Abstract
Background
S0124 was a large North American phase III trial that failed to confirm a survival benefit for cisplatin/irinotecan over cisplatin/etoposide in patients with extensive stage small cell lung cancer (E-SCLC). These results were contrary to J9511, a phase III trial exclusively in Japanese patients. Since S0124 and J9511 used identical treatment regimens and similar eligibility criteria, patient-level data were pooled from both trials and a “common arm” analysis was performed to explore potential reasons for the divergent results.
Methods
Patients with documented E-SCLC and adequate end-organ function were randomized to intravenously receive either Cisplatin 60 mg/m2 day 1 + Irinotecan 60 mg/m2 days 1, 8, & 15 every 4 weeks or Cisplatin 80 mg/m2 day 1 + Etoposide 100 mg/m2 days 1-3 every 3 weeks. Demographics and outcomes data were compared among 805 patients enrolled in J9511 and S0124 receiving identical treatment using a logistic model adjusted for age, sex, and performance status (PS).
Results
Of 671 patients in S0124, 651 eligible patients were included as were all 154 patients from J9511. Significant differences in sex and PS distribution as well as toxicity were seen between trials. There were also significant differences in response rates (87% vs. 60%, p<0.001) and median overall survival (12.8 vs. 9.8 months, p<0.001) when the cisplatin/irinotecan arms from both trials were compared.
Conclusions
Significant differences in patient demographics, toxicity, and efficacy were identified in the J9511 and S0124 populations. These results, relevant in the current era of clinical trials globalization, warrant: 1) consideration of differential patient characteristics and outcomes amongst populations receiving identical therapy; 2) utilization of the “common arm” model in prospective trials; and 3) inclusion of pharmacogenomic correlates in cancer trials where ethnic/racial differences in drug disposition are expected.
Background
Lung cancer represents the most common cause of malignant disease globally. Almost 1.4 million new cases of lung cancer are diagnosed annually worldwide, with nearly 1.2 million deaths.1 Small cell lung cancer (SCLC) is a unique subtype of lung cancer which accounts for approximately 15% of all new cases.2 Unfortunately, most SCLC patients succumb to the disease, due commonly to systemic metastasis (defined as “extensive stage”).3,4 Over the past twenty years, standard therapy for most patients with extensive stage SCLC has been either carboplatin or cisplatin in combination with etoposide.5
This paradigm was challenged in 2002 when the results of the Japanese phase III study JCOG 9511 (J9511) comparing etoposide-cisplatin (EP) with cisplatin-irinotecan (IP) in 174 patients demonstrated that tumor response, progression-free survival (PFS), and overall survival (OS) rates were significantly higher in the IP group.6 It must be noted that J9511 was stopped early at interim analysis by its Data Safety Monitoring Board when prospectively pre-specified efficacy parameters were met.
Subsequently, the Southwest Oncology Group (SWOG) conducted a large phase III trial (S0124) involving 671 patients which employed virtually the same eligibility criteria and treatment regimens as the Japanese trial to confirm the results of J9511 in North American patients.7 As reported previously, S0124 found no statistical differences in tumor response, PFS, and OS between the two arms, contrary to the results of J9511.
To explore potential reasons for the divergent results of these identically designed phase III trials, a pooled comparative outcomes analysis inclusive of patient-level data from both trials was conducted.
Patients and Methods
Patients in both trials had cytologically or histologically confirmed small-cell lung cancer and clinical evidence of extensive stage disease (defined by distant metastasis, contralateral hilar-node metastasis, or malignant pleural effusion). Eligibility criteria for both trials were similar and have been previously reported. Patients were randomly assigned to receive either a combination of EP or IP. The IP regimen consisted of four cycles of 60 mg of irinotecan per square meter of bodysurface area on days 1, 8, and 15 and 60 mg of cisplatin per square meter on day 1. Cycle length for this arm was four weeks. The EP regimen consisted of four cycles of 100 mg of etoposide per square meter on days 1, 2, and 3 and 80 mg of cisplatin per square meter on day 1. Cycle length for this arm was three weeks.
All patients underwent evaluations every cycle that included an assessment of symptoms, a physical examination, a complete blood count, and blood chemistry studies. Tumor response was assessed after every two cycles. In the S0124 trial, tumor response was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) while in the J9511 trial, the World Health Organization criteria were used.8 Overall survival was calculated as the time between trial registration and death or date of last contact. Progression free survival was calculated as the time between trial registration and death or progression, with censoring if alive without progression at last contact.
