir and allowed to attach. We applied Orange (CMRA) Cell Tracker prior to fluorescently labeling cells. In addition, seeking to investigate the optical clarity of fibroblasts cultured on the membrane inside our device, we performed a control experiment in parallel using a Transwell support (0.4 μm pore diameter), a platform commonly utilized for cell growth and microscopy. Phase-contrast and fluorescence micrographs of Transwell-cultured fibroblasts [Figs. 3a, 3b] show cells clearly attached and spread on the membrane. Note that images in Figs. 3a, 3b were taken at the same magnification (30×) and position on the membrane. Cell resolution was similar in both phase-contrast and fluorescence micrographs of cultured fibroblasts inside the device [see Figs. 3c, 3d and compare with Figs. 3a, 3b, respectively]. The lack of observable difference in cell resolution between Transwell and device fluorescent micrographs qualitatively suggests that our device is a suitable platform for adherent cell imaging with high resolution.
Figure 3.
Fluorescently labeled cells cultured on the PE membrane of a Transwell support and inside our microfluidic device. Both phase-contrast and fluorescence micrograph pairs were taken at 30× and at the same location on the membrane. Fibroblasts stained with Orange Cell Tracker emitted strongly at 576 nm. [(a) and (b)] Phase-contrast and fluorescence micrographs showed fibroblasts densely seeded on a Transwell support. Fibroblast visibility is comparable in (c) phase-contrast of cells in microfluidic device and (d) fluorescence micrograph of cells in the microfluidic device. While the fibroblast nucleus is not prominent in either Transwell or device micrographs, cellular outlines and cytoplasm are easily visible in both, demonstrating that optical clarity is not compromised when imaging inside our microfluidic device compared with traditional Transwell supports.
Diffusion of fluorescent tracers across the polyester membrane for a cellular uptake assay
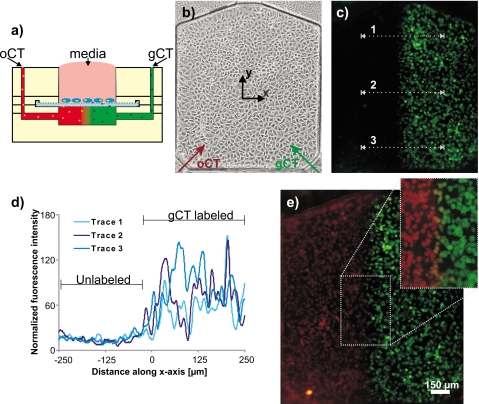
Solutions of orange and green fluorescent markers were employed to demonstrate cellular uptake across the polyester membrane interface. Cell-activated tracers, which fluoresce when cleaved by enzymes inside a cell, were used to indicate probe diffusion through the polyester membrane. By pressurizing both inlets equally, we attempted to create two symmetric, but distinct fluorescent regions beneath the membrane, eliciting a sharp gradient in the center of the channel parallel to the direction of flow [Fig. 4a]. For the experiment, fibroblasts suspended in phenol red-free media were plated on the membrane housed inside the reservoir and were allowed 3–4 h to attach and spread [Fig. 4b]. The cells took up Cell Tracker molecules for the extent of the 30 min experiment, as fluorescence emission intensity increased throughout the time course. Figure 4c shows that after the system was flushed with D-PBS, any remaining fluorescence is restricted within the cells, matching the flow pattern of Green Cell Tracker (Orange Cell Tracker not shown for clarity). Concentration profile scans along the numbered lines quantitatively demonstrate that the underlying gradient region containing Green Cell Tracker was undoubtedly localized across the membrane to attached cells; no fluorescence attributable to green tracer was observed above the background in the unlabeled (orange tracer) region [Fig. 4d]. The mirroring pattern was observed in fluorescence micrographs demonstrating cellular mapping of Orange Tracker [Fig. 4e]. An overlay of the two images highlights a narrow band of yellow colored cells [Fig. 4e, inset] representing the distance both (green and orange) fluorescent tracer molecules diffuse before being taken up by membrane-attached cells. The observed diffusion distance, or gradient width, corresponds to 10–20 μm, approximately 1–2 cell diameters. This experiment demonstrates that sharp gradients can be precisely delivered onto cultured cells across a membrane boundary.
Figure 4.
