Abstract
Objectives
To describe the cerebrospinal fluid (CSF) profiles of febrile infants 1–90 days with negative bacterial cultures and negative testing for enteroviruses by polymerase chain reaction.
Study design
Statistical analysis of a retrospective cohort.
Results
CSF profiles from 823 infants with negative evaluations for infection were analyzed. For 677 with atraumatic lumbar punctures (RBC < 1,000/mm3), the mean and median CSF WBC counts were 4.3/mm3 and 3.0/mm3 respectively with a range from 0–12/mm3. Mean CSF WBC counts (6.1/mm3 vs. 3.1/mm3 and 3.0/mm3) and protein levels (75.4 mg/dl vs. 58.9 mg/dl and 39.2 mg/dl) were higher in the first month compared with months two and three, respectively (p<0.001 for all). CSF glucose levels were lower in the first month compared with month three (45.3 mg/dl vs. 48.0 mg/dl and 57.7 mg/dl) (p< 0.001). Increasing RBC counts were statistically associated with increasing WBC counts (p< 0.001). However, the contribution of RBC < 10,000/mm3 was small and the normal range for WBC in uninfected infants with traumatic lumbar punctures was 0–16/mm3.
Conclusions
CSF WBC counts in febrile infants without evidence of bacterial or enteroviral infection, even in those with traumatic lumbar puncture, are lower than reported in pediatric references.
Fever in infants 1–90 days of age is a commonly encountered clinical problem. The medical evaluation of these infants often includes an analysis of cerebrospinal fluid (CSF) to exclude bacterial meningitis.(1) Controversy exists regarding the interpretation of CSF profiles from young infants. We identified eighteen publications that analyzed the CSF profiles in presumptively uninfected infants.(2–19) Many did not include the precise age of the infants and most did not document all bacterial and viral testing performed. The majority of studies included data from 50 or fewer infants and only three studies included data for 100 or more infants making it difficult to determine normative values for the CSF profile in young infants.(2, 9, 19)
The normal values referenced in US pediatric textbooks for infant CSF profile are based on four studies with small numbers of infants.(10, 15–17) Three studies were performed before polymerase chain reaction (PCR) testing for enteroviruses (EV), the most common cause of aseptic meningitis in this age group, was available.(10, 15, 16) A recent study describing normative CSF white blood cell counts in infants ≤ 56 days reported negative EV PCR testing for ~ 20% of the cohort and no EV PCR testing for the remainder.(19) Further, though controversial(20), recent literature has also associated CSF pleocytosis with urinary tract infection (UTI)(21–25), the most common cause of serious bacterial infection (SBI) in this age group.(26, 27) Two of the commonly referenced studies describing normal infant CSF white blood cell (WBC) values did not report the infants’ status regarding UTI.(15, 16) Finally, the presence of red blood cells (RBC) in CSF may alter the number of WBC observed. Traumatic lumbar punctures are common in pediatric practice.(28) Several authors have evaluated the contribution of RBCs in older infants, children, and adults(29–34), however, there is limited information regarding effect CSF RBCs on the CSF WBC counts in infants 1–90 days of age.
The objectives of this study were: (1) describe the cerebrospinal fluid (CSF) profiles of febrile infants with negative bacterial cultures of blood, urine, and CSF and negative testing for enteroviruses (EV) by the polymerase chain reaction (PCR); and (2) determine the contribution of CSF RBCs to the CSF WBCs in these infants.
METHODS
Approval to conduct this study was granted by the Institutional Review Boards of the University of Utah and Primary Children’s Medical Center (PCMC) in Salt Lake City, Utah.
Medical records of infants undergoing lumbar puncture as part of an evaluation for fever at Primary Children’s Medical Center (PCMC) in Salt Lake City, UT between December 1996 and June 2002 were evaluated. The 1779 febrile infants enrolled in this study have been previously described(27). Infants were eligible for enrollment in the original study if they: 1) were discharged from the hospital after birth and presented to PCMC between 1–90 days with rectal temperatures ≥ 38° C, 2) had received no antibiotics in the previ ous 48 hours, and 3) had not received the oral polio vaccine (a live enteroviral vaccine). All infants had an evaluation for SBI including bacterial cultures of blood, urine, and cerebrospinal fluid (CSF). 1061infants (60%) with sufficient CSF or whole blood had PCR testing for enteroviruses (EV).(27) A CSF profile (WBC, RBC, glucose and protein) was not required for entry in the study, but most infants had these data.