Study Design and Statistical Analysis
The primary objective of both studies was to compare the survival in patients with extensive small cell lung cancer treated with EP (standard arm) with that in comparable patients treated with the IP (experimental) on an intent-to-treat basis. As S0124 and J9511 protocols used identical treatment regimens and similar eligibility criteria, patient-level data from both trials were pooled to explore potential reasons for the divergent results. Final results of both trials have previously been reported. Of 671 patients in S0124, 651 were eligible and included in this analysis as were all 154 patients from J9511. Patient demographics, toxicity, and outcomes were compared among 805 patients receiving identical treatment using a “common arm” analysis. Overall and progression free survival as compared between the Japan and U.S. trials for both treatment arms in the combined sample were analyzed using Cox proportional hazards regression, adjusted for age, sex, and performance status. A logistic model adjusted for age, sex, and performance status was used to compare response to treatment between the two trials for the two treatment arms. The existence of possible interactions between trial (JCOG vs. SWOG) and treatment arm was evaluated for all endpoints, using data pooled over both arms. Significance was set at p < 0.05.
Results
Patient Demographics
Median age in J9511 and S0124 were 61 and 62 years, respectively. There were proportionally more males in J9511 (86%, n=132) compared to S0124 (57%, n=370). There were more patients with Zubrod performance status 0 in S0124 (211, 32%) compared to J9511 (19, 12%). Demographics are summarized in Table 1.
Table 1.
Patient Demographics
| JCOG-9511 | SWOG-0124 | |||||
|---|---|---|---|---|---|---|
| Cisplatin + Etopside |
Cisplatin + Irinotecan |
Total | Cisplatin + Etoposide |
Cisplatin + Irinotican |
Total | |
| Age, years | ||||||
| Median | 63 | 63 | 63 | 63 | 62 | 63 |
| Minimum | 41 | 30 | 30 | 35 | 22 | 22 |
| Maximum | 70 | 70 | 70 | 86 | 86 | 86 |
| Sex | ||||||
| % Male | 90% | 82% | 86% | 56% | 58% | 57% |
|
Performance
Status (PS) |
||||||
| % PS 0 | 12% | 13% | 12% | 33% | 32% | 32% |
| % PS 1 | 75% | 79% | 77% | 66% | 67% | 66% |
| % PS 2 | 13% | 8% | 10% | 0% | 0% | 0% |
Comparisons of the JCOG and SWOG populations with respect to differences in sex and performance status were significant by chi-square test (p<0.0001 for both comparisons).
Toxicity
Common arm comparisons of select attributable hematologic toxicities are summarized in Table 2. Regardless of treatment arm, patients in J9511 experienced significantly more hematologic toxicity consisting of neutropenia, leucopenia, and anemia than S0124. Other than a difference in infection rates in the cisplatin/etoposide arm, no differences in non-hematologic toxicities between the two trials were seen.
Table 2.
Common Arm Comparative Toxicity Analysis
| ≥ Grade 3 Toxicity |
Cisplatin + Etoposide | Cisplatin + Irinotecan | ||||
|---|---|---|---|---|---|---|
| J9511 | S0124 | p-value | J9511 | S0124 | p-value | |
| Infection | 3 (4%) | 52 (16%) | 0.01 | 4 (5%) | 36 (11%) | 0.23 |
| Neutropenia | 71 (92%) | 220 (68%) | <0.001 | 49 (65%) | 107 (34%) | < 0.001 |
| Leukopenia | 41 (53%) | 109 (34%) | 0.006 | 20 (27%) | 57 (18%) | 0.04 |
| Anemia | 25 (32%) | 39 (12%) | <0.001 | 21 (28%) | 18 (6%) | < 0.001 |
Treatment Delivery and Dose Intensity (DI)
In the original J9511 and S0124 papers, there were no significant differences reported between the two arms in terms of treatment delivery. A preliminary common arm comparison of treatment delivery and DI was performed in the current analysis. These results are summarized in Table 3. There were no clear differences in the proportion of patients completing all four cycles of therapy. However, a higher proportion of patients completed all four cycles of EP in J9511 versus S0124 (38% vs. 29%). A more modest difference was seen in the IP arm (29% vs. 23%). When comparing the published DI data (J9511 vs. S0124), there was a numerical difference in the proportion of irinotecan (80.4% vs. 66%) and cisplatin (95.3% vs. 78%) DI.
Table 3.
