Abstract
Objective
IGF-1 stimulates cartilage repair but is not a practical therapy due to its short half-life. We have previously modified IGF-1 by adding a heparin-binding domain and have shown that this fusion protein (HB-IGF-1) stimulates sustained proteoglycan synthesis in cartilage. Here, we first examined the mechanism by which HB-IGF-1 is retained in cartilage. We then tested whether HB-IGF-1 provides sustained growth factor delivery to cartilage in vivo and to human cartilage explants.
Methods
Retention of HB-IGF-1 and IGF-1 was analyzed by Western blotting. The requirement of heparan sulfate (HS) or chondroitin sulfate (CS) glycosaminoglycans for binding was tested using enzymatic removal and cells with genetic deficiency of HS. Binding affinities of HB-IGF-1 and IGF-1 proteins for isolated glycosaminoglycans were examined by surface plasmon resonance and ELISA.
Results
In cartilage explants, chondroitinase treatment decreased binding of HB-IGF-1, whereas heparitinase had no effect. Furthermore, HS was not necessary for HB-IGF-1 retention on cell monolayers. Binding assays showed that HB-IGF-1 bound both CS and HS, whereas IGF-1 did not bind either. After intra-articular injection in rat knees, HB-IGF-1 was retained in articular and meniscal cartilages, but not in tendon, consistent with enhanced delivery to CS-rich cartilage. Finally, HB-IGF-1 but not IGF-1 was retained in human cartilage explants.
Conclusions
After intra-articular injection in rats, HB-IGF-1 is specifically retained in cartilage through its high abundance of CS. Modification of growth factors with heparin-binding domains may be a new strategy for sustained and specific local delivery to cartilage.
Insulin-like growth factor-I (IGF-1) is known to be an important anabolic factor in cartilage homeostasis (1). IGF-1 not only promotes synthesis of aggrecan, link protein, and hyaluronan (2–4), it also inhibits proteoglycan degradation (5–7). IGF-1 is primarily produced by the liver and reaches cartilage through the synovial fluid (8–10), acting on chondrocytes through both autocrine and paracrine mechanisms (11,12). In multiple animal models of cartilage injury, chondrocytes transfected to overexpress IGF-1 have been successfully used to enhance cartilage repair (13,14).
While IGF-1 may therefore be a potential therapeutic for cartilage repair, a clinically useful technique for acellular IGF-1 delivery to cartilage has yet to be developed. A successful IGF-1 delivery strategy must overcome two major obstacles. First, IGF-1 has a short half-life of 8–16 hours in vivo when delivered systemically (15). Second, systemic delivery of IGF-1 must be minimized since long-term excess circulating IGF-1 has been linked to increased risk for cancer (16) and high-dose systemic IGF-1 administration causes significant adverse events (17). Studies delivering IGF-1 directly to the joint through fibrin constructs (18–20) have been promising, but rapid clearance of IGF-1 from the joint has prevented intra-articular injections of IGF-1 without a carrier from being effective (9), and has been a limiting factor in delivery methods proposed to date.
We have focused on the family of heparin-binding growth factors as a model for sequestration and sustained local delivery of growth factors to cartilage. Basic fibroblast growth factor (bFGF or FGF-2), vascular endothelial growth factor (VEGF), heparin-binding epidermal growth factor-like growth factor (HB-EGF), pleiotrophin, midkine, and platelet-derived growth factor (PDGF) are all members of the heparin-binding growth factor family and have been extensively studied for their ability to be retained in the extracellular matrix (ECM) of various tissues through their highly positively charged heparin-binding domains (21,22). Heparin-binding domains may be particularly relevant for localizing growth factors in cartilage. In particular, FGF-2 has been shown to bind to isolated highly negatively charged small leucine rich proteoglycan fibromodulin (23) and to the heparan sulfate proteoglycan perlecan (24,25) in cartilage. Binding to ECM maintains a reservoir of FGF-2 that is released from the tissue upon cartilage injury or degradation (23,26,27), and binding to perlecan has been shown to protect FGF-2 from proteolytic degradation (28,29).
Motivated by these considerations, we have designed a new strategy for local delivery of IGF-1 in various tissues: we added the heparin-binding domain of HB-EGF to the amino-terminus of IGF-1 to create a new heparin-binding IGF-1 fusion protein, HB-IGF-1 (30). We have previously shown that HB-IGF-1 produces long-term delivery of bioavailable IGF-1 to bovine cartilage explants and a single dose stimulates a sustained increase in proteoglycan synthesis compared to IGF-1. However, the mechanism by which HB-IGF-1 is retained in tissues is not yet clear. Heparin-binding domains are all highly positively charged but the rigidity of their secondary structure varies, leading to different specificities for binding to heparan sulfate as opposed to other negatively charged sulfated glycosaminoglycans (31,32). Cartilage extracellular matrix (ECM) contains primarily chondroitin sulfate (CS), while the pericellular matrix is rich in heparan sulfate (HS) (21,25).
We hypothesized that HB-IGF-1 is retained in cartilage by binding heparan sulfate proteoglycans in the matrix and at the cell surface. In the present study, we tested this hypothesis by measuring release of bound HB-IGF-1 following chondroitinase or heparitinase treatment of cartilage explants, binding of HB-IGF-1 to cells unable to produce heparan sulfate, and the binding affinities of HB-IGF-1 for isolated heparan sulfate and chondroitin sulfate. Surprisingly, we found that HB-IGF-1 was retained primarily by binding to chondroitin sulfate, whereas heparan sulfate was not required. This result led us to test whether intra-articular injection of HB-IGF-1 allows sustained in vivo delivery preferentially to CS-rich rat knee cartilage and whether HB-IGF-1 can bind adult human cartilage.
Materials and Methods
Protein Production
HB-IGF-1 and IGF-1 were expressed in E. coli as Xpress and hexahistidine tagged proteins and were purified by Ni-NTA affinity followed by reverse-phase chromatography, as previously described in detail (30).