Determination of Normative CSF Values from Presumptively Uninfected Infants
All infants with full CSF profiles were eligible to be included in this study (n=1186). We then selected infants who fulfilled all of the following criteria for analysis of CSF profiles to determine normative values: (1) term birth (≥ 37 weeks gestation); (2) negative bacterial cultures of blood, urine, and CSF; (3) negative EV PCR of CSF; (4) negative EV PCR of blood when obtained; and (5) no clinical evidence of herpes simplex infection including no history of acyclovir treatment. 823 infants fulfilled all criteria and were classified as presumptively uninfected.
Analysis of CSF Profile in Infants without Evidence of Infection to Determine Normative Values
Infants who were classified as uninfected and who had atraumatic lumbar punctures were used to establish normative values for CSF profiles. Traumatic lumbar puncture was defined as CSF RBC count ≥1000/mm3 (32). Of the 823 infants identified as uninfected, 743 (90%) had CSF RBC counts < 1000/mm3. We defined the normal range as upper quartile (Q) 3 ±1.5 IQR, with the upper bound being Q3 + 1.5 IQ and the lower bound Q1 – 1.5 IRQ (truncated at 0).(35) Data points beyond the upper bound were statistical outliers.(35) The definition of the normal range as upper quartile (Q3) ±1.5 IQR is widely accepted in the statistical literature.(36, 37) Using the upper one-sided 95% confidence interval is another option(19), however, this method should be reserved for data that follows a Normal (Gaussian) distribution or known parametric distributions and the method does not identify statistical outliers.(38) The CSF WBC counts in our data set were highly skewed and did not follow a Normal distribution. In this setting, normal values can be approximated by a log-Normal distribution. In a log-Normal distribution, Q3+1.5 IQR corresponds to the ~ the 92 percentile. We elected to include all CSF profiles from uninfected infants with atraumatic lumbar punctures in the initial analysis and to identify statistical outliers. Because we were unable to test for all viral pathogens known to cause CSF pleocytosis, it is possible that some infants included in our analysis as having no identified infection actually had infections with known viruses such as parechovirus or human herpesvirus 6 or unknown viruses.(39–41) Infants with undiagnosed viral infection of the central nervous system could be expected to produce data points that were statistical outliers. After testing for statistical outliers, 677 infants remained and were used to determine normative values.
Statistical analysis included calculating the means, standard deviations, medians and interquartile ranges (IQR) for CSF WBC, RBC, glucose and protein concentrations were performed by week of age. Comparison of continuous variables was accomplished using analysis of variance (ANOVA) method as well as regression models. A p value of ≤ 0.05 was considered significant.
Analysis of Effect of RBC Count on CSF WBC Count in Infants without Evidence of SBI, EV, or HSV Infection
For the analysis of RBC effect on WBC count, we used the entire cohort of presumptively uninfected infants (N=823). These infants had CSF RBC counts that ranged from 0–9,999cells/mm3. The strength of correlation between the number of RBC and the number of WBC was determined using linear regression and the coefficient of determination (R2). An R2 value of 1 indicates that the regression model predicts the observed data perfectly.(42)
RESULTS
Normative Values for CSF Profiles in Infants Without Identified Infection and Atraumatic Lumbar Punctures
Of the 823 term infants without infection identified, 743 (90%) had atraumatic lumbar punctures. The mean, median, and range of CSF WBC counts for these infants are shown in Table I. Analysis of the CSF WBC counts from these infants identified CSF WBC counts > 14.5/mm3 as statistical outliers. Of the 743 uninfected infants with atraumatic lumbar punctures, 677 (91%) had CSF WBC counts < 14.5/mm3. The CSF profiles from these 677 infants were used to determine normative values for CSF profiles including WBC counts, glucose and protein (Table II).