Common Arm Analysis of Treatment Delivery and Dose Intensity
| Cisplatin (P) + Etoposide (E) | Cisplatin (P) + Irinotecan (I) | |
|---|---|---|
| Completed all 4 cycles | ||
| JCOG 9511 | 55/77 (71.4%) | 53/77 (68.8%) |
| SWOG 0124 | 218/327 (66.6%) | 213/324 (65.8%) |
| Completed 4 cycles without dose modification | ||
| JCOG 9511 | 29/77 (38%) | 22/77 (29%) |
| SWOG 0124 | 94/327 (29%) | 76/324 (23%) |
| Reported average dose intensity (% of total planned dose) | ||
| JCOG 9511 | E: 83.9% P: 84.6% | I: 80.4% P: 95.3% |
| SWOG 0124 | E: 78% P: 81% | I: 66% P: 78% |
Efficacy
Common arm comparisons of efficacy endpoints including response rate, progression free survival, and overall survival are summarized in Table 4 and Figure 1. Ten patients (2 from JCOG and 8 from SWOG) were excluded from the analysis of treatment response because they did not receive treatment. Significant differences in response rates were seen in the common arm comparisons when evaluated in multivariate logistic regression models, which enabled adjustment for age, sex and performance status. Specifically, for the EP arm, response rates were 68% in J9511 and 57% in S0124 (p=0.02). For the IP arm, response rates were 87% for the J9511 and 60% in S0124 (p<0.001). In an expanded logistic regression model which pooled the data for both treatment arms, there was a significant arm by trial interaction, indicating that the difference in response between the Japanese and U.S. patients is significantly greater in the IP-arm patients. (P-value for interaction=.03)
Table 4.
Common Arm Analysis of Efficacy
| Efficacy Measure | Cisplatin + Etoposide | Cisplatin + Irinotecan | ||||
|---|---|---|---|---|---|---|
| J9511 | S0124 | p-value | J9511 | S0124 | p-value | |
| Response Rate | 68% | 57% | 0.01 | 87% | 60% | < 0.001 |
|
Progression-free Survival, median (months) |
4.7 | 5.2 | 0.18 | 6.8 | 5.8 | 0.6 |
|
Overall survival, median
(months) |
9.4 | 9.1 | 0.5 | 12.8 | 9.9 | < 0.001 |
| One-year survival | 38% | 34% | 58% | 41% | ||
Figure 1.
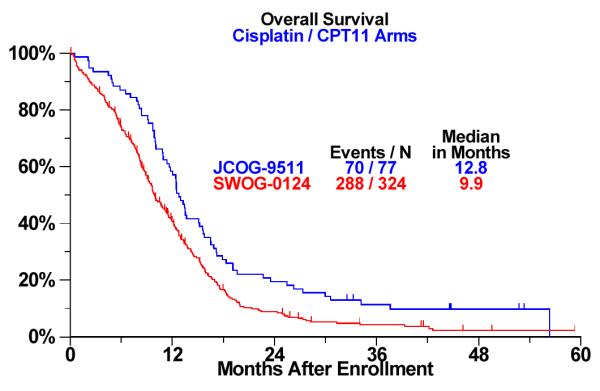
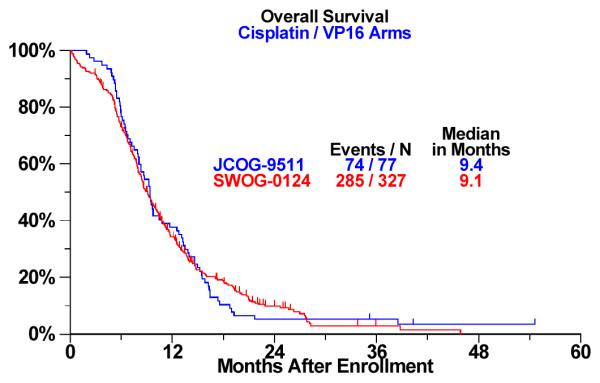
a: Overall Survival Analysis by Trial: Cisplatin / Irinotecan (CPT-11) Arm b: Overall Survival Analysis by Trial: CDDP/VP-16 Arm
There were no differences in PFS and OS for the EP arm across trials. However, significant differences were seen for OS for the IP arm. Specifically, median OS was 12.8 months for J9511 and 9.9 months for S0124 (p<0.001, adjusted for age, sex, and performance status via Cox proportional hazards regression). Similarly, one-year survival rates were 58% and 41%, respectively .The one-month numerical difference in PFS in the IP arm was not statistically significant. Kaplan Meier survival curves of OS common arm comparisons in the IP arm are shown in Figure 1. In a multivariate proportional hazards regression model including trial (Japan vs. U.S.) treatment arm, age, sex, and performance status, the interaction between trial and treatment arm is significant, confirming that the survival difference by site (Japan vs. U.S.) depends on treatment arm (P-value for interaction term = .01). A performance status of 0 (versus 1 or 2) was also independently prognostic for increased survival in multivariate modeling (P<.001). Age and sex were not.