Binding in Bovine Cartilage with GAG-ase Treatments
Cartilage disks (3 mm diameter, 0.5 mm thick, surface removed, first and second slices) from calf femoropatellar grooves were cultured in serum-free low glucose-DMEM with 500 nM HB-IGF-1 or IGF-1 for 2 days. At Day 2, disks were washed with PBS and treated for an additional 48 h with either no enzyme, chondroitinase ABC (E.C. 4.2.2.4, Associates of Cape Cod, Inc., East Falmouth, MA) (0.4 U/mL), or heparitinase (4:1 mixture of heparitinase I and II, E.C. 4.2.2.8, Associates of Cape Cod, Inc.) (0.036 U/mL). These enzymes have been shown to be specific for chondroitin sulfate (33) and heparan sulfate (34), respectively. Buffers were chosen based on recommendations by the manufacturer. Chondroitinase buffer was: 0.15 M NaCl, 0.05 M Tris, pH 8.0, 1 mM PMSF, 2 mM EDTA, 5 mM benzamidine HCl, 10 mM N-Ethylmaleimide (NEM). Heparitinase buffer was: 0.1 M sodium acetate pH 7.0, 10 mM calcium acetate, 1 mM PMSF, 2 mM EDTA, 5 mM benzamidine HCl, 10 mM NEM. Disks not exposed to enzymes were cultured in PBS with 1 mM PMSF, 2 mM EDTA, 5 mM benzamidine HCl, and 10 mM NEM. To rule out the possibility that the high abundance of CS in the tissue could sterically block penetration of heparitinase into cartilage and therefore block heparitinase action, at day 4 half of the explants treated with chondroitinase were incubated with heparitinase (0.036 U/mL); all other disks were incubated in enzyme-free solution (n=4) (days 4–6). On Day 6, disks were flash frozen and protein was extracted by pulverization and by incubation with 100 mM NaCl, 50 mM Tris, 0.5% TritonX-100, pH 7.0 with protease inhibitor cocktail (Roche, Basel, Switzerland) rotating at 4 °C overnight. Protein was quantified by BCA assay (Thermo Fisher Scientific Inc., Rockford, IL) and equal amounts of protein were loaded on a 4–12% SDS-PAGE gel and analyzed by Western blot with anti-IGF-1 (1:500, Abcam Inc., Cambridge, MA, recognizes both HB-IGF-1 and IGF-1 (30)). 5 ng of recombinant HB-IGF-1 was loaded as a protein standard and equal protein of an explant incubated with 500 nM IGF-1 for 2 days without enzyme treatment or washing was loaded as a positive control. HB-IGF-1 released to the buffer solution was analyzed by ELISA as previously described (30). Heparitinase activity was confirmed by assaying conditioned buffer solution using an anti-HS-stub antibody 3G10 (1:500, Associates of Cape Cod, Inc.) by Western blot. This antibody has been shown previously to only detect neoepitopes generated by heparitinase cleavage and not by chondroitinase (35). Chondroitinase activity was confirmed by assaying for GAG loss in treated explants using the DMMB dye binding assay, which showed that >75% sGAG was removed after 48 h.
Binding to Chinese hamster ovary (CHO) cell surfaces
Mutant CHO cells unable to produce heparan sulfate (strain pgsD-677) (36) and wildtype CHO (K1) cells were cultured in F12 medium supplemented with 10% FBS. At confluence, cells were washed with PBS and incubated in serum-free medium with 100 nM HB-IGF-1 or IGF-1. After 3 hours, cells were washed 3×10 min with PBS and lysed with 50 mM Tris, 150 mM NaCl, 10 mM EDTA, 1% Triton X-100, 1% Igepal CA-630 (Sigma-Aldrich, St. Louis, MO), 2 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, and protease inhibitor cocktail. Protein was quantified by BCA assay, and equal amounts of protein were analyzed by Western blot using anti-IGF-1 as described above. 5 ng of recombinant IGF-1 was loaded as a control.
Biotinylation of Glycosaminoglycans (GAGs)
Heparan sulfate (HS) (bovine kidney, Sigma, #H7640, 0.88 sulfates/disaccharide (37)) and chondroitin sulfate C (CS) (CS-C from shark cartilage, Sigma, #C4384, 0.99 sulfates/disaccharide (37)) (0.5 mg) were biotinylated mid-chain with Ez-Link-biotin hydrazide (Thermo Fisher Scientific Inc.) as previously described (38) and purified following manufacturer instructions. Biotinylation was confirmed by dot blot using anti-biotin (1:500, Cell Signaling Technology, Danvers, MA).
Binding Analysis via Surface Plasmon Resonance
All binding experiments were performed at room temperature at a flow rate of 20 µL/min on a Biacore2000 system (GE Healthcare, Buckinghamshire, UK). Biotinylated HS and CS were immobilized on separate flow cells of a streptavidin-coated Biacore chip (GE Healthcare, Piscataway, NJ) and coated with ~600 RU. Another flow cell was left untreated as a control. HB-IGF-1 or IGF-1 was injected in running buffer consisting of 0.01M HEPES, 0.15M NaCl, 3mM EDTA, 0.005% Tween20, pH 7.4. KinInject was used to inject each IGF-1 over the chip with association and dissociation times of 5 min. The surface was regenerated by flowing 1 M NaCl over the chip between experiments. Three to four concentrations of each IGF-1, with three repeats at each concentration, were performed for kinetic analyses. Control flow cell curves were subtracted from all binding curves in order to account for non-specific binding and refractive index change. Association and dissociation rate constants (ka and kd respectively) were determined by fitting the measured binding curves globally with a 1:1 binding model using BIAevaluation software v4.1 and floating Rmax as a local parameter (39). The equilibrium dissociation constant (KD) was calculated as kd/ka.
ELISA Analysis of Binding to Biotinylated GAGs
Coating, blocking and washing buffers, secondary antibody, substrate, and stop solutions were purchased from KPL, Inc. (Gaithersburg, MD). Streptavidin-coated microplates (R&D Systems, Inc., Minneapolis, MN) were coated with biotinylated HS and CS at 20 µg/mL overnight at 4 °C. Plates were blocked for 15 min at room temperature before incubation with 0–500 nM HB-IGF-1 or IGF-1 for one hour. Plates were washed three times and incubated with rabbit anti-IGF-1 (10 µg/mL) (Abcam Inc.) for one hour at room temperature to detect protein bound to the biotinylated GAGs. After additional washes, anti-rabbit HRP (1:500) was applied for one hour at room temperature. Following final washes, color was developed by addition of ABTS peroxidase substrate solution. Absorbance was measured at 405 nm after quenching the wells with stop solution.
Rat Intra-articular Injection
10 µg HB-IGF-1 in 50 µl saline (14.3 µM), 10 µg IGF-1 in 50 µl saline (17.4 µM), or 50 µl saline alone was injected into the right knee joint of 2-month-old male Sprague-Dawley rats. After 24 hours, we harvested articular cartilage, medial meniscus, patella, and patellar tendon from the injected joint as well as a sample of the quadriceps muscle. Tissues were weighed, pulverized while frozen in liquid nitrogen, and extracted with 10 µl lysis buffer (100 mM NaCl, 50 mM Tris, 0.5% Triton X-100, 5 mM EDTA, 1 mM PMSF, and protease inhibitor cocktail (Roche)) per mg of tissue. Portions of extracts with equal total protein mass were analyzed by Western blot. 5 ng of recombinant HB-IGF-1 or IGF-1 were loaded as standards. All animal procedures were approved by the Harvard Medical Area Standing Committee on Animals.