Cellular uptake assay. Fibroblasts cultured on the PE membrane were exposed to Orange and Green CT pressurized with compressed air at 0.5 psi. (a) Side profile schematic showing experimental setup. Cells are added by pipette in the open well (top center) and fluorescent probes via cored inlets on the top layer (top left and right). (b) 15× magnification of fibroblasts seeded at 600 000 cells/ml. Orange and green arrows show Cell Tracker flow paths (oCT and gCT, respectively). (c) After exposing cells to the oCT and gCT gradients for 30 min, cells that took up gCT fluoresced (orange fluorescing cells not shown for clarity). Concentration profile scans were taken along the dotted lines. (d) Fluorescence intensity graphed as a measure of distance along the x-axis. The labeled and unlabeled sections of the chamber show that very low gCT uptake levels were reported in cells on the left side of the membrane (those exposed to oCT). (e) Overlay of Green and Orange CT fluorescence micrographs. A narrow yellow band at the gradient interface (inset), approximately 10–20 μm wide, indicates cells exposed to both fluorescent probes.
The width of the uptake gradient (10–20 μm) agrees well with our experiments with fluorescein (χ90-10=33 μm), which is a faster-diffusing molecule than Orange or Green Cell Tracker (the media, the device, and the inlet pressurization were the same in both experiments). These results can be partially explained by considering that fluorescein (376 Da) is smaller than either Orange (550 Da) or Green (464 Da) Cell Tracker and should diffuse further in the same amount of time. In addition, when fibroblasts are seeded and spread, they flatten and should partially or completely block membrane pores, limiting or eliminating fluorescent probe diffusion. To validate this model, the diffusion distance of a single Green Cell Tracker molecule was estimated using δ, the free-diffusion distance (δ=(2Dt)1∕2), where D represents the diffusion coefficient of the Green Cell Tracker32 (D=3.4×10−8 cm2 s−1) and t equals 30 min (duration of flow). From the equation, the diffusion distance of a single molecule is approximately 110 μm, well above the experimentally reported distance (χ90-10=33 μm). This simple estimate does not take into account that diffusion is restricted to a cylindrical conduit and does not account for possible interactions between the Green Cell Tracker molecules with the polymer walls of the pore. However, the difference is very large, suggesting that membrane pore blockage from fibroblast spreading is likely a contributing factor in reducing molecular diffusion in the cell uptake assay.
CONCLUSIONS
In summary, we have demonstrated that our device is capable of delivering stable gradients with steep interfaces to the basal surface of cultured cells. Our fabrication method also facilitates cell access by external proximal probes, such as patch-clamp pipettes, atomic force microscopes, or near-field scanning optical microscopes. Lastly, we have demonstrated the successful uptake of fluorescent molecules by cells cultured atop a membrane interface, an experiment that can be generalized virtually to any biomolecule. Although in these experiments we used a two-channel system for gradient generation, more complex arrays can be easily integrated, including 3D networks capable of delivering multiple signaling factors simultaneously. We believe that this device will enhance our understanding of gradient-responsive cell types and should have general applicability in cell biology, tissue engineering, and biotechnology applications where flow-induced shear forces and enclosed volumes are believed to have detrimental effects on cells.
ACKNOWLEDGMENTS
We gratefully acknowledge Nirveek Bhattacharjee, Chi-Tim Chang, and William Watt for their critical comments and helpful insight. This work was partially funded by NIH under Grant No. R33 EB003307.
