Abstract
BACKGROUND
Endothelial gene silencing via small interfering RNA (siRNA) transfection represents a promising strategy for the control of vascular disease. Here, we demonstrate endothelial gene silencing in human saphenous vein using three rapid siRNA transfection techniques amenable for use in the operating room.
MATERIALS AND METHODS
Control siRNA, Cy5 siRNA, or siRNA targeting glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or endothelial specific nitric oxide synthase (eNOS) were applied to surplus human saphenous vein for 10 minutes by (i) soaking, (ii) applying 300 mmHg hyperbaric pressure, or (iii) 120 mmHg luminal distending pressure. Transfected vein segments were maintained in organ culture. siRNA delivery and gene silencing were assessed by tissue layer using confocal microscopy and immunohistochemistry.
RESULTS
Distending pressure transfection yielded the highest levels of endothelial siRNA delivery (22% pixels fluorescing) and gene silencing (60% GAPDH knockdown, 55% eNOS knockdown) as compared to hyperbaric (12% pixels fluorescing, 36% GAPDH knockdown, 30% eNOS knockdown) or non-pressurized transfections (10% pixels fluorescing, 30% GAPDH knockdown, 25% eNOS knockdown). Cumulative endothelial siRNA delivery (16% pixels fluorescing) and gene silencing (46% GAPDH knockdown) exceeded levels achieved in the media/adventitia (8% pixels fluorescing, 24% GAPDH knockdown) across all transfection methods.
CONCLUSION
Endothelial gene silencing is possible within the timeframe and conditions of surgical application without the use of transfection reagents. The high sensitivity of endothelial cells to siRNA transfection marks the endothelium as a promising target of gene therapy in vascular disease.
INTRODUCTION
Gene silencing by small interfering RNA (siRNA) transfection holds promise for the control of vascular disease.1, 2 In one extension of vascular RNA interference technology, vein grafts could be treated at the time of surgery with siRNA cocktails designed to interrupt the signaling pathways leading to intimal hyperplasia, stenosis, and graft failure.3 Our group recently demonstrated the first transfection of human saphenous vein with siRNA targeting the myristoylated alanine-rich C kinase substrate (MARCKS), a gene product shown to reduce intimal hyperplasia by inhibiting smooth muscle cell migration and proliferation when silenced.2 However, these data, as well as an earlier in vitro study, revealed a heightened susceptibility of endothelial cells to siRNA transfection and gene silencing compared to other vascular cells.1, 2 Given the inciting role of endothelial cells in the pathogenesis of vascular disease, the endothelium is an ideal target of vascular gene therapy.4–6 In this study, we further characterize ex vivo siRNA delivery and gene silencing in human saphenous vein endothelium, comparing both pressurized and non-pressurized delivery methods amenable for use in the operating room.
We employ three noninvasive techniques for the local administration of siRNA to human saphenous vein suitable for surgical application. These techniques involve soaking vein in siRNA solution without the use of pressure or transfection reagents, soaking vein in siRNA solution in the presence of 300 mmHg hyperbaric pressure using a custom fabricated pressure chamber, or distending vein segments with siRNA solution at 120 mmHg luminal distending pressure. Hyperbaric transfection is designed to be analogous to the nondistending external pressure technique described by Mann and colleagues for DNA oligonucleotide delivery and uses the optimal pressure level previously identified.7 Distending pressure transfection was used by our group for MARCKS silencing in human saphenous vein2 and utilizes a pressure level intended not to exceed the pressures experienced by vein grafts after implantation into the arterial circulation or pressures where graft injury has been shown to occur.8–10 The genes chosen for silencing are glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and endothelial specific nitric oxide synthase (eNOS). GAPDH is an abundant housekeeping gene expressed ubiquitously by all cells in the vessel wall. eNOS is an endothelial-specific gene product found only in the endothelium.11
MATERIALS AND METHODS
siRNA design
Control siRNA (sense 5′ - CGC ACC AGA ACA AAC ACA C - 3′), Cy5 siRNA (sense 5′ - Cy5 - CGC ACC AGA ACA AAC ACA C - 3′), and eNOS siRNA (sense 5′ - CGA GGA GAC UUC CGA AUC UUU - 3′) were purchased from Dharmacon (Lafayette, CO, USA) as described previously.1 GAPDH siRNA (Silencer GAPDH siRNA - Human) was purchased from Ambion (proprietary sequence; Austin TX, USA). Control and Cy5 siRNA sequences do not harbor any homology with the human genome.