Human Cartilage Binding Assay
Joints from 4 human subjects were obtained postmortem from the Gift of Hope Organ and Tissue Donor Network (Elmhurst, IL). Cartilage disks (3 mm diameter, 0.8 mm thick, with and without intact superficial zone was used) were harvested from femoropatellar grooves of 26-year old (Collins grade 0) (40), 49-year old (Collins grade 0), and 42-year old female (Collins grade 2) knee joints and from a 28-year old (Collins grade 0) male knee joint and cultured in 1% ITS serum-free high glucose DMEM (41) supplemented with 500 nM HB-IGF-1 or IGF-1. After 48 h (Day 0), disks were washed with PBS and incubated in IGF-1 free medium. Disks were collected on Days 0, 1, 2 and 4. Protein was extracted from pulverized disks with lysis buffer containing nonionic detergent as described above for bovine cartilage. Portions of extracts containing equal amounts of total protein were analyzed for IGF-1 by Western blot. 5 ng of recombinant HB-IGF-1 or IGF-1 was loaded as standards. Procedures were approved by the Office of Research Affairs at Rush–Presbyterian–St. Luke’s Medical Center and the Committee on Use of Humans as Experimental Subjects at Massachusetts Institute of Technology.
Statistical Analyses
All data are presented as mean ± standard error of the mean. Surface plasmon resonance binding constants were log-transformed and evaluated by Student’s t-test without assuming equal variances. Densitometry data for HB-IGF-1 binding to human cartilage were rank-transformed and evaluated by paired t-test. Densitometry data for HB-IGF-1 binding to CHO cells were rank-transformed and evaluated by Student’s t-test without assuming equal variances. HB-IGF-1 loss to medium ELISA data were analyzed by one-way ANOVA. Immobilized GAG ELISA data were analyzed by two-way ANOVA followed by Posthoc Tukey tests. All tests were performed with acceptance level α=0.05.
Results
HB-IGF-1 Is Retained in Cartilage Explants through Binding to Chondroitin Sulfate
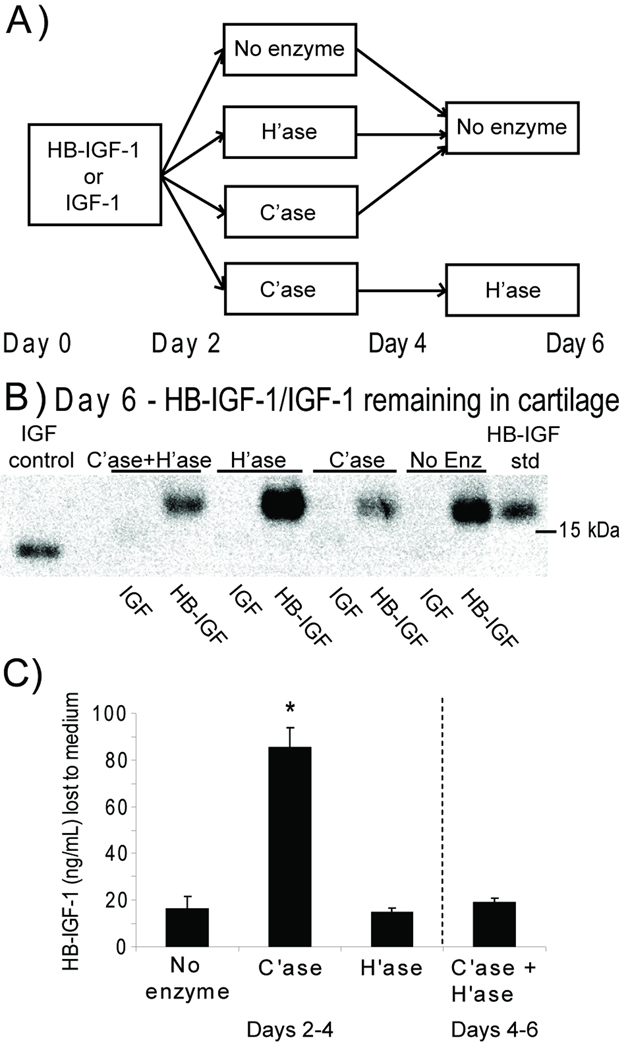
To determine which GAG was more important for HB-IGF-1 retention in cartilage, we first tested whether removal of HS or CS affects HB-IGF-1 retention in cartilage explants (Fig 1A). As shown previously (30), cartilage explants incubated with IGF-1 in the medium for two days retained no IGF-1 in the tissue after 4 days of incubation in the absence of exogenous IGF-1 (Fig 1B). In contrast, in the absence of enzymatic treatment, HB-IGF-1 was strongly retained in cartilage explants. Unexpectedly, treatment with heparitinase had no effect on the retention of HB-IGF-1 in the cartilage, whereas treatment with chondroitinase ABC caused a substantial decrease in HB-IGF-1 remaining in the tissue. While some HB-IGF-1 remained in the explants after treatment with chondroitinase, treatment of these explants with heparitinase did not reduce retention of HB-IGF-1 any further (Fig 1B, “C’ase+H’ase”). ELISA of conditioned solution confirmed that significantly more HB-IGF-1 was released from chondroitinase-treated cartilage than from heparitinase-treated cartilage, indicating comparatively low amounts of HB-IGF-1 bound to heparan sulfate ex vivo (Fig 1C).
Figure 1.
Retention of HB-IGF-1 in bovine cartilage explants following enzymatic digestion of glycosaminoglycans. (A) HB-IGF-1 or IGF-1 was incubated with cartilage disks for two days (Day 0 to Day 2) followed by treatment with no enzyme, chondroitinase (C’ase), or heparitinase (H’ase) for an additional two days (Day 2 to Day 4). At Day 4, a subset of chondroitinase-treated disks was incubated with heparitinase (C’ase+H’ase) for two days while all remaining disks were kept in enzyme-free medium. (B) Western analysis of HB-IGF-1 or IGF-1 remaining in the cartilage tissue at Day 6. The blot shown is representative of four repeats. (C) ELISA of HB-IGF-1 released to the medium following 48-hour enzyme treatment of cartilage explants (Days 2–4 for No enzyme, C’ase, and H’ase conditions; Days 4–6 for C’ase + H’ase). mean ± SEM, * vs. No enzyme, n=4, p<0.001.