Table 1.
CSF WBC counts in infected febrile infants 1–90 days with atraumatic lumbar punctures*
| Age (N) | CSF WBC/mm3 Mean Median (Range) |
|---|---|
| Month One 1–28 Days (n=297) | 8.9, 5.0 (0–20.5) |
| Month Two 29–60 Days (n=357) | 8.3, 3.0 (0–11.0) |
| Month Three 61–90 days (n=89) | 4.5, 3.0 (0–11.0) |
| All (n=743) | 8.1, 4.0 (0–14.5) |
All infants had no evidence of SBI, EV or HSV infection. All infants had atraumatic lumbar punctures with CSF RBC counts < 1000/mm3. Statistical outliers are included.
Table 2.
Normative values for CSF profiles by age for febrile infants 1–90 days*
| Age (N) | CSF WBC/mm3 Mean Median (Range) |
CSF RBC/mm3 Mean Median (Range) |
Glucose mg/dl Mean Median (Range) |
Protein mg/dl Mean Median (Range) |
|---|---|---|---|---|
| Month One 1–28 days (n=278) | 6.1, 5.0 (0–18.0) | 95.5, 5.5 (0–236.0) | 45.3, 46.0 (30.0–61.0) | 75.4, 73.0 (15.8–131.0) |
| Month Two 29–60 days (n=318) | 3.1, 3.0 (0–8.5) | 75.5, 2.0 (0–64.5) | 48.0, 48.0 (30.5–65.5) | 58.9, 54.0 (5.5–105.5) |
| Month Three 61–90 days (n=81) | 3.0, 3.0 (0–8.5) | 31.2, 2.0 (0–30.0) | 57.7, 51.0 (33.5–69.5) | 39.2, 38.0 (7.0–71.0) |
| All ages (n=677) | 4.3, 3.0 (0–12.0) | 78.4, 3.0 (0–85.0) | 48.0, 47.0 (29.5–65.5) | 63.3, 61.0 (0.0–126.5) |
All infants had no evidence of SBI, EV or HSV infection. All infants had atraumatic lumbar punctures with CSF RBC counts < 1000/mm3. Statistical outliers have been removed.
When analyzed by week of age, mean CSF WBC counts during weeks one through four were 6.3/mm3, 6.8/mm3, 6.9/mm3, and 5.0/mm3 respectively. The mean CSF WBC counts during weeks one through three were statistically higher (p< 0.05 for all) than the mean WBC counts observed in later weeks. There were no statistical differences in WBC counts after week four. For this reason, normative data are summarized by month (Table II).
CSF WBC counts were higher in the first month when compared with months two and three (p < 0.001 for both). The mean CSF glucose concentration was lowest and the mean CSF protein concentration was highest for infants in the first month. The CSF glucose value increased significantly in month three (p< 0.001), and the mean CSF protein value decreased in months two and three (both p-values <0.001).
Variation of CSF WBC Count Related to CSF RBC Count
As described above, 823 presumptively uninfected infants had results from full testing of CSF. We analyzed CSF RBC count as a continuous variable in these infants all of whom had CSF RBC counts < 10,000/mm3 to determine the effect of RBC count on CSF WBC count.
Mean and median CSF WBC increased with increasing CSF RBCs in all age groups (p for trend, < 0.001). For infants with CSF RBC < 1000 (n=743), R2=0.012. For infants with a CSF RBC count between 1000–9999 (n=80), R2=0.104. These data indicate that the proportion of variation in the WBCs explained by the presence of RBCs is small (R2=0.034 overall), but the linear association between WBC and RBC count is statistically significant (p<0.001). The mean and median CSF WBC counts in febrile infants with traumatic lumbar punctures and CSF RBC counts < 10,000/mm3 were 6.5/mm3 and 5/mm3, respectively, with a range of 0–16/mm3.