siRNA transfection of human saphenous vein
Freshly harvested surplus human saphenous vein was procured from the operating room with institutional review board approval from patients undergoing cardiac or vascular procedures. Vein tissue was transported to the laboratory in Plasmalyte A solution (140 mEq/L sodium, 5 mEq/L potassium, 3 mEq/L magnesium, 98 mEq/L chloride, 27 mEq/L acetate, 23 mEq/L gluconate, 294 mOsmol/L, pH 7.4) for immediate transfection. Specimens were divided into 3–4 cm segments and transfected with siRNA resuspended in Plasmalyte A by soaking segments for 10 minutes in siRNA solution in the absence of pressure, soaking vein segments in siRNA solution for 10 minutes in the presence of 300 mmHg (1 mmHg = 133 Pa) hyperbaric pressure, or distending vein segments with siRNA solution for 10 minutes at 120 mmHg luminal distending pressure. For the soak treatment, vein segments were submerged in 500 μl siRNA solution in the wells of 24-well plates. For hyperbaric pressure transfection, vein segments were submerged in 500 μl siRNA solution in the wells of 24-well plates, with the plates then placed in a custom fabricated pressure chamber (Fig. 1) and the pressure in the chamber raised to 300 mmHg using wall air. For distending pressure transfection, 3–4 cm vein segments were cannulated distally using a vein graft cannula secured with 3-0 silk ties, and flushed with siRNA solution. The veins were then clamped proximally using a spring-loaded crossover clamp (bulldog clamp) and siRNA solution was infused via the cannula to a pressure of 120 mmHg using a standard angioplasty insufflator.7 After all transfections, vein segments were rinsed, divided into 5–10 mm segments, and placed in organ culture consisting of RPMI 1640 media supplemented with 30% FBS, 2 mM L-glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B with media changes performed every 2 days, as previously described.12, 13 Preliminary dose-response experiments achieved eNOS and GAPDH mRNA and protein knockdown in whole vein homogenate by qRT-PCR and western blotting using 25 μM siRNA and a 10 minute soak treatment. Immunohistochemistry experiments were then performed using 25 μM control, GAPDH, and eNOS siRNA to evaluate gene knockdown by tissue layer and transfection method.
Figure 1.

Custom pressure chamber designed to expose standard tissue culture plates to hyperbaric pressure.
Confocal microscopy
Confocal microscopy to assess Cy5 siRNA delivery was performed as previously described.2 Briefly, cultured vein segments transfected with 25 μM Cy5 siRNA were snap-frozen after 24 hours in organ culture, sectioned, mounted, and imaged under confocal microscopy. Micrographs with excitation wavelengths for red, green, and blue fluorescence emission were acquired to show Cy5 fluorescence (blue), as well as the vessel architecture (green and red auto-fluorescence of the elastic fibers). Cy5 fluorescence in confocal micrographs was analyzed using Adobe Photoshop CS2 (Adobe Systems, San Jose, CA, USA) to quantify the percentage of pixels fluorescing in a given area, as previously described.14
Immunohistochemistry
Vein segments transfected with control or GAPDH siRNA were cultured for 5 days, formalin fixed, and embedded in paraffin. Serial sections were stained with anti-GAPDH (AM4300; Ambion), anti-CD31 (ab9498; Abcam, Cambridge, MA, USA) or anti-actin (A4700; Sigma, St. Louis, MO, USA) antibodies according to manufacturers’ protocols. Vein segments transfected with control or eNOS siRNA were cultured for 0, 1, 2, or 3 days. Serial sections were fixed with acetone and stained with anti-eNOS (610291, BD Transduction Laboratories) or anti-CD31 antibodies.
For assessment of GAPDH knockdown, quantitative morphometric analysis was performed to cover the entire cross-sectional area of the vein segments. Images were analyzed using Adobe Photoshop CS4 (Adobe Systems) to quantify pixels with DAB chromagen luminosity.14–16 The total area in pixels for the media and adventitia was determined using the histogram function. Stained pixels were selected according to hue, color intensity, and saturation, discriminating unstained portions of the specimen using the Magic Wand tool with the Select Similar function.17 Results were calculated as total pixels stained divided by media and adventitial pixel area and standardized to global actin staining in the media and adventitia. Next, the endothelial area was manually selected using the Lasso tool, inverted, and the non-endothelial area deleted. Stained endothelial pixels were divided by total endothelial pixels and standardized to CD31 staining. Assessment of eNOS knockdown in the endothelial layer was performed in the same manner and standardized to CD31 staining.