Heparan Sulfate is Not Required for Retention of HB-IGF-1 on Cell Monolayers
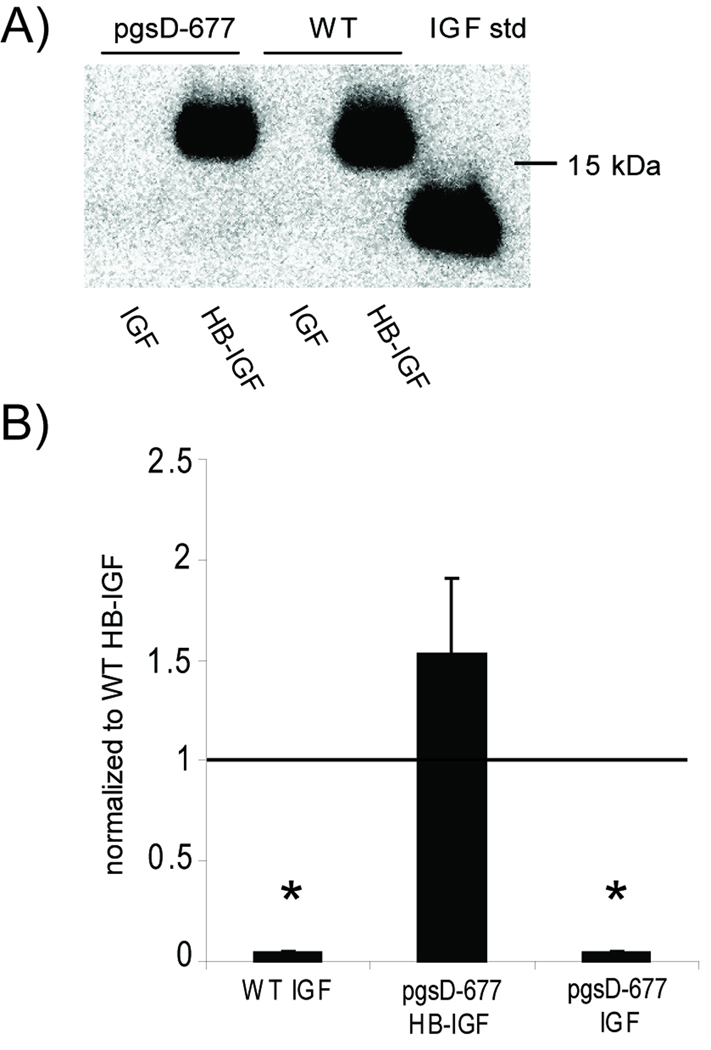
To confirm that heparan sulfate is not required, we tested retention of HB-IGF-1 and IGF-1 to mutant Chinese hamster ovary (CHO) cells (strain pgsD-677) that are unable to make HS due to a genetic defect in HS chain polymerization (36). The mutant cells upregulate synthesis of CS and thus produce similar amounts of total sulfated GAG as the wild-type cells (36). HB-IGF-1 was retained in the wildtype CHO cells after washing, whereas no IGF-1 was retained (Fig 2A,B). However, HB-IGF-1 was also retained in the HS-deficient cells, confirming that HB-IGF-1 can be retained by binding to CS in the absence of HS (Fig 2A,B).
Figure 2.
Retention of HB-IGF-1 on cells lacking heparan sulfate. (A) HB-IGF-1 (HB-IGF) and IGF-1 (IGF) were incubated with mutant CHO cells unable to produce heparan sulfate (pgsD-677) and wildtype CHO cells (WT), then washed in PBS. Western analysis of the cell lysates for IGF showed that HB-IGF-1 remained bound to cells with or without the presence of heparan sulfate, whereas IGF-1 binding was not detectable. (B) Densitometry of Western blots from four repeated experiments, each normalized to wild-type HB-IGF-1 binding. mean ± SEM, * vs. WT HB, p<0.005.
HB-IGF-1 but not IGF-1 Binds Immobilized Glycosaminoglycans (GAGs)
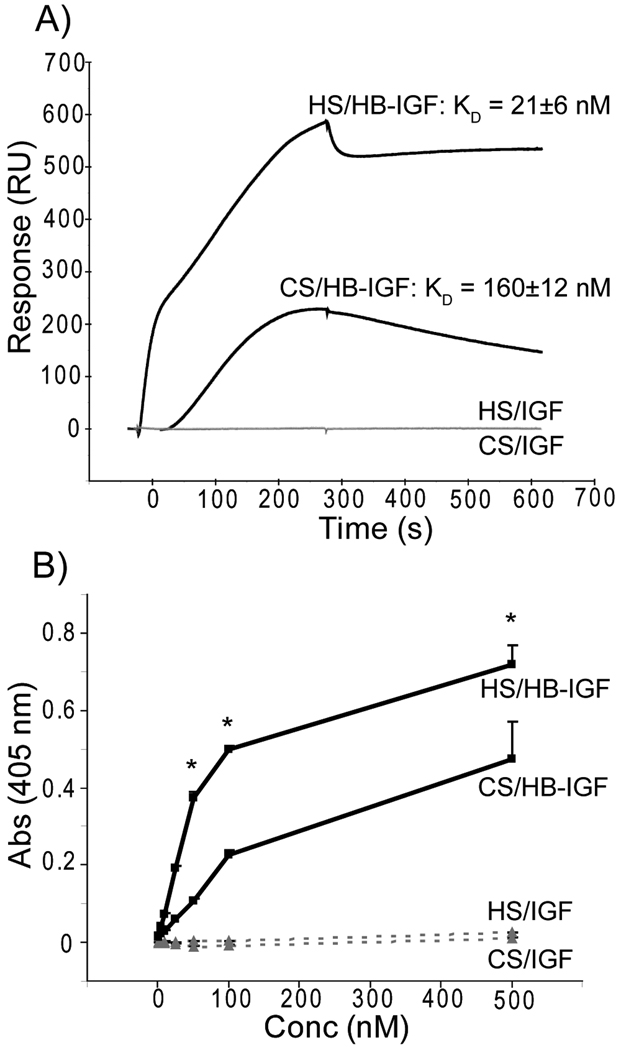
In order to quantify the binding affinities of HB-IGF-1 and IGF-1 for immobilized CS and HS, we used surface plasmon resonance. Comparable levels of biotinylated HS and CS were attached to a streptavidin-coated Biacore chip. A representative sensorgram is shown in Fig 3A, demonstrating that while IGF-1 does not bind to either GAG (response units (RU) <10), HB-IGF-1 binds to both HS and CS. Kinetic analysis of the surface plasmon resonance curves confirmed that although HB-IGF-1 bound HS significantly more strongly (KD = 21 ± 6 nM) than CS (KD = 160 ± 12 nM) (p = 0.012). This difference in KD values resulted from significantly different association rate constants, (ka = 16×104 ± 6.6×104 (1/M·s) for HS vs. ka = 1.5×104 ± 0.06×104 (1/M·s) for CS, p=0.035); the dissociation rates kd were similar (2.7×10−3 ± 0.93×10−3 (1/s) for HS vs. 2.5×10−3 ± 0.16×10−3 (1/s) for CS), p=0.85).
Figure 3.
Binding analysis of HB-IGF-1 and IGF-1 to isolated glycosaminoglycans. (A) Representative sensorgram for Biacore kinetic analysis over HS or CS surfaces using 250 nM HB-IGF-1 or IGF-1. HB-IGF-1 is shown in the top two curves (black) with corresponding equilibrium dissociation constants, KD, determined from a minimum of three concentrations used during three experimental repeats. IGF-1 (bottom two curves, grey) was unable to bind either surface (RU < 10). (B) Sandwich ELISA detecting absorbance at a given HB-IGF-1 (solid line, black) or IGF-1 (dashed line, grey) concentration resulting from binding HS or CS. Representative of two repeats, each with duplicate wells. n=2, mean ± SEM, * vs. CS, p<0.05.