DISCUSSION
We report CSF data from young febrile term infants who all had uniform testing for SBI and enteroviuses. The CSF profiles from infants with no evidence of bacterial or enteroviral infection and who had non-traumatic lumbar punctures were used to establish normative values by age. We determined that infants in the first month have higher CSF WBC counts than those in months two and three. Likewise, infants with traumatic lumbar punctures may have slightly higher values for CSF WBC. However, the mean and median CSF WBC values observed in this study, even in infants with traumatic lumbar puncture, are lower than published norms.(10, 16, 17)
The interpretation of the CSF profile in young infants is challenging. Normal values in pediatric references are based on small sample sizes, with most studies reporting data for fewer than 100 infants.(10, 15–17) Further, commonly cited references may have inadvertently included infants with EV infection or those with UTI, potentially falsely elevating mean CSF WBC counts. We recognized an opportunity to analyze the CSF profiles in a large cohort of febrile infants who had evaluations for sepsis that included testing for SBI and enteroviruses.(27)
We identified CSF WBC counts > 14.5/mm3 as outliers in infants with atraumatic lumbar punctures. Normative values were then determined using only CSF profiles from presumptively uninfected infants with atraumatic lumbar punctures and WBC counts < 14.5/mm3. The analysis demonstrated that infants in the first month had the highest CSF WBC counts, but had a mean of only 6.1/mm3 with an upper limit of 18/mm3. This range is significantly lower than several common references that indicate infants in the first week to month may have CSF WBC counts up to 22/mm3 (16, 43, 44). In fact, our data indicate that even when statistical outliers are included, the range for CSF WBC count for infants in the first month was 0–20.5/mm3, again lower than published norms.
In our study, infants in the first month had a mean CSF WBC count of 6.1/mm3 most similar to those reported in the two of the largest studies identified in the review of the literature, both of which examined CSF from newborns on day one and found mean WBC counts of 6.3/mm3 and 5.0/mm3 respectively.(2, 9) The 272 infants 1–28 days in our study had lower mean WBC (6.1/mm3 vs. 9.1/mm3) and range (0–18/mm3 vs. 0–19/mm3) than the 142 infants of the same age reported by Kestenbaum et al.(19) The lower values seen in our study are likely due to differences in enrollment criteria, viral diagnostic testing, and statistical methodology. It is possible that the Kestenbaum study included infants with enteroviral or other viral infections that were misclassified as uninfected. They note that they included infants with CSF WBC counts “far outside the range most physicians would consider normal” and that these infants may have had pathology.(19) Our results for CSF WBC in the fist month are very similar to the results reported by Kestenbaum et al when statistical outliers were included in our data set (Table I).
In our study, infants in the second and third months had similar CSF WBC counts, within normal range. The mean CSF WBC counts for infants in the second month was 3.1/mm3 and in the third month was 3.0/mm3. Both groups had a median CSF WBC count of 3.0/mm3 and upper limits of 8.5/mm3. These data, in spite of the methodological differences noted above, are very similar to the data reported by Kestenbaum et al for infants 29–56 days of age.(19) The upper range in both our study and in the Kestenbaum study is ~ 9/mm3 and is slightly higher than the range of 0–7/mm3 reported for infants and children older than eight weeks(16, 43, 44).
The contamination of CSF by blood is common in pediatrics, occurring in 20%–30% of lumbar punctures.(28) The interpretation of a blood contaminated CSF profile is difficult. Multiple ratios have been published in an effort to help interpret the CSF WBC in blood-contaminated specimens.(29, 30, 34, 45, 46) When we analyzed the variation of CSF WBC in uninfected infants related to the CSF RBC count, we found a statistically significant increase in the CSF WBCs for infants with traumatic lumbar punctures. However, the CSF RBC contribution to the model was low with an R2 value of only 3.4% overall. These data are similar to those reported by Bonsu et al (42). Our data indicate that for CSF profiles with RBC counts < 10,000/mm3 adjustments to the CSF WBC count are not warranted and that blood in the CSF is not sufficient to explain CSF pleocytosis. CSF WBC counts > 16/mm3 in traumatic lumbar punctures were identified as statistical outliers and represent CSF pleocytosis in young febrile infants.