Statistical methods
All experiments are representative of a cohort of three patient samples per group unless otherwise noted. Data are presented as mean ± standard deviation. Statistical analysis was performed using STATA software (STATA Corporation, College Station, TX, USA). Significance of association was assessed using one-way ANOVA with Bonferroni correction (Fig. 2), unmatched two-way ANOVA with Bonferroni correction (Fig. 3), or repeated measures two-way ANOVA with Bonferroni correction (Fig. 4).
Figure 2.
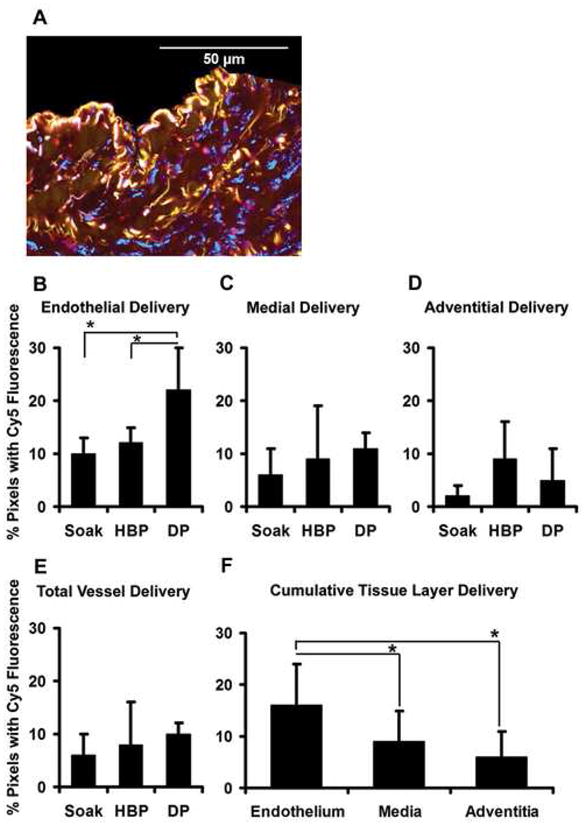
Endothelial siRNA delivery exceeds medial and adventitial delivery and is increased using distending pressure transfection. (A) Vein segments transfected with Cy5 siRNA demonstrate discrete areas of blue fluorescence independent of the red/green autofluorescence of the elastic fibers (representative micrograph, ×600). Quantitation of blue pixels by tissue layer demonstrates (B) maximal Cy5 fluorescence in the endothelium after distending pressure (DP) transfection as compared to the soak and hyperbaric pressure (HBP) transfections (*P < 0.05 for both comparisons). (C–E) Cy5 fluorescence in the medial layer, adventitial layer, and total vessel wall was statistically equivalent between the three transfection conditions. (F) Cumulative analysis of images from all transfection conditions demonstrates greater Cy5 fluorescence in the endothelial layer as compared to the media and the adventitia (*P < 0.05 for both comparisons). For all data, n = 5 to 6 vein segments per condition.
Figure 3.
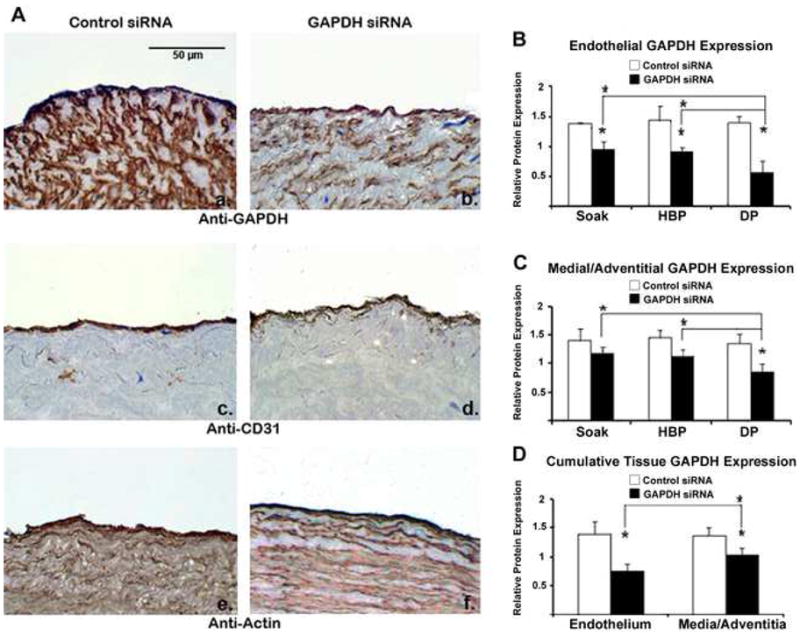
Endothelial GAPDH protein knockdown exceeds medial and adventitial GAPDH knockdown and is increased using distending pressure transfection. (A) Immunohistochemistry demonstrates GAPDH knockdown within all tissue layers after distending pressure (DP) transfection with GAPDH siRNA (panel b) as compared to vein segments treated with control siRNA (panel a). Protein knockdown is specific to GAPDH as CD31 (panels c,d) and actin (panels e,f) levels are preserved. Quantitation demonstrates (B) greater GAPDH knockdown in the endothelium using distending pressure as compared to hyperbaric (HBP) and non-pressurized (soak) transfection. (C) Medial/adventitial GAPDH levels were significantly reduced after distending pressure transfection, but not with hyperbaric or non-pressurized transfection. (D) Cumulative analysis of all transfections revealed greater knockdown in the endothelium as compared to the media/adventitia. n = 3 vein segments per condition. Micrographs (×400) correspond to one representative image of three experiments performed. (* denotes P < 0.05 for comparisons).
