Abstract
The transmembrane (TM) domains of receptor tyrosine kinases (RTKs) are believed to be important players in RTK signal transduction. However, the degree of specificity and promiscuity of RTK TM domain lateral interactions in mammalian membranes has not been assessed in detail in the literature. A technique to probe the occurrence of interactions between TM domains and their biological significance is to evaluate the propensity for formation of heterodimers of a full-length RTK and its TM domain. Here we examine if specific inhibition of two RTK pathogenic mutants, Neu/V664E and FGFR3/A391E, can be achieved by the TM domains of Neu, Neu/V664E, FGFR3 and FGFR3/A391E. We show that the TM domain of Neu/V664E specifically inhibits the phosphorylation of full-length Neu/V664E, while the wild-type Neu TM domain does not. In addition, Neu/V664E TM domain does not affect the phosphorylation levels of full-length FGFR3/A391E. The results suggest that TM domain peptides could be exploited in the future for the development of specific inhibitors of mutant RTKs.
Introduction
RTKs are single-pass transmembrane (TM) proteins which are composed of four distinct domains: an extracellular (ligand-binding) domain, a single TM domain, a juxtamembrane, and an intracellular catalytic domain. They transduce biochemical signals via lateral dimerization in the plasma membrane. The dimerization process is controlled by the presence of ligands [1], which stabilize the RTK dimers upon binding to their extracellular domains. RTK dimerization is tightly linked to RTK activity, because the contact between the two catalytic domains in the dimer stimulates catalytic activity, and triggers signaling cascades [2–4].
The role of the TM domains in the dimerization process has been highly controversial. While in some studies TM domains have had negligible effects on signaling, suggesting that the TM domains are passive anchors [5, 6], in other cases changes in the sequences of the TM domains have affected signaling [7, 8]. Studies of the isolated TM domains in lipid bilayers or bacterial membranes have showed that RTK domains can dimerize by themselves [9–13], suggesting that the TM domains contribute to the energetics of RTK dimerization. Most of these studies have utilized genetic two-hybrid assays (ToxR, TOXCAT, GALLEX) that measure the interaction of membrane spanning helices linking a periplasmic maltose binding protein (MBP) with a cytosolic DNA-binding domain that is activated upon dimerization [9, 14–17]. In addition, FRET-based dimerization measurements [18–20] of the isolated TM domains of ErbB1, FGFR3 and EphA1 [10, 11, 21] have demonstrated that these TM domains can form homodimers in lipid bilayers[10, 11, 21]. Another very strong argument for the important roles of the TM domains in signaling is the finding that pathogenic mutations in TM domains promote ligand-independent dimerization and cause disease [22–25]. In model systems such pathogenic mutants can exhibit higher dimerization propensity than the wild-type RTK TM domains [12, 25, 26].
While dimerization between isolated TM domains has been shown to occur in model systems, and to be enhanced due to pathogenic single amino acid mutations, direct demonstrations of dimerization of isolated RTK TM domains in mammalian membranes is lacking. Here we employ chemical cross-linking and verify that dimerization between isolated RTK TM domains can occur in mammalian membranes. Another technique to probe the occurrence of interactions between TM domains and their biological significance is to evaluate the propensity for formation of heterodimers of a full-length RTK and its TM domain [27–29]. The presence of such heterodimers, which are inactive, is expected to decrease RTK phosphorylation if RTK TM domain interactions are significant for biological function. This approach could also be developed into a targeted treatment strategy [29]. However, this strategy will be viable only if the interactions between RTK TM domains are highly specific (i.e. homodimerization strengths greatly exceed heterodimerization strengths). The question of specificity, however, has not been investigated much in the literature. One paper reports that the phosphorylation level of EGFR can only be inhibited by the TM domain of EGFR, but not the TM domains of an EGFR mutant, ErbB2 or the insulin receptor [27]. A second study suggests that the activation of ErbB2 can only be targeted by its own TM domain [30]. Yet, others argue that RTK interactions are, in fact, quite promiscuous [31]. For instance, many RTK TM domains have GxxxG-like interaction motifs and capabilities for hydrogen bonding with backbone donors and acceptors that in principle could drive promiscuous TM domain heterodimerization [9, 25, 32].