This study has several limitations. First, it is a single center study. However, our dataset is many times larger than the datasets used to establish modern normal values.(10, 15–17) Second, our rate of traumatic lumbar punctures is lower than that reported by others, thus our ability to evaluate the proportion of variation of the CSF WBC due to the CSF RBC may have been limited. Third, we did not test all infants for herpes simplex virus (HSV), a known cause of CSF pleocytosis. However, no infant included in the analysis had skin lesions or received acyclovir and all were discharged home in good condition, indicating the likelihood of undiagnosed HSV infection was extremely low. Finally, it is possible that infants included in this study were infected with viral pathogens we did not identify and the effect on CSF profiles is unknown. We believe our statistical methodology identifying outliers reduced the influence this factor might have on the data. In spite of these limitations, this study remains one of the largest of CSF profiles from febrile infants reported. We believe the data presented will be useful to clinicians as they evaluate CSF obtained from young infants with fever.
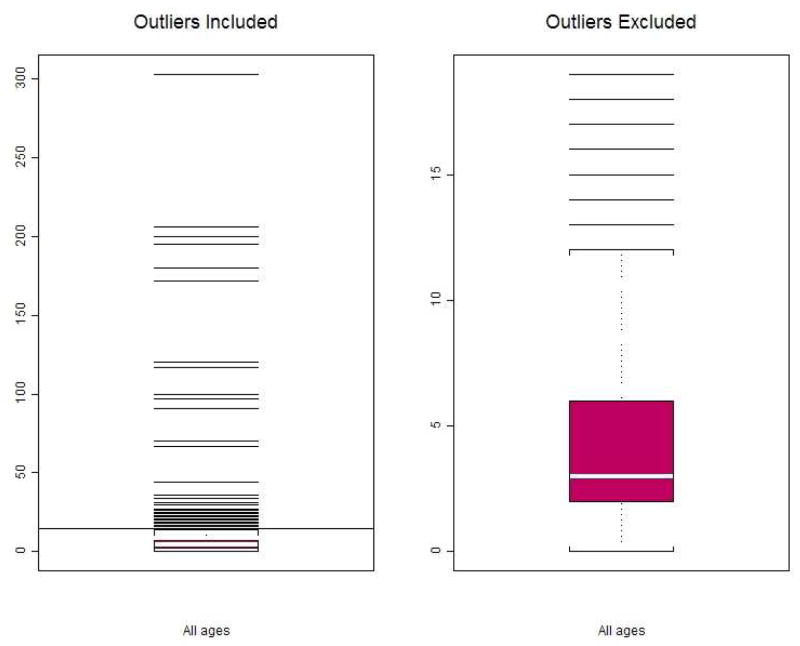
Figure 1.
Left panel: Boxplot of CSF WBC counts in febrile infants 1–90 days. Statistical outliers are included, N=743. Minimum(=0/mm3), Q1(=2/mm3), Median(=4/mm3), and Q3(=7/mm3) and are too close to be distinguished on this plot. The horizontal line at 14.5/mm3 is the cut-point of statistical outliers from all-age group.
(Note the outlier cut-points are different for each age group and for all-age groups combined (as shown Table 1).
Right panel: Boxplot of CSF WBC counts in febrile infants 1–90 days. Statistical outliers for each age groups are excluded, N=677. The cut point for statistical outliers for infants of all ages was 14.5/mm3, however infants in the first 28 days had higher CSF WBC values and outliers were identified as those with CSF WBC counts greater than 20.5/mm3. The box and whiskers represent Minimum (=0/mm3), Q1(=2/mm3), Median(=3/mm3), Q3(=6/mm3), and a few outliers beyond the upper limit of the normal range at 12/mm3.
Acknowledgments
Supported by the Robert Wood Johnson Generalist Physician Faculty Scholar Program (C.B.), National Institute of Child Health and Human Development (K24 HD047249-01A1 to C.B.), and Public Health Services research grant UL1-RR025764 from the National Center for Research Resources.