The relative differences in binding affinities of HB-IGF-1 and IGF-1 to HS and CS were confirmed by a sandwich ELISA. Binding of HB-IGF-1 to immobilized HS and CS increased with concentration, with more binding to HS at the same given concentration of HS or CS contained in each well (Fig 3B). Therefore, although HB-IGF-1 binds HS with higher affinity, the data suggest that binding to CS would dominate in cartilage tissue, where CS is ~500–1000 more abundant than HS (25,42).
HB-IGF-1 Is Preferentially Retained in Cartilage after Intra-articular Injection
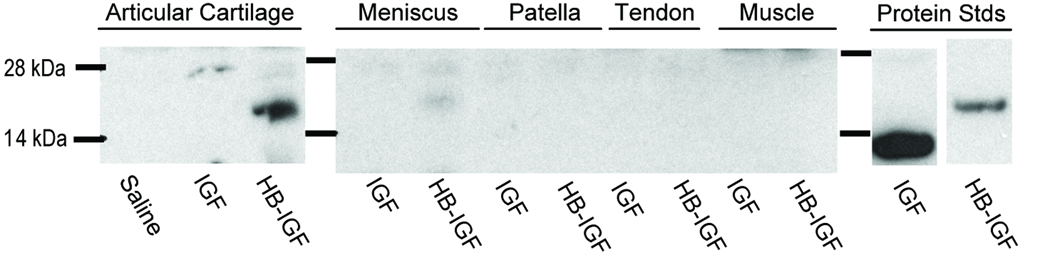
The ability of HB-IGF-1 to bind CS led us to hypothesize that it would be retained preferentially in the GAG-rich cartilage tissues if delivered by intra-articular injection. Consistent with this hypothesis, we found that one day after intra-articular injection in a rat knee, HB-IGF-1 remained strongly detectable in articular cartilage extracts (Fig 4, Articular Cartilage), despite stronger immunoreactivity of the IGF-1. HB-IGF-1 was also slightly detectable in extracts of the fibrocartilaginous meniscus (Fig 4, Meniscus). In contrast, HB-IGF-1 was not detectable in patella, patellar tendon, or muscle extracts (Fig 4). IGF-1 was not detectable in any of the tissues one day after injection (Fig 4).
Figure 4.
Retention of HB-IGF-1 in vivo. Western blot showing retained IGF-1 (IGF) or HB-IGF-1 (HB-IGF) in rat articular cartilage, meniscus, patella, patellar tendon, or muscle extracts one day after intra-articular injection of IGF-1, HB-IGF-1, or saline.
HB-IGF-1 Is Retained in Human Cartilage
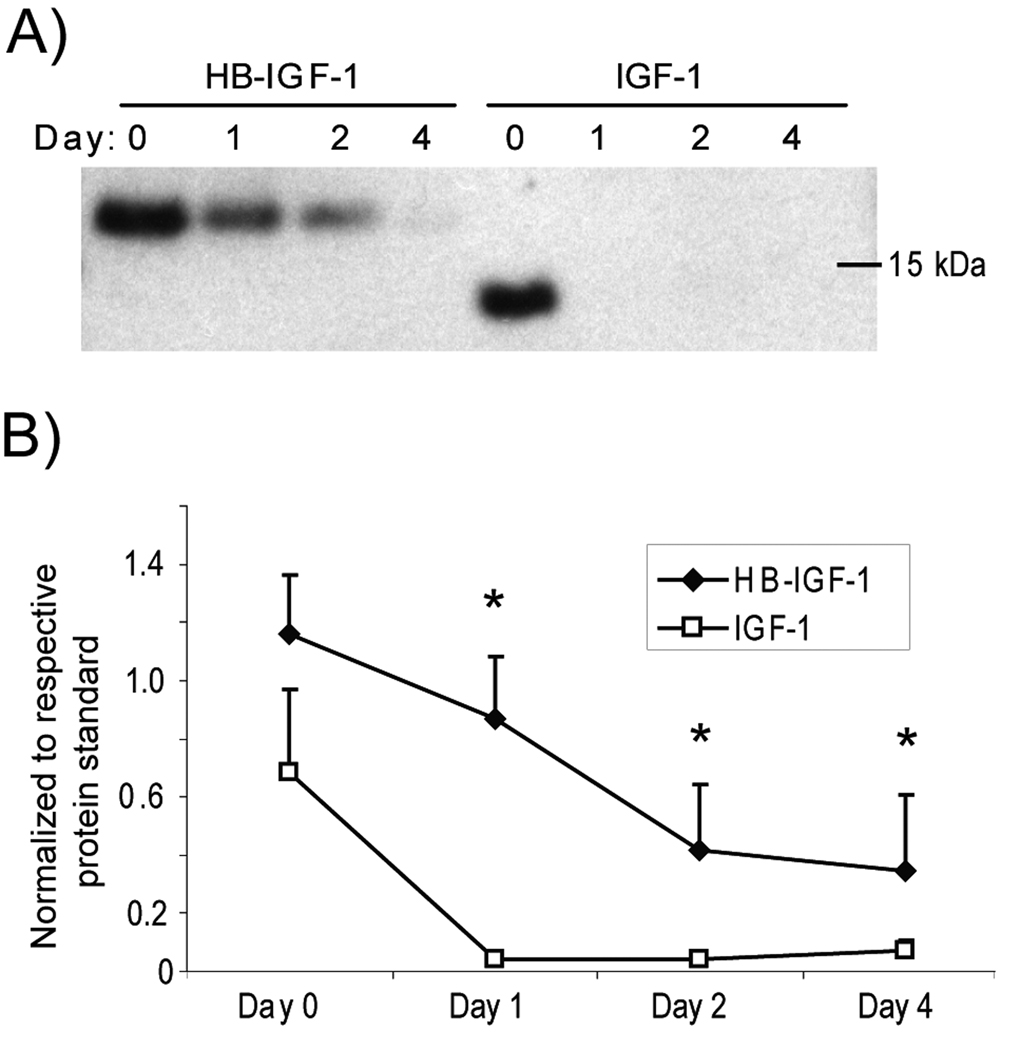
To examine whether HB-IGF-1 may be relevant as a strategy for clinical delivery of growth factors to cartilage, we tested whether HB-IGF-1 could also be retained in post-mortem adult human cartilage explants. Human knee cartilage obtained from three Collins grade 0 donor joints (both male and female) and one Collins grade 2 joint (female) was incubated with 500 nM HB-IGF-1 or IGF-1 for two days, washed with PBS, and incubated in no-IGF-1 medium for an additional 0, 1, 2, or 4 days. Binding of IGF-1 was only detectable immediately after washing out the IGF (Fig 5A, Day 0). In contrast, HB-IGF-1 was retained after further incubation for up to 4 days (Fig 5A). Analysis of Western blots from the four donor cartilages by densitometry demonstrated that retention of HB-IGF-1 was significantly higher than IGF-1 after 1, 2, and 4 days of incubation (Fig 5B).
Figure 5.