Figure 4.
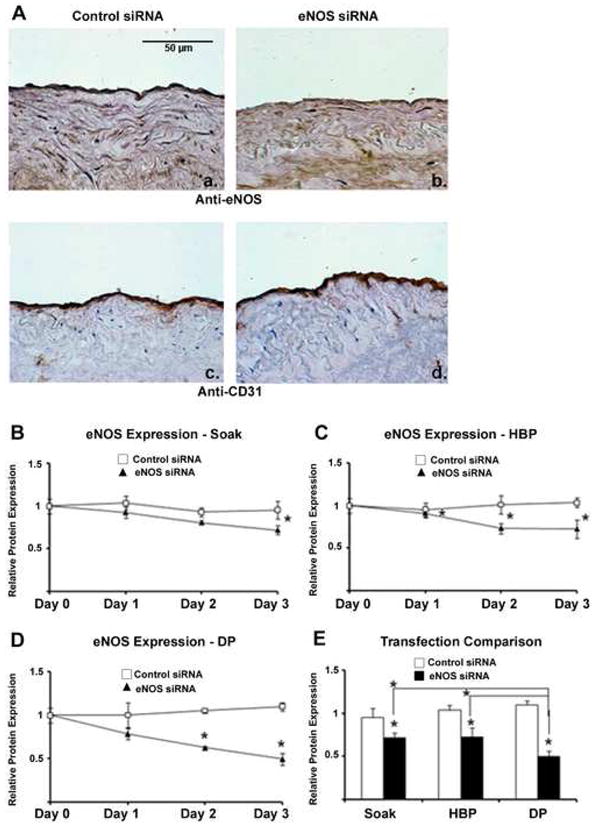
eNOS knockdown is achieved using all three transfection methods and is increased using distending pressure transfection. (A) Immunohistochemistry demonstrates greatest endothelial eNOS knockdown after distending pressure (DP) transfection with eNOS siRNA (panel b) as compared to vein segments treated with control siRNA (panel a). Protein knockdown is specific to eNOS as CD31 levels (panels c,d) are preserved. (B–E) Quantitation demonstrates greater eNOS knockdown using distending pressure as compared to hyperbaric (HBP) and non-pressurized (soak) transfection. n = 3 vein segments per condition. Micrographs (×400) correspond to one representative image of three experiments performed. (* denotes P < 0.05 for comparisons).
RESULTS
Endothelial siRNA delivery exceeds medial and adventitial delivery and is increased using distending pressure transfection
Confocal micrographs of vein segments maintained in organ culture for 24 hours following 10 minute siRNA transfections with 25 μM Cy5 siRNA demonstrated discrete areas of blue Cy5 fluorescence within all tissue layers (Fig. 2A).
Quantitation of blue fluorescence in micrographs using image analysis software demonstrated significantly greater uptake of Cy5 siRNA in the endothelial layer using distending pressure transfection as compared to the soak or hyperbaric transfection treatments [Fig. 2B, P < 0.05 for the difference between distending pressure transfection (22 ± 11% pixels fluorescing) vs. soak (10 ± 6% pixels fluorescing) or hyperbaric (12 ± 9% pixels fluorescing) treatments]. Quantitative analysis of medial, adventitial and total vessel Cy5 fluorescence statistically failed to demonstrate additional differences in siRNA delivery between the three transfection methods. However, trends for greater medial layer and total vessel delivery after distending pressure transfection (Fig. 2C, 2E), as well as greater adventitial delivery after hyperbaric pressure transfection (Fig. 2D) were observed. Cumulative data from all transfection images revealed significantly greater uptake of siRNA by the endothelial layer as compared to the media and adventitia (Fig. 2F, P < 0.05 for the difference between endothelial delivery [16 ± 8% pixels fluorescing] vs. medial [9 ± 6% pixels fluorescing] or adventitial delivery [6 ± 5% pixels fluorescing]).