We thank Drs. Paul C. Young, Chuck Norlin, and Andrew T. Pavia for their careful review of the manuscript.
Abbreviations
- WBC
white blood cell
- RBC
red blood cell
- EV
enteroviruses
- PCR
polymerase chain reaction
- SBI
serious bacterial infection
- IQR
inter quartile range
Footnotes
The authors declare no conflicts of interest.
Portions of the data were presented at the Pediatric Academic Societies Annual Meeting, May 15, 2005, Washington, DC.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baraff LJ. Management of fever without source in infants and children. Ann Emerg Med. 2000;36(6):602–14. doi: 10.1067/mem.2000.110820. [DOI] [PubMed] [Google Scholar]
- 2.Roberts M. The spinal fluid in the newborn with especial reference to intracranial hemorrhage. JAMA. 1925;85:500–3. [Google Scholar]
- 3.Stewart D. The normal cerebrospinal fluid in children. Arch Dis Child. 1928;3:96–108. doi: 10.1136/adc.3.14.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waitz R. Le liquide cephalorachidien du nouveau-ne. Rev Franc Pediatr. 1928;4:1–56. [Google Scholar]
- 5.Samson K. Die liquordiagnostik im kindesaltor. Ergeb Inn Med Kinderheilkd. 1931;41:553. [Google Scholar]
- 6.Otila E. Studies on the cerebrospinal fluid in premature infants. Acta Paediatr. 1948;35 (Suppl):8–100. [Google Scholar]
- 7.Wyers H, Bakker J. De liquor cerebro-spinalis van normale, a terme geboren neonati. Maandschrift Kindergenceskunde. 1954;22:253–63. [PubMed] [Google Scholar]
- 8.Widell S. On the cerebrospinal fluid in normal children and in patients with acute abacterial meningo-encephalitis. Acta Paediatr. 1958;47 (Suppl 115):1–102. [PubMed] [Google Scholar]
- 9.Naidoo BT. The cerebrospinal fluid in the healthy newborn infant. S Afr Med J. 1968;42(35):933–5. [PubMed] [Google Scholar]
- 10.Sarff LD, Platt LH, McCracken GH., Jr Cerebrospinal fluid evaluation in neonates: comparison of high-risk infants with and without meningitis. J Pediatr. 1976;88(3):473–7. doi: 10.1016/s0022-3476(76)80271-5. [DOI] [PubMed] [Google Scholar]
- 11.Vaz FA, Livramento JA, Spina-Franca A. Cerebrospinal fluid in the healthy preterm newborn infant. Arq Neuropsiquiatr. 1977;35(3):183–8. doi: 10.1590/s0004-282x1977000300001. [DOI] [PubMed] [Google Scholar]
- 12.Pappu LD, Purohit DM, Levkoff AH, Kaplan B. CSF cytology in the neonate. Am J Dis Child. 1982;136(4):297–8. doi: 10.1001/archpedi.1982.03970400015004. [DOI] [PubMed] [Google Scholar]
- 13.Statz A, Felgenhauer K. Development of the blood-CSF barrier. Dev Med Child Neurol. 1983;25(2):152–61. doi: 10.1111/j.1469-8749.1983.tb13738.x. [DOI] [PubMed] [Google Scholar]
- 14.Fukunaga FH. Cerebrospinal fluid--a re-examination. Hawaii Med J. 1984;43(6):194, 6, 8. [PubMed] [Google Scholar]
- 15.Portnoy JM, Olson LC. Normal cerebrospinal fluid values in children: another look. Pediatrics. 1985;75(3):484–7. [PubMed] [Google Scholar]
- 16.Bonadio WA, Stanco L, Bruce R, Barry D, Smith D. Reference values of normal cerebrospinal fluid composition in infants ages 0 to 8 weeks. Pediatr Infect Dis J. 1992;11(7):589–91. doi: 10.1097/00006454-199207000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed A, Hickey SM, Ehrett S, Trujillo M, Brito F, Goto C, et al. Cerebrospinal fluid values in the term neonate. Pediatr Infect Dis J. 1996;15(4):298–303. doi: 10.1097/00006454-199604000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Nascimento-Carvalho CM, Moreno-Carvalho OA. Normal cerebrospinal fluid values in full-term gestation and premature neonates. Arq Neuropsiquiatr. 1998;56(3A):375–80. doi: 10.1590/s0004-282x1998000300005. [DOI] [PubMed] [Google Scholar]