Retention of HB-IGF-1 in human cartilage explants. Human cartilage (grade 0 to 2) was incubated with HB-IGF-1 or IGF-1 for two days (Day -2 to 0), washed, and incubated in IGF-free medium for 4 days (Days 0–4). (A) Amount of HB-IGF-1 or IGF-1 remaining in cartilage after 0–4 days. The experiment was performed on cartilage from four donors and a representative Western blot is shown. (B) Analysis of four Western blots by densitometry, normalized to the density of 5 ng of the respective protein standard. mean ± SEM, * vs. IGF-1, p<0.05.
Discussion
We demonstrate here that adding a heparin-binding motif to IGF-1 converts it from a short-acting growth factor to one that can be locally delivered and retained in articular cartilage in vivo. Contrary to our initial expectations, HB-IGF-1 does not require heparan sulfate for retention in either cell culture or in cartilage explants. While we show that HB-IGF-1 does have a higher affinity for heparan sulfate, its affinity for chondroitin sulfate is within an order of magnitude. Therefore, in cartilage, where CS concentrations are 500–1000 times higher than HS (25,42), binding to CS dominates and retention of HB-IGF-1 is independent of HS.
These results led us to hypothesize that HB-IGF-1 could be preferentially delivered and retained in articular cartilage due to its high concentration of sulfated glycosaminoglycans (1). We tested this hypothesis in rats and showed that after intra-articular injection, HB-IGF-1 remains bound to the CS-rich articular cartilage, but not to the adjacent patella, patellar tendon, or muscle tissue. In contrast, unmodified IGF-1 is not able to bind either CS or HS, and was not detectable in either tissue after intra-articular injection. Finally, we demonstrated that HB-IGF-1 is retained in adult human cartilage whereas unmodified IGF-1 is not. Taken together, the results suggest that modification of growth factors with heparin-binding domains may be a clinically relevant strategy for local delivery to cartilage.
Our finding that HB-IGF-1 is retained primarily by chondroitin sulfate contrasts with the extensive work demonstrating that the heparin-binding domain of FGF-2 binds primarily to heparan sulfate proteoglycans in cartilage (24,25). This is likely explained by differences in the binding specificities of the heparin-binding domains. In general, specificity for HS depends on not only the heparin binding domains, but also on the secondary and/or tertiary structure of the native proteins. The heparin-binding domain we used here to make HB-IGF-1 came from HB-EGF. Structure-function analysis of HB-EGF has shown that in addition to the heparin-binding domain, a portion of the EGF-like domain (32) is required for binding specifically to heparan sulfate. Similarly, FGF-2 affinity for HS has been shown to depend on the spatial distribution of basic amino acids within the heparin-binding loops of this molecule and on the specific conformation and topological arrangement of these loops (43). Since we added only the heparin-binding domain of HB-EGF to IGF-1, it is likely that charge plays a primary role in the interactions of HB-IGF-1, allowing it to bind other negatively charged glycosaminoglycans such as CS.
There may be additional mechanisms that contribute to the differences seen here between HB-IGF-1 and unmodified IGF-1. We have previously shown that insertion of the HB domain to the amino terminus of IGF does not affect signaling through the IGF receptor (30). However, insertion of the HB domain may also increase bioavailability of IGF by decreasing its affinity for the IGF binding proteins (IGFBPs). In cartilage, endogenous IGF-1 binds primarily to IGFBPs, and not to ECM constituents (44,45), potentially decreasing its availability. Increased levels of IGFBPs may be one reason for the decreased response of cartilage from osteoarthritic patients to IGF-1 (1,46–49). However, deletion of just the first three amino acids of IGF-1 decreases binding to IGFBPs by 100-fold (50), suggesting that the interaction may be very sensitive to alterations at the amino terminus of IGF-1 where the HB domain is inserted. Further studies will therefore be required to address whether HB-IGF-1 has altered binding to IGFBPs.
Although we have shown that retention of HB-IGF-1 is accompanied by extended stimulation of biosynthesis in cartilage explants (30), further in vivo studies of HB-IGF-1 distribution, kinetics, and effects will be needed to establish its potential therapeutic value. In particular, despite the strong retention of HB-IGF-1 in cartilage, it will be necessary to verify whether HB-IGF-1 significantly raises IGF-1 levels in the circulation after relevant doses. We anticipate that treatment with HB-IGF-1 would likely be most helpful before extensive cartilage damage and proteoglycan loss occurs, and where acute rather than long-term therapy may be useful, such as in traumatic joint injuries.
In conclusion, HB-IGF-1 may be a new therapeutic for sustained and relatively specific local delivery of IGF-1 to cartilage through its preferential retention in CS-rich tissues. Modification of growth factors by addition of heparin binding domains may therefore be a novel strategy for targeted delivery to cartilage after intra-articular injection.
Acknowledgements
The authors thank Dr. Jeff Esko for very helpful discussions.
AJG, RTL: NIH-NIBIB Grant EB003805, AJG: NIH-NIAMS Grant AR045779
REM: NDSEG and NSF graduate fellowships
KC, PP, RTL: a collaborative research grant from the Massachusetts Life Sciences Center and funding from Biomeasure, Inc.
Footnotes
Disclosure: RTL is listed as the inventor of a patent related to HB-IGF.