Endothelial GAPDH knockdown exceeds medial and adventitial GAPDH knockdown and is increased using distending pressure transfection
To assess protein knockdown levels throughout the vessel wall after rapid siRNA transfection, vein segments were transfected with siRNA targeting the ubiquitous metabolic enzyme GAPDH using all three transfection methods. GAPDH protein expression was quantified by immunohistochemistry after 5 days in organ culture. Distending pressure transfection produced the greatest degree of GAPDH knockdown in all tissue layers (Fig 3A, panels a–b). Gene silencing was specific to GAPDH and not indicative of global protein degradation as CD31 and actin levels were maintained (Fig 3A, panels c–f). In the endothelium, distending pressure achieved a 60 ± 13% reduction in GAPDH protein levels followed by hyperbaric (36 ± 4%), and non-pressurized transfection (30 ± 9%; Fig. 3B; P < 0.05 for all). In the media/adventitia, distending pressure achieved a 36 ± 9% reduction in target protein levels (Fig. 3C; P < 0.05), whereas hyperbaric and non-pressurized transfection did not significantly reduce GAPDH protein. Cumulative analysis of images from all transfection methods demonstrated heightened GAPDH knockdown in the endothelial layer compared to the media/adventitia (Fig. 3D; P < 0.05 for endothelial pixels stained [46 ± 13%] vs. medial/adventitial pixels stained [24 ± 9%]).
eNOS knockdown is achieved using all three transfection methods and is increased using distending pressure transfection
To further confirm endothelial gene silencing in human saphenous vein using rapid siRNA transfection, vein segments were transfected with siRNA targeting eNOS, a gene product found only within the endothelium. eNOS protein expression was quantified by immunohistochemistry after 0 to 3 days in organ culture. eNOS knockdown was achieved using all three transfection methods and was greatest using distending pressure transfection (Figure 4A, panels a–b). Gene silencing was specific to eNOS and not indicative of global protein degradation as CD31 levels were maintained (Fig 4A, panels c–d). At day 3, eNOS knockdown levels reached 25 ± 6% for non-pressurized, 30 ± 10% for hyperbaric, and 55 ± 6% for distending pressure transfection (Fig 4. B–D, P < 0.05 for all). Distending pressure transfection produced significantly greater eNOS knockdown than the other two methods (Fig. 4E, P < 0.05).
DISCUSSION
Previous work from our group reported an increased susceptibility of human endothelial cells to siRNA transfection and gene silencing in vitro using lipid transfection reagents,1 as well as greater siRNA delivery to the endothelium in human saphenous vein segments transfected ex vivo using distending pressure.2 This study now confirms the feasibility of intraoperative vascular gene therapy directed towards the endothelium by demonstrating robust endothelial gene silencing of globally expressed and endothelial-specific gene products in intact human saphenous vein transfected using rapid siRNA transfection techniques.
This study compares three methods for rapid siRNA delivery that could be used in the operating room. The simplest transfection method would entail incubating vein grafts in siRNA solution for short time periods. Local administration of naked siRNA to solid tissues without the use of transfection reagents or pressure has been shown in other systems, including the eye, lung, CNS, and tumors, with comparable levels of gene silencing between 40–75% achieved in experimental animals.18, 19 Conversely, the initial report by Mann and colleagues demonstrating oligodeoxynucleotide (ODN) transfection of human vein indicated minimal ODN delivery and no ODN activity in vein transfected without the use of pressure.7 This apparent discrepancy between siRNA and ODN delivery could be explained by a heightened activity of siRNA versus ODN20 or by the existence of cell surface receptors that assist in siRNA uptake from the environment.21 Methodological differences could also complicate the comparison between siRNA and ODN delivery given that ODN localizes to the nucleus and is detected with a nuclear counterstain whereas siRNA localizes and functions in the cytoplasm. However, other investigators have achieved high levels of ODN delivery to both porcine and human vein using non-pressurized transfection, suggesting the uptake of DNA and RNA oligonucleotides by vein may proceed in a similar fashion.22, 23
Hydrodynamic pressure transfection with siRNA has been performed in liver, muscle, kidney, lung, and pancreatic tissues producing gene silencing levels as high as 90% in the liver.19 Hyperbaric and distending pressure transfection are more complex than non-pressurized transfection, however, both have been used successfully for the preparation of vein bypass grafts in the operating room.24–26 Advantages to these methods suggested by our data include greater siRNA delivery and protein knockdown, consistent with results reported from other tissue types using pressure. The tissue layer delivery patterns uncovered also appear to logically follow from the mechanism of pressure delivery. Distending pressure transfection places the endothelial cell at the forefront of the pressure gradient and led to the highest delivery in the endothelium, followed by the media and the adventitia. Hyperbaric transfection exposes both sides of the vessel wall to elevated pressure equally and appears to preferentially transfect both the endothelial and adventitial surfaces in direct contact with the siRNA solution. Nonetheless, these data suggest differing transfection patterns produced by the various techniques, and the final choice of transfection method could be selected and optimized to match the pattern and degree of silencing desired for different target genes throughout the vessel wall.