Discussion
This “common arm” comparison of J9511 and S0124 utilizing pooled patient-level data provides unique insights into potential reasons for the divergent results of these trials. In addition, this analysis highlights the issue of whether in the current era of clinical trials globalization, the results of randomized oncology studies conducted outside the Unites States are directly translatable to North American populations.9 These issues obviously have regulatory implications.
This analysis is unparalleled because S0124 was designed a priori as a confirmatory trial for J9511, albeit accruing from a different ethnic patient pool. The design of the S0124 protocol was modeled directly on J9511, including similar eligibility criteria and identical treatment doseschedules. The observed differences in demographics, toxicity and efficacy outcomes between these trials can be attributed to many factors, some of which were previously discussed in the S0124 paper. With the pooled multivariate analysis we were able to investigate (and rule out) the possibility that the different outcomes between trials in the IP arms were attributable to clear differences in patient populations with respect to gender and performance status. Our analysis of both survival and response showed that although performance status was prognostic for survival, the differences between trials in the IP arm persisted even after adjusting for this imbalance. Other potential factors included the smaller sample size and/or the early stopping of J9511 which may have overestimated the treatment effect. 10
This common arm analysis demonstrates that the principal difference in OS was seen only in the IP arms. The control EP arms in both S0124 and J9511 had identical OS results. In the context of irinotecan-based therapy, one hypothesis that has been posited is that there are inherent genetic differences related to genes involved in irinotecan drug disposition between patient populations. Although a preliminary pharmacogenomic analysis of specimens from S0124 patients was performed to investigate some of these irinotecan-related genes, no specimens were available from the older J9511 trial for similar pharmacogenomic investigations. Hence, no direct comparison of relevant genotypes between trials is possible. However, insights on this issue can be derived from prior “common arm” joint collaborations between SWOG and Japanese investigators wherein patients with advanced non-small cell lung cancer were enrolled in SWOG and Japanese trials onto a common arm of paclitaxel and carboplatin.11 In that experience, genes relevant to chemotherapy metabolism and transport were analyzed in both American and Japanese populations. Significant differences in toxicity, efficacy, and allelic distribution for genes involved in paclitaxel disposition or DNA repair were observed between Japanese and US patients, supporting the hypothesis that pharmacogenomics may in part be responsible for outcome divergence amongst patient populations. This may also partly explain the toxicity differences seen between the Japanese and North American populations, wherein Japanese patients apparently had increased hematologic toxicity (neutropenia, leucopenia, and anemia) in both treatment arms when compared with the North Americans.
In addition, there appears to be some apparent differences in the delivered DI in the IP arms of both trials (as reported in the published papers). Specifically, more J9511 patients achieved a higher DI for both irinotecan and cisplatin as compared to S0124 patients. Enhanced DI for J9511 patients may potentially explain the differences in toxicity and efficacy between the trials. A more detailed and expansive analysis of dose delivery using individual patient data is required, but is beyond the scope of this manuscript. Finally, it must be noted that other trials comparing similar chemotherapy regimens in SCLC have previously been published.12 13 Some of us (PNL, RN, and DRG) have previously discussed these trials in the context of S0124 and J9511 in a recent editorial.14 We refer readers to that editorial for additional details.
In conclusion, EP remains the reference treatment standard in North America. In Japan, IP remains a standard treatment option. Significant differences in patient demographics, toxicity, and efficacy exist between Japanese and North American SCLC patients receiving identical treatment. These results, relevant in the current era of clinical trials globalization, warrant 1) consideration of differential patient characteristics and outcomes amongst patients receiving identical therapy, 2) utilization of the “common arm” model in prospective trials, and 3) inclusion of pharmacogenomic correlates in cancer trials where ethnic/racial differences in drug disposition are expected.