References
- 1.Goldring MB. Update on the biology of the chondrocyte and new approaches to treating cartilage diseases. Best Practice & Research Clinical Rheumatology. 2006;20:1003–1025. doi: 10.1016/j.berh.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Bonassar LJ, Grodzinsky AJ, Frank EH, Davila SG, Bhaktav NR, Trippel SB. The effect of dynamic compression on the response of articular cartilage to insulin-like growth factor-I. J Orthop Res. 2001;19:11–17. doi: 10.1016/S0736-0266(00)00004-8. [DOI] [PubMed] [Google Scholar]
- 3.McQuillan DJ, Handley CJ, Campbell MA, Bolis S, Milway VE, Herinton AC. Stimulation of proteoglycan biosynthesis by serum and insulin-like growth factor-I in cultured bovine articular cartilage. Biochem J. 1986;240:423–430. doi: 10.1042/bj2400423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curtis AJ, Devenish RJ, Handley CJ. Modulation of aggrecan and link-protein synthesis in articular cartilage. Biochem J. 1992;288:721–726. doi: 10.1042/bj2880721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hui W, Rowan AD, Cawston T. Modulation of the expression of matrix metalloproteinase and tissue inhibitors of metalloproteinases by TGF-β1 and IGF-1 in primary human articular and bovine nasal chondrocytes stimulated with TNF-α. Cytokine. 2001;16:31–35. doi: 10.1006/cyto.2001.0950. [DOI] [PubMed] [Google Scholar]
- 6.Luyten FP, Hascall VC, Nissley SP, Morales TI, Reddi AH. Insulin-like growth factors maintain steady-state metabolism of proteoglycans in bovine articular cartilage explants. Arch Biochem Biophys. 1988;267:416–425. doi: 10.1016/0003-9861(88)90047-1. [DOI] [PubMed] [Google Scholar]
- 7.Tyler JA. Insulin-like growth factor I can decrease degradation and promote synthesis of proteoglycan in cartilage exposed to cytokines. Biochem J. 1989;260:543–548. doi: 10.1042/bj2600543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneiderman R, Rosenberg N, Hiss J, Lee P, Liu F, Hintz RL, et al. Concentration and size distribution of insulin-like growth factor-1 in human normal and osteoarthritic synovial fluid and cartilage. Arch Biochem Biophys. 1995;324:173–188. doi: 10.1006/abbi.1995.9913. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt MB, Chen EH, Lynch SE. A review of the effects of insulin-like growth factor and platelet derived growth factor on in vivo cartilage healing and repair. Osteoarthritis Cartilage. 2006;14:403–412. doi: 10.1016/j.joca.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto T, Gargosky SE, Iwasaki K, Rosenfeld RG. Identification and characterization of insulin-like growth factors (IGFs), IGF-binding proteins (IGFBPs), and IGFBP proteases in human synovial fluid. J Clin Endocrinol Metab. 1996;81:150–155. doi: 10.1210/jcem.81.1.8550744. [DOI] [PubMed] [Google Scholar]
- 11.Loeser RF, Shanker G. Autocrine stimulation by insulin-like growth factor 1 and insulin-like growth factor 2 mediates chondrocyte survival in vitro. Arthritis Rheum. 2000;43:1552–1559. doi: 10.1002/1529-0131(200007)43:7<1552::AID-ANR20>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 12.Nixon A, Saxer R, Brower-Toland BD. Exogenous insulin-like growth factor-I stimulates an autoinductive IGF-I autocrine/paracrine response in chondrocytes. J Orthop Res. 2001;19:26–32. doi: 10.1016/S0736-0266(00)00013-9. [DOI] [PubMed] [Google Scholar]
- 13.Madry H, Kaul G, Cucchiarini M, Stein U, Zurakowski D, Remberger K, et al. Enhanced repair of articular cartilage defects in vivo by transplanted chondrocytes overexpressing insulin-like growth factor I (IGF-I) Gene Ther. 2005;12:1171–1179. doi: 10.1038/sj.gt.3302515. [DOI] [PubMed] [Google Scholar]
- 14.Goodrich LR, Hidaka C, Robbins PD, Evans CH, Nixon AJ. Genetic modification of chondrocytes with insulin-like growth factor-1 enhances cartilage healing in an equine model. J Bone Joint Surg Br. 2007;89-B:672–685. doi: 10.1302/0301-620X.89B5.18343. [DOI] [PubMed] [Google Scholar]
- 15.Laron Z. Somatomedin-1 (Recombinant Insulin-Like Growth Factor-1): Clinical Pharmacology and Potential Treatment of Endocrine and Metabolic Disorders. BioDrugs. 1999;11:55–70. doi: 10.2165/00063030-199911010-00006. [DOI] [PubMed] [Google Scholar]
- 16.Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, et al. Plasma Insulin-Like Growth Factor-I and Prostate Cancer Risk: A Prospective Study. Science. 1998;279:563–566. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 17.Jabri N, Schalch D, Schwartz S, Fischer J, Kipnes M, Radnik B, et al. Adverse effects of recombinant human insulin-like growth factor I in obese insulin-resistant type II diabetic patients. Diabetes. 1994;43:369–374. doi: 10.2337/diab.43.3.369. [DOI] [PubMed] [Google Scholar]
- 18.Fortier LA, Mohammed HO, Lust G, Nixon AJ. Insulin-like growth factor-I enhances cell-based repair of articular cartilage. J Bone Joint Surg Br. 2002;84-B:276–288. doi: 10.1302/0301-620x.84b2.11167. [DOI] [PubMed] [Google Scholar]
- 19.Nixon AJ, Fortier LA, Williams J, Mohammed H. Enhanced repair of extensive articular defects by insulin-like growth factor-I-laden fibrin composites. J Orthop Res. 1999;17:475–487. doi: 10.1002/jor.1100170404. [DOI] [PubMed] [Google Scholar]
- 20.Hunziker EB, Rosenberg LC. Repair of partial-thickness defects in articular cartilage: cell recruitment from the synovial membrane. J Bone Joint Surg Am. 1996;78:721–733. doi: 10.2106/00004623-199605000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Farach-Carson MC, Hecht JT, Carson DD. Heparan Sulfate Proteoglycans: Key Players in Cartilage Biology. Critical Reviews in Eukaryotic Gene Expression. 2005;15:29–48. doi: 10.1615/critreveukaryotgeneexpr.v15.i1.30. [DOI] [PubMed] [Google Scholar]
- 22.Kirn-Safran CB, Gomes RR, Brown AJ, Carson DD. Heparan sulfate proteoglycans: Coordinators of multiple signaling pathways during chondrogenesis. Birth Defects Research Part C: Embryo Today: Reviews. 2004;72:69–88. doi: 10.1002/bdrc.20005. [DOI] [PubMed] [Google Scholar]
- 23.Heinegard D. Proteoglycans and more - from molecules to biology. Int J Exp Path. 2009;90:575–586. doi: 10.1111/j.1365-2613.2009.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Govindraj P, West L, Smith S, Hassell JR. Modulation of FGF-2 binding to chondrocytes from the developing growth plate by perlecan. Matrix Biol. 2006;25:232–239. doi: 10.1016/j.matbio.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Vincent TL, McLean CJ, Full LE, Peston D, Saklatvala J. FGF-2 is bound to perlecan in the pericellular matrix of articular cartilage, where it acts as a chondrocyte mechanotransducer. Osteoarthritis Cartilage. 2007;15:752–763. doi: 10.1016/j.joca.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 26.Vincent T, Hermansson M, Bolton M, Wait R, Saklatvala J. Basic FGF mediates an immediate response of articular cartilage to mechanical injury. Proc Natl Acad Sci U S A. 2002;99:8259–8264. doi: 10.1073/pnas.122033199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vincent TL, Hermansson MA, Hansen UN, Amis AA, Saklatvala J. Basic fibroblast growth factor mediates transduction of mechanical signals when articular cartilage is loaded. Arthritis Rheum. 2004;50:526–533. doi: 10.1002/art.20047. [DOI] [PubMed] [Google Scholar]