This study does not directly explore cell viability or vessel damage induced by the various transfection methods, although global cellular protein expression as evidenced by housekeeper gene levels remained stable after transfection. Previous studies from our laboratory demonstrated preservation of endothelial morphology by electron microscopy after distending veins to pressures as high as 500 mmHg during graft preparation when using a warm nutritive medium and papaverine.10 However, other authors demonstrated morphologic vessel damage after distending grafts to 500 and 600 mmHg during preparation.8, 9 The hyperbaric transfection technique was used in the PREVENT III and PREVENT IV clinical trails of edifoligide for vein graft protection.24, 25 Despite demonstration of safety in phase II testing,27 the rate of graft failure in veins pressure-treated with placebo in the PREVENT IV trial was found to be higher than in other studies, and the transfection technique was questioned to have heightened the rate of vein graft failure.28 Given these conflicting reports, the possibility of vessel damage from pressure transfection remains, and rigorous testing of transfection techniques is warranted before future clinical trials.
Gene silencing levels in this study ranged from 25–60%, depending on the transfection method and gene targeted. Quantitative immunohistochemistry suggested greater protein knockdown at delayed time points, consistent with prior data.1 Although further optimization of transfection methods could strive to fully silence any targeted gene, the gene silencing levels achieved thus far are comparable with other reports of gene silencing in solid tissues.18 Furthermore, phenotypic relief from disease has been shown in experimental models following targeted gene silencing as low as 40%, suggesting RNAi-based therapeutics could be successful at alleviating disease with less than 100% suppression of involved genes.2, 29
In this study, tissue layer gene knockdown levels are measured by immunohistochemistry without confirmatory assessment of mRNA knockdown. However, prior studies from our group rigorously demonstrated the relationship between mRNA silencing and protein knockdown in vitro in both human endothelial and smooth muscle cells.1 Confirmation of mRNA knockdown by tissue layer would require quantitative in situ hybridization or laser capture microdissection of cell populations from each distinct tissue layer followed by qRT-PCR. These experiments are challenging to perform using operative tissue samples in limited supply. This study is similarly limited by small sample sizes for each of the experimental conditions, owing to the challenges of obtaining appreciable quantities of human vein from surgical patients in a timely manner. Despite these disadvantages, use of human vein tissue in these early studies will ease the transition from bench to bedside as the technology will have proven successful in vein tissue from the full range of patients undergoing bypass operations. Nonetheless, the ex vivo transfection of vein, while an important prerequisite step before further study, fails to provide insight into the time course, degree of gene silencing, and phenotypic effects on vein graft remodeling that would be achieved in veins grafted into a physiologic flow environment. Thus, further experimentation with siRNA transfection of vein grafts in animal models is justified by this study.
To conclude, here we demonstrate the feasibility of siRNA delivery and gene silencing in human vein endothelium within the timeframe and conditions of surgical application. The high sensitivity of endothelial cells to gene silencing marks the endothelium as a promising target of intraoperative vascular gene therapy. Distending pressure transfection produced the greatest degree of siRNA delivery and protein knockdown, although various transfection methods are available and could be optimized on a case-by-case basis to intelligently manipulate target gene expression within the different layers of the vessel wall.