- 19.Kestenbaum LA, Ebberson J, Zorc JJ, Hodinka RL, Shah SS. Defining cerebrospinal fluid white blood cell count reference values in neonates and young infants. Pediatrics. 2010;125(2):257–64. doi: 10.1542/peds.2009-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wald ER. Aseptic meningitis and urinary infection. Pediatr Infect Dis J. 2004;23(5):480. doi: 10.1097/00006454-200405000-00026. author reply -1. [DOI] [PubMed] [Google Scholar]
- 21.Syrogiannopoulos GA, Grivea IN, Anastassiou ED, Triga MG, Dimitracopoulos GO, Beratis NG. Sterile cerebrospinal fluid pleocytosis in young infants with urinary tract infection. Pediatr Infect Dis J. 2001;20(10):927–30. doi: 10.1097/00006454-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Adler-Shohet FC, Cheung MM, Hill M, Lieberman JM. Aseptic meningitis in infants younger than six months of age hospitalized with urinary tract infections. Pediatr Infect Dis J. 2003;22(12):1039–42. doi: 10.1097/01.inf.0000100576.99266.07. [DOI] [PubMed] [Google Scholar]
- 23.Shah SS, Zorc JJ, Levine DA, Platt SL, Kuppermann N. Sterile cerebrospinal fluid pleocytosis in young infants with urinary tract infections. J Pediatr. 2008;153(2):290–2. doi: 10.1016/j.jpeds.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 24.Yam AO, Andresen D, Kesson AM, Isaacs D. Incidence of sterile cerebrospinal fluid pleocytosis in infants with urinary tract infection. J Paediatr Child Health. 2009 doi: 10.1111/j.1440-1754.2009.01502.x. [DOI] [PubMed] [Google Scholar]
- 25.Finkelstein Y, Mosseri R, Garty BZ. Concomitant aseptic meningitis and bacterial urinary tract infection in young febrile infants. Pediatr Infect Dis J. 2001;20(6):630–2. doi: 10.1097/00006454-200106000-00019. [DOI] [PubMed] [Google Scholar]
- 26.Baraff LJ, Oslund SA, Schriger DL, Stephen ML. Probability of bacterial infections in febrile infants less than three months of age: a meta-analysis. Pediatr Infect Dis J. 1992;11(4):257–64. doi: 10.1097/00006454-199204000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Byington CL, Enriquez FR, Hoff C, Tuohy R, Taggart EW, Hillyard DR, et al. Serious bacterial infections in febrile infants 1 to 90 days old with and without viral infections. Pediatrics. 2004;113(6):1662–6. doi: 10.1542/peds.113.6.1662. [DOI] [PubMed] [Google Scholar]
- 28.Baxter AL, Fisher RG, Burke BL, Goldblatt SS, Isaacman DJ, Lawson ML. Local anesthetic and stylet styles: factors associated with resident lumbar puncture success. Pediatrics. 2006;117(3):876–81. doi: 10.1542/peds.2005-0519. [DOI] [PubMed] [Google Scholar]
- 29.Novak RW. Lack of validity of standard corrections for white blood cell counts of blood-contaminated cerebrospinal fluid in infants. Am J Clin Pathol. 1984;82(1):95–7. doi: 10.1093/ajcp/82.1.95. [DOI] [PubMed] [Google Scholar]
- 30.Rubenstein JS, Yogev R. What represents pleocytosis in blood-contaminated (“traumatic tap”) cerebrospinal fluid in children? J Pediatr. 1985;107(2):249–51. doi: 10.1016/s0022-3476(85)80137-2. [DOI] [PubMed] [Google Scholar]