- 28.Saksela O, Moscatelli D, Sommer A, Rifkin DB. Endothelial cell-derived heparan sulfate binds basic fibroblast growth factor and protects it from proteolytic degradation. J Cell Biol. 1988;107:743–751. doi: 10.1083/jcb.107.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flaumenhaft R, Moscatelli D, Saksela O, Rifkin DB. Role of extracellular matrix in the action of basic fibroblast growth factor: Matrix as a source of growth factor for long-term stimulation of plasminogen activator production and DNA synthesis. J Cellular Physiology. 1989;140:75–81. doi: 10.1002/jcp.1041400110. [DOI] [PubMed] [Google Scholar]
- 30.Tokunou T, Miller R, Patwari P, Davis ME, Segers VF, Grodzinsky AJ, et al. Engineering insulin-like growth factor-1 for local delivery. FASEB J. 2008;22:1886–1893. doi: 10.1096/fj.07-100925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cardin AD, Weintraub HJ. Molecular modeling of protein-glycosaminoglycan interactions. Arterioscler Thromb Vasc Biol. 1989;9:21–32. doi: 10.1161/01.atv.9.1.21. [DOI] [PubMed] [Google Scholar]
- 32.Thompson SA, Higashiyama S, Wood K, Pollitt NS, Damm D, McEnroe G, et al. Characterization of sequences within heparin-binding EGF-like growth factor that mediates interaction with heparin. J Biol Chem. 1994;269:2541–2549. [PubMed] [Google Scholar]
- 33.Oike Y, Kimata K, Shinomura T, Nakazawa K, Suzuki S. Structural analysis of chick-embryo cartilage proteoglycan by selective degradation with chondroitin lyases (chondroitinases) and endo-beta-D-galactosidase (keratanase) Biochem J. 1980;191:193–207. doi: 10.1042/bj1910193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silverberg I, Havsmark B, Fransson L-Å. The substrate specificity of heparan sulphate lyase and heparin lyase from Flavobacterium heparinum. Carbohydrate Research. 1985;137:227–238. [Google Scholar]
- 35.David G, Bai XM, Van der Schueren B, Cassiman JJ, Van den Berghe H. Developmental changes in heparan sulfate expression: in situ detection with mAbs. J. Cell Biol. 1992;119:961–975. doi: 10.1083/jcb.119.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lidholt K, Weinke JL, Kiser CS, Lugemwa FN, Bame KJ, Cheifetz S, et al. A single mutation affects both N-acetylglucosaminyltransferase and glucuronosyltransferase activities in a Chinese hamster ovary cell mutant defective in heparan sulfate biosynthesis. Proc Natl Acad Sci U S A. 1992;89:2267–2271. doi: 10.1073/pnas.89.6.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ley K, Cerrito M, Arfors K-E. Sulfated polysaccharides inhibit leukocyte rolling in rabbit mesentery venules. Am J Physiol. 1991;260:H1667–H1673. doi: 10.1152/ajpheart.1991.260.5.H1667. [DOI] [PubMed] [Google Scholar]
- 38.Friedrich U, Blom AM, Dahlback B, Villoutreix BO. Structural and Energetic Characteristics of the Heparin-binding Site in Antithrombotic Protein C. J. Biol. Chem. 2001;276:24122–24128. doi: 10.1074/jbc.M011567200. [DOI] [PubMed] [Google Scholar]
- 39.Ricard-Blum S, Feraud O, Lortat-Jacob H, Rencurosi A, Fukai N, Dkhissi F, et al. Characterization of Endostatin Binding to Heparin and Heparan Sulfate by Surface Plasmon Resonance and Molecular Modeling. J Biol Chem. 2004;279:2927–2936. doi: 10.1074/jbc.M309868200. [DOI] [PubMed] [Google Scholar]
- 40.Muehleman C, Bareither D, Huch K, Cole AA, Kuettner KE. Prevalence of degenerative morphological changes in the joints of the lower extremity. Osteoarthritis Cartilage. 1997;5:23–37. doi: 10.1016/s1063-4584(97)80029-5. [DOI] [PubMed] [Google Scholar]
- 41.Sui Y, Lee JH, DiMicco MA, Vanderploeg EJ, Blake SM, Hung HH, et al. Mechanical injury potentiates proteoglycan catabolism induced by interleukin-6 with soluble interleukin-6 receptor and tumor necrosis factor alpha in immature bovine and adult human articular cartilage. Arthritis Rheum. 2009;60:2985–2996. doi: 10.1002/art.24857. [DOI] [PubMed] [Google Scholar]
- 42.Govindraj P, West L, Koob TJ, Neame P, Doege K, Hassell JR. Isolation and Identification of the Major Heparan Sulfate Proteoglycans in the Developing Bovine Rib Growth Plate. J Biol Chem. 2002;277:19461–19469. doi: 10.1074/jbc.M200786200. [DOI] [PubMed] [Google Scholar]
- 43.Raman R, Venkataraman G, Ernst S, Sasisekharan V, Sasisekharan R. Structural specificity of heparin binding in the fibroblast growth factor family of proteins. Proc Natl Acad Sci U S A. 2003;100:2357–2362. doi: 10.1073/pnas.0437842100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia A, Szasz N, Trippel SB, Morales T, Grodzinsky AJ, Frank E. Transport and binding of insulin-like growth factor I through articular cartilage. Arch Biochem Biophys. 2003;415:69–79. doi: 10.1016/s0003-9861(03)00215-7. [DOI] [PubMed] [Google Scholar]
- 45.Bhakta NR, Garcia AM, Frank EH, Grodzinsky AJ, Morales TI. The Insulin-like Growth Factors (IGFs) I and II Bind to Articular Cartilage via the IGF-binding Proteins. J Biol Chem. 2000;275:5860–5866. doi: 10.1074/jbc.275.8.5860. [DOI] [PubMed] [Google Scholar]
- 46.Martel-Pelletier J, Di Battista JA, Lajeunesse D, Pelletier JP. IGF/IGFBP axis in cartilage and bone in osteoarthritis pathogenesis. Inflamm Res. 1998;47:90–100. doi: 10.1007/s000110050288. [DOI] [PubMed] [Google Scholar]
- 47.Martin JA, Ellerbroek SM, Buckwalter JA. Age-related decline in chondrocyte response to insulin-like growth factor-I: The role of growth factor binding proteins. J Orthop Res. 1997;15:491–498. doi: 10.1002/jor.1100150403. [DOI] [PubMed] [Google Scholar]
- 48.Loeser RF, Shanker G, Carlson C, Gardin J, Shelton B, Sonntag W. Reduction in the chondrocyte response to insulin-like growth factor in aging and osteoarthritis. Arthritis Rheum. 2000;43:2110–2120. doi: 10.1002/1529-0131(200009)43:9<2110::AID-ANR23>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 49.Dore S, Pelletier JP, DiBattista JA, Tardif G, Brazeau P, Martel-Pelletier J. Human osteoarthritic chondrocytes possess an increased number of insulin-like growth factor 1 binding sites but are unresponsive to its stimulation. Possible role of IGF-1-binding proteins. Arthritis Rheum. 1994;37:253–263. doi: 10.1002/art.1780370215. [DOI] [PubMed] [Google Scholar]
- 50.Bagley CJ, May BL, Szabo L, McNamara PJ, Ross M, Francis GL, et al. A key functional role for the insulin-like growth factor 1 N-terminal pentapeptide. Biochem J. 1989;259:665–671. doi: 10.1042/bj2590665. [DOI] [PMC free article] [PubMed] [Google Scholar]