Acknowledgments
Grant Support: 1) SWOG - This investigation was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA32102, CA38926, CA46441, CA58882, CA35261, CA35431, CA35119, CA22433, CA58658, CA11083, CA46441, CA37981, CA45560, CA58861, CA04919, CA67663, CA12644, CA45807, CA67575, CA35281, CA20319, CA45808, CA35178, CA58416, CA14028, CA76448, CA35090, CA52654, CA58882, CA76447, CA76429, CA35128, CA46282, CA63848, CA46113, CA58723, CA63844, CA46368, CA35192, CA68183, CA45450, CA35176, CA76132, CA13612, CA16385, CA45377, CA63850, CA74647, CA58348, CA42777, CA35279; CA25224, CA27525, CA21115 and in part by Pfizer, Inc. and CA114771 (NIH SPECS in Lung Cancer)
2) JCOG - This work was supported in part by the Grants-in-Aid for Cancer Research (5S-1, 8S-1, 11S-2, 11S-4, 14S-2, 14S-4, 17S-2, 17S-5, 20S-2 and 20S-6) and for the Second-Term Comprehensive 10-Year Strategy for Cancer Control from the Ministry of Health, Labour, and Welfare.
Footnotes
Presented in part at the American Society of Clinical Oncology annual meeting (Chicago 2009) and at the World Conference on Lung Cancer in (San Francisco 2009)
ClinicalTrials.govIdentifier: NCT00045162
References
- 1.Parkin JM, Bray F, Ferlay J, Pisani P. Global cancer statistics. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Govindan R, Page N, Morgensztern N, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the Surveillance, Epidemiologic, and End Results database. J Clin Oncol. 2006;24(28):4539–4544. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 3.Clark R, Ihde DC. Small cell lung cancer: treatment progress and prospects. Oncology (Huntington) 1998;12(5):647–58. [PubMed] [Google Scholar]
- 4.Albain KS, Crowley JJ, LeBlanc M, Livingston RB. Determinants of improved outcome in small cell lung cancer: an analysis of the 2,580 patient Southwest Oncology Group database. J Clin Oncol. 1990;8(9):1563–74. doi: 10.1200/JCO.1990.8.9.1563. [DOI] [PubMed] [Google Scholar]
- 5.Davies AM, Lara PN, Lau DH, Gandara DR. Treatment of extensive small cell lung cancer. Hemat Oncol Clin N Am. 2004;18:373–385. doi: 10.1016/j.hoc.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus Cisplatin Compared with Etoposide plus Cisplatin for Extensive Small-Cell Lung Cancer. N Engl J Med. 2002;346(2):85–91. doi: 10.1056/NEJMoa003034. [DOI] [PubMed] [Google Scholar]
- 7.Lara PN, Jr, Natale R, Crowley J, Lenz HJ, Redman MW, Carleton JE, Jett J, Langer CJ, Kuebler JP, Dakhil SR, Chansky K, Gandara DR. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol. 2009;27(15):2530–5. doi: 10.1200/JCO.2008.20.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 9.Thiers F, Sinskey AJ, Berndt ER. From the analyst’s couch: trends in the globalization of clinical trials. Nature Reviews Drug Discovery. 2008;7:13–14. [Google Scholar]
- 10.Wilcox R, Djulbegovic B, Guyatt GH, Montori VM. Randomized Trials in Oncology Stopped Early for Benefit. J Clin Oncol. 2008;26(1):18–19. doi: 10.1200/JCO.2007.13.6259. [DOI] [PubMed] [Google Scholar]
- 11.Gandara DR, Kawaguchi T, Crowley J, Moon J, Furuse K, Kawahara M, Teramukai S, Ohe Y, Kubota K, Williamson SK, Gautschi O, Lenz H, McLeod HL, Lara PN, Coltman C, Fukuoka M, Saijo N, Fukushima M, Mack PC. Japanese-US Common Arm Analysis of Paclitaxel-Carboplatin in Advanced Non-Small Cell Lung Cancer: A Model for Assessing Population-Related Pharmacogenomics. J Clin Oncol. 2009;27(21):3540–6. doi: 10.1200/JCO.2008.20.8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hermes A, Bergman B, Bremnes R, et al. Irinotecan plus carboplatin versus oral etoposide plus carboplatin in extensive small-cell lung cancer: A randomized phase III trial. J Clin Oncol. 2008;26:4261–4267. doi: 10.1200/JCO.2007.15.7545. [DOI] [PubMed] [Google Scholar]
- 13.Hanna N, Bunn P, Jr, Langer C, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol. 2006;24:2038–2043. doi: 10.1200/JCO.2005.04.8595. [DOI] [PubMed] [Google Scholar]
- 14.Gandara DR, Lara PN, Natale R, Belani C. Progress in small-cell lung cancer: the lowest common denominator. J Clin Oncol. 2008;26(26):4236–8. doi: 10.1200/JCO.2008.17.2692. [DOI] [PubMed] [Google Scholar]