Acknowledgments
We would like to thank Ian R. Driscoll, MD, and Faramarz Edalat, MD for help with transfection experiments. This work was supported by NIH R01 grants HL021796, HL086741 (to CF, and FWL), and HL080130 (to CF), NIH T32 Harvard-Longwood Research Training in Vascular Surgery grant HL007734 (to TSM, and JYM), a Howard Hughes Medical Institute Research Training Fellowship for Medical Students (to NDA), The von Liebig Foundation (to IRD, FE, MJ, LP, and FWL), and the Royal College of Surgeons in Ireland Annual Appeal Alumni Research Fund (to AC).
Footnotes
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andersen ND, Monahan TS, Malek JY, Jain M, Daniel S, Caron LD, Pradhan L, Ferran C, Logerfo FW. Comparison of gene silencing in human vascular cells using small interfering RNAs. J Am Coll Surg. 2007;204(3):399–408. doi: 10.1016/j.jamcollsurg.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 2.Monahan TS, Andersen ND, Martin MC, Malek JY, Shrikhande GV, Pradhan L, Ferran C, LoGerfo FW. MARCKS silencing differentially affects human vascular smooth muscle and endothelial cell phenotypes to inhibit neointimal hyperplasia in saphenous vein. FASEB J. 2009;23(2):557–564. doi: 10.1096/fj.08-114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conte MS, Mann MJ, Simosa HF, Rhynhart KK, Mulligan RC. Genetic interventions for vein bypass graft disease: a review. J Vasc Surg. 2002;36(5):1040–1052. doi: 10.1067/mva.2002.129112. [DOI] [PubMed] [Google Scholar]
- 4.Esper RJ, Nordaby RA, Vilarino JO, Paragano A, Cacharron JL, Machado RA. Endothelial dysfunction: a comprehensive appraisal. Cardiovasc Diabetol. 2006;5:4. doi: 10.1186/1475-2840-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubanyi GM. The role of endothelium in cardiovascular homeostasis and diseases. J Cardiovasc Pharmacol. 1993;22 (Suppl 4):S1–14. doi: 10.1097/00005344-199322004-00002. [DOI] [PubMed] [Google Scholar]
- 6.Friedewald VE, Giles TD, Pool JL, Yancy CW, Roberts WC. The Editor’s Roundtable: Endothelial dysfunction in cardiovascular disease. Am J Cardiol. 2008;102(4):418–423. doi: 10.1016/j.amjcard.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Mann MJ, Gibbons GH, Hutchinson H, Poston RS, Hoyt EG, Robbins RC, Dzau VJ. Pressure-mediated oligonucleotide transfection of rat and human cardiovascular tissues. Proc Natl Acad Sci U S A. 1999;96(11):6411–6416. doi: 10.1073/pnas.96.11.6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramos JR, Berger K, Mansfield PB, Sauvage LR. Histologic fate and endothelial changes of distended and nondistended vein grafts. Ann Surg. 1976;183(3):205–228. doi: 10.1097/00000658-197603000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boerboom LE, Olinger GN, Bonchek LI, Gunay II, Kissebah AH, Rodriguez ER, Ferrans VJ. The relative influence of arterial pressure versus intraoperative distention on lipid accumulation in primate vein bypass grafts. J Thorac Cardiovasc Surg. 1985;90(5):756–764. [PubMed] [Google Scholar]
- 10.LoGerfo FW, Quist WC, Crawshaw HM, Haudenschild C. An improved technique for preservation of endothelial morphology in vein grafts. Surgery. 1981;90(6):1015–1024. [PubMed] [Google Scholar]
- 11.Chatterjee A, Black SM, Catravas JD. Endothelial nitric oxide (NO) and its pathophysiologic regulation. Vascul Pharmacol. 2008;49(4–6):134–140. doi: 10.1016/j.vph.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soyombo AA, Angelini GD, Bryan AJ, Jasani B, Newby AC. Intimal proliferation in an organ culture of human saphenous vein. Am J Pathol. 1990;137(6):1401–1410. [PMC free article] [PubMed] [Google Scholar]
- 13.Soyombo AA, Angelini GD, Bryan AJ, Newby AC. Surgical preparation induces injury and promotes smooth muscle cell proliferation in a culture of human saphenous vein. Cardiovasc Res. 1993;27(11):1961–1967. doi: 10.1093/cvr/27.11.1961. [DOI] [PubMed] [Google Scholar]