- 31.Mehl AL. Interpretation of traumatic lumbar puncture. A prospective experimental model. Clin Pediatr (Phila) 1986;25(10):523–6. doi: 10.1177/000992288602501008. [DOI] [PubMed] [Google Scholar]
- 32.Bonadio WA, Smith DS, Goddard S, Burroughs J, Khaja G. Distinguishing cerebrospinal fluid abnormalities in children with bacterial meningitis and traumatic lumbar puncture. J Infect Dis. 1990;162(1):251–4. doi: 10.1093/infdis/162.1.251. [DOI] [PubMed] [Google Scholar]
- 33.Mazor SS, McNulty JE, Roosevelt GE. Interpretation of traumatic lumbar punctures: who can go home? Pediatrics. 2003;111(3):525–8. [PubMed] [Google Scholar]
- 34.Mayefsky JH, Roghmann KJ. Determination of leukocytosis in traumatic spinal tap specimens. Am J Med. 1987;82(6):1175–81. doi: 10.1016/0002-9343(87)90221-x. [DOI] [PubMed] [Google Scholar]
- 35.Barnett V, Lewis T. Outliers in Statistical Data. Wiley; 1994. [Google Scholar]
- 36.Iglewicz B, Hoaglin D. How to Detect and Handle Outliers. Milwaukee, WI: 1993. [Google Scholar]
- 37.Smithson M. Quantitative Applications in the Social Sciences Series. Belmont, CA: SAGE Publications; 2003. Confidence intervals. [Google Scholar]
- 38.Sheskin D. Handbook of Parametric and Nonparametric Statistical Procedures. Chapman and Hall/CRC; 2007. [Google Scholar]
- 39.Harvala H, Robertson I, Chieochansin T, McWilliam Leitch EC, Templeton K, Simmonds P. Specific association of human parechovirus type 3 with sepsis and fever in young infants, as identified by direct typing of cerebrospinal fluid samples. J Infect Dis. 2009;199(12):1753–60. doi: 10.1086/599094. [DOI] [PubMed] [Google Scholar]
- 40.Verboon-Maciolek MA, Krediet TG, Gerards LJ, de Vries LS, Groenendaal F, van Loon AM. Severe neonatal parechovirus infection and similarity with enterovirus infection. Pediatr Infect Dis J. 2008;27(3):241–5. doi: 10.1097/INF.0b013e31815c1b07. [DOI] [PubMed] [Google Scholar]
- 41.Hall CB, Long CE, Schnabel KC, Caserta MT, McIntyre KM, Costanzo MA, et al. Human herpesvirus-6 infection in children. A prospective study of complications and reactivation. N Engl J Med. 1994;331(7):432–8. doi: 10.1056/NEJM199408183310703. [DOI] [PubMed] [Google Scholar]
- 42.Bonsu BK, Harper MB. Corrections for leukocytes and percent of neutrophils do not match observations in blood-contaminated cerebrospinal fluid and have no value over uncorrected cells for diagnosis. Pediatr Infect Dis J. 2006;25(1):8–11. doi: 10.1097/01.inf.0000195624.34981.36. [DOI] [PubMed] [Google Scholar]
- 43.Rowe PC. Laboratory Values. In: DeAngelis C, Feigin R, Warshaw J, editors. Principles and Practice of Pediatrics. Philadelphia: JB Lippincott Company; 1990. p. 1980. [Google Scholar]
- 44.Choukair M. Blood Chemistries/Body Fluids. In: Siberry G, Iannone R, editors. The Harriet Lane Handbook. 15. St Louis, MI: Mosby; 2000. [Google Scholar]
- 45.Osborne JP, Pizer B. Effect on the white cell count of contaminating cerebrospinal fluid with blood. Arch Dis Child. 1981;56(5):400–1. doi: 10.1136/adc.56.5.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feigin R. Bacterial Meningitis Beyond the Newborn Period. In: DeAngelis C, Feigin R, Warshaw J, editors. Principles and Practice of Pediatrics. Philadelphia: JB Lippincott Company; 1990. p. 1031. [Google Scholar]