References
- Boyden S., J. Exp. Med. 115, 453 (1962). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond S. H., J. Cell Biol. 75, 606 (1977). 10.1083/jcb.75.2.606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zicha D., Dunn G. A., and Brown A. F., J. Cell Sci. 99, 769 (1991). [DOI] [PubMed] [Google Scholar]
- Brown A. F., J. Cell Sci. 58, 455 (1982). [DOI] [PubMed] [Google Scholar]
- Wilkinson P. C. and Lackie J. M., Exp. Cell Res. 145, 255 (1983). [DOI] [PubMed] [Google Scholar]
- Chung B. G., Lin F., and Jeon N. L., Lab Chip 6, 764 (2006). 10.1039/b512667c [DOI] [PubMed] [Google Scholar]
- Dertinger S. K. W., Chiu D. T., Jeon N. L., and Whitesides G. M., Anal. Chem. 73, 1240 (2001). 10.1021/ac001132d [DOI] [Google Scholar]
- Irimia D., Geba D. A., and Toner M., Anal. Chem. 10, 78 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon N. L., Dertinger S. K. W., Chiu D. T., Choi I. S., Stroock A. D., and Whitesides G. M., Langmuir 16, 8311 (2000). 10.1021/la000600b [DOI] [Google Scholar]
- Keenan T. M. and Folch A., Lab Chip 8, 34 (2008). 10.1039/b711887b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan T. M., Hsu C. H., and Folch A., Appl. Phys. Lett. 89, 114103 (2006). [Google Scholar]
- Li C. W., Chen R., and Yang M., Lab Chip 7, 1371 (2007). 10.1039/b705525k [DOI] [PubMed] [Google Scholar]
- Shamloo A., Ma N., Poo M., Sohn L. L., and Heilshorn S. C., Lab Chip 8, 1292 (2008). 10.1039/b719788h [DOI] [PubMed] [Google Scholar]
- Atencia J., Morrow J., and Locascio L. E., Lab Chip 9, 2707 (2009). 10.1039/b902113b [DOI] [PubMed] [Google Scholar]
- Mosadegh B., Huang C., Park J. W., Shin H. S., Chung B. G., Hwang S. K., Lee K. H., Kim H. J., Brody J., and Jeon N. L., Langmuir 23, 10910 (2007). 10.1021/la7026835 [DOI] [PubMed] [Google Scholar]
- Abhyankar V., Toepke M. W., Cortesio C. L., Lokuta M. A., Huttenlocher A., and Beebe D. J., Lab Chip 8, 1507 (2008). 10.1039/b803533d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abhyankar V. V., Lokuta M. A., Huttenlocher A., and Beebe D. J., Lab Chip 6, 389 (2006). 10.1039/b514133h [DOI] [PubMed] [Google Scholar]
- Chueh B. H., Huh D. G., Kyrtsos C. R., Houssin T., Futai N., and Takayama S., Anal. Chem. 79, 3504 (2007). 10.1021/ac062118p [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong J., Lammertink R. G. H., and Wessling M., Lab Chip 6, 1125 (2006). 10.1039/b603275c [DOI] [PubMed] [Google Scholar]
- Kim T., Pinelis M., and Maharbiz M. M., Biomed. Microdevices 11, 65 (2010). 10.1007/s10544-008-9210-7 [DOI] [PubMed] [Google Scholar]
- Aran K., Sasso L. A., Kamdar N., and Zahn J. D., Lab Chip 10, 548 (2010). 10.1039/b924816a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C. H., Chen C. C., and Folch A., Lab Chip 4, 420 (2004). 10.1039/b404956j [DOI] [PubMed] [Google Scholar]
- Xia Y. N. and Whitesides G. M., Angew. Chem., Int. Ed. 37, 551 (1998). [Google Scholar]
- Cai Z. -X., Fang Q., Chen H. W., and Fang Z. L., Anal. Chim. Acta 556, 151 (2006). 10.1016/j.aca.2005.06.028 [DOI] [PubMed] [Google Scholar]
- Hediger S., Fontannaz J., Sayah A., Hunziker W., and Gijs M., Sens. Actuators B 63, 63 (2000). 10.1016/S0925-4005(00)00292-6 [DOI] [Google Scholar]
- Thorslund S., Klett O., Nikolajeff F., Markides K., and Bergquist J., Biomed. Microdevices 8, 73 (2006). 10.1007/s10544-006-6385-7 [DOI] [PubMed] [Google Scholar]
- Xiang F., Lin Y., Wen J., Matson D., and Smith R., Anal. Chem. 71, 1485 (1999). 10.1021/ac981400w [DOI] [PubMed] [Google Scholar]
- Bancaud A., Wagner G., Dorfman K. D., and Viovy J. L., Anal. Chem. 77, 833 (2005). 10.1021/ac048996+ [DOI] [PubMed] [Google Scholar]
- See: http://www.microchem.com/products/su_eight.htm for outlined photolithography protocols for the entire SU-8 line of negative photoresists, including silicon wafer preparation and cross-linked SU-8 removal.
- Zhu X., Chu L. Y., Chueh B., Shen M., Hazarika B., Phadke N., and Takayama S., Analyst (Cambridge, U.K.) 129, 1026 (2004). 10.1039/b407623k [DOI] [PubMed] [Google Scholar]
- Jackson G. W. and James D. F., Can. J. Chem. Eng. 64, 364 (1986). 10.1002/cjce.5450640302 [DOI] [Google Scholar]
- Wartenberg M., Donmez F., Ling F. C., Acker H., Hescheler J., and Sauer H., FASEB J. 15, 995 (2001). 10.1096/fj.00-0350com [DOI] [PubMed] [Google Scholar]