- 14.Kirkeby S, Thomsen CE. Quantitative immunohistochemistry of fluorescence labelled probes using low-cost software. J Immunol Methods. 2005;301(1–2):102–113. doi: 10.1016/j.jim.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Matkowskyj KA, Schonfeld D, Benya RV. Quantitative immunohistochemistry by measuring cumulative signal strength using commercially available software photoshop and matlab. J Histochem Cytochem. 2000;48(2):303–312. doi: 10.1177/002215540004800216. [DOI] [PubMed] [Google Scholar]
- 16.Hollenbeck ST, Sakakibara K, Faries PL, Workhu B, Liu B, Kent KC. Stem cell factor and c-kit are expressed by and may affect vascular SMCs through an autocrine pathway. J Surg Res. 2004;120(2):288–294. doi: 10.1016/j.jss.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Russ JC. The image processing handbook. 2. Boca Raton: CRC Press; 1995. [Google Scholar]
- 18.de Fougerolles A, Manoharan M, Meyers R, Vornlocher HP. RNA interference in vivo: toward synthetic small inhibitory RNA-based therapeutics. Methods Enzymol. 2005;392:278–296. doi: 10.1016/S0076-6879(04)92016-2. [DOI] [PubMed] [Google Scholar]
- 19.Aigner A. Delivery Systems for the Direct Application of siRNAs to Induce RNA Interference (RNAi) In Vivo. J Biomed Biotechnol. 2006;2006(4):71659. doi: 10.1155/JBB/2006/71659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyagishi M, Hayashi M, Taira K. Comparison of the suppressive effects of antisense oligonucleotides and siRNAs directed against the same targets in mammalian cells. Antisense Nucleic Acid Drug Dev. 2003;13(1):1–7. doi: 10.1089/108729003764097296. [DOI] [PubMed] [Google Scholar]
- 21.Sundaram P, Echalier B, Han W, Hull D, Timmons L. ABC Transporters Are Required for Efficient RNAi in Caenorhabditis elegans. Mol Biol Cell. 2006 doi: 10.1091/mbc.E06-03-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mannion JD, Ormont ML, Shi Y, O’Brien JE, Jr, Chung W, Roque F, Zalewski A. Saphenous vein graft protection: effects of c-myc antisense. J Thorac Cardiovasc Surg. 1998;115(1):152–161. doi: 10.1016/s0022-5223(98)70453-2. [DOI] [PubMed] [Google Scholar]
- 23.Kodama T, Tan PH, Offiah I, Partridge T, Cook T, George AJ, Blomley MJ. Delivery of oligodeoxynucleotides into human saphenous veins and the adjunct effect of ultrasound and microbubbles. Ultrasound Med Biol. 2005;31(12):1683–1691. doi: 10.1016/j.ultrasmedbio.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, Lorenz TJ, Namini H, Hamdan AD, Roddy SP, Belkin M, Berceli SA, DeMasi RJ, Samson RH, Berman SS. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;43(4):742–751. doi: 10.1016/j.jvs.2005.12.058. discussion 751. [DOI] [PubMed] [Google Scholar]
- 25.Alexander JH, Hafley G, Harrington RA, Peterson ED, Ferguson TB, Jr, Lorenz TJ, Goyal A, Gibson M, Mack MJ, Gennevois D, Califf RM, Kouchoukos NT. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005;294(19):2446–2454. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 26.LoGerfo FW, Haudenschild CC, Quist WC. A clinical technique for prevention of spasm and preservation of endothelium in saphenous vein grafts. Arch Surg. 1984;119(10):1212–1214. doi: 10.1001/archsurg.1984.01390220086020. [DOI] [PubMed] [Google Scholar]
- 27.Mann MJ, Whittemore AD, Donaldson MC, Belkin M, Conte MS, Polak JF, Orav EJ, Ehsan A, Dell’Acqua G, Dzau VJ. Ex-vivo gene therapy of human vascular bypass grafts with E2F decoy: the PREVENT single-centre, randomised, controlled trial. Lancet. 1999;354(9189):1493–1498. doi: 10.1016/S0140-6736(99)09405-2. [DOI] [PubMed] [Google Scholar]
- 28.Desai ND, Fremes SE. Efficacy and safety of edifoligide. JAMA. 2006;295(13):1514. doi: 10.1001/jama.295.13.1514-a. author reply 1514–1515. [DOI] [PubMed] [Google Scholar]
- 29.Dorn G, Patel S, Wotherspoon G, Hemmings-Mieszczak M, Barclay J, Natt FJ, Martin P, Bevan S, Fox A, Ganju P, Wishart W, Hall J. siRNA relieves chronic neuropathic pain. Nucleic Acids Res. 2004;32(5):e49. doi: 10.1093/nar/gnh044. [DOI] [PMC free article] [PubMed] [Google Scholar]