Of the different 59 RTKs found in humans, two RTK sub-families have been studied most extensively: ErbB (ErbB1, ErbB2, ErbB3, and ErbB4) and FGFR (FGFR1, FGFR2, FGFR3, and FGFR4). ErbBs play a role in cancer progression and metastasis [33–35]. On the other hand, FGFRs have been linked to skeletal dysplasias, craniosynostoses, and cancer [36–39]. Many of these abnormalities have been linked to mutations in the TM domains of these receptors. One such mutation is the V664E mutation in rat Neu/ErbB2, shown to be oncogenic [40, 41]. In humans, a different mutation to glutamic acid, A391E in FGFR3, has been identified as a germ-line mutation in Crouzon syndrome with acanthosis nigricans [24] and as a somatic mutation in bladder cancer [38]. Both mutations are believed to stabilize the mutant RTK dimers via hydrogen bonding between the two TM domains [42]. Since both Neu and FGFR3 TM domains have GxxxG-motifs and amundant moieties with hydrogen bonding capabilities (every peptide bond in a TM helix can serve as hydrogen bond donor or acceptor), a question arises as to whether promiscuous hetero-interactions may occur between these two TM domains in the plasma membrane, or whether alternatively these TM domains engage only in specific homodimerization interactions in the plasma membrane. To start addressing these important questions of specificity and promiscuity in cellular membranes, here we examine if specific inhibition of the two pathogenic mutants, Neu/V664E and FGFR3/A391E, can be achieved by the TM domains of Neu, Neu/V664E, FGFR3 and FGFR3/A391E.
While some of the experiments involving FGFR3 TM domains are inconclusive due to the impaired trafficking of these TM domains to the plasma membrane, here we show that the TM domain of Neu/V664E has a modest, yet specific inhibitory effect on the phosphorylation of full-length Neu/V664E. In addition, Neu/V664E TM domain does not affect the phosphorylation levels of full-length FGFR3/A391E, indicating that the interactions between mutant RTKs and their TM domains are specific. Thus, the mutant TM domain peptides could be exploited in the development of specific inhibitors of mutant RTKs.
Materials and Methods
Cell Culture
B104-1-1 cells, a NIH 3T3 transfectant that stably expresses the Neu/V664E mutant, were cultured in DMEM (Invitrogen, CA) supplemented with 10% Bovine Calf Serum (Hyclone Laboratories, UT). Human Embryonic Kidney (HEK) 293 cells were cultured in DMEM supplemented with 10% Fetal Bovine Serum (FBS). Cells were maintained in an incubator with 5% CO2 at 37°C.
Construction of Plasmids Encoding RTK TM domains
Genes encoding the TM sequences of Fibroblast growth factor receptor 3 (FGFR3), FGFR3/A391E, Neu and Neu/V664E were inserted in a pcDNA 3.1(+) vector (Invitrogen, CA) by ligation of the transmembrane PCR products using the HindIII and XbaI restriction sites. The final sequences contained the FGFR3 signal peptide, a VSV-G-tag and the sequences of the TM domains.
Transfection and Immunostaining
Transfection of the plasmids into B104-1-1 cells was performed using Lipofectamine™ 2000 transfection reagent (Invitrogen, CA) according to the manufacturer’s protocol. Transfection of the plasmids into HEK 293 cells was performed using Fugene HD (Roche Applied Science, IN). Cells were cultured in the incubator for 24 hours after transfection. After being fixed by 3% paraformaldehyde (PFA), cells were blocked using bovine serum albumin (BSA) for 1 hour. Cells were then incubated with anti-VSV-G antibodies (Sigma-Aldrich, MO) overnight at 4°C. The secondary antibody used was Alexa Fluor 647 goat anti rabbit IgG (H+L) (Invitrogen, CA).
Western Blotting
After culturing the cells in the incubator for 24 hours following transfection, cells were lysed with lysis buffer (25 mM Tris-HCl, 0.5% TritonX-100, 20 mM NaCl, 2 mM EDTA, 2 mM NaVO4 and protease inhibitor (Roche Applied Science, IN). After spinning at 15,000g for 15 minutes at 4°C, the supernatant was collected and the pellet was discarded. Protein concentration was determined using the BCA™ assay kit (Pierce, IL). The lysate was loaded into 3–8% NuPAGE® Novex® Tris-Acetate mini gels (Invitrogen, CA). Proteins were separated by electrophoresis, and transferred onto a nitrocellulose membrane. The membrane was blocked with milk for one hour at room temperature, and then stained with one of the following antibodies: anti-C-Neu (Santa Cruz Technology, CA), anti-N-FGFR3 (Santa Cruz Technology, CA), anti-p-ErbB2 (Cell Signaling Technology, MA), anti-p-FGFR3 (Cell Signaling Technology, MA) and anti-VSV-G antibodies (Sigma-Aldrich, MO). The secondary antibodies were anti-mouse or anti-rabbit HRP conjugated antibody (Promega, WI). ECL™ detection reagent (GE Healthcare life sciences, UK) was used to reveal the bands on the films. The bands were quantified using ImageQuant TL (GE Healthcare life sciences, UK).
Cross-linking of TM domains using membrane permeable linkers
Twenty-four hours after transfection, cells were incubated in 2 mM Ethylene Glycol Bis (sulfosuccinimidyl) substrate (EGS, Pierce, IL) for one hour at room temperature, and then quenched in 20 mM Tris-HCl for 15 minutes. After two rinses with ice-cold PBS, cells were lysed as described above.
Quantification of Western blots
The western blot films were scanned and processed with ImageQuant TL. At least three sets of independent experiment were performed in order to determine the average and the standard deviations. The Student T-test was performed to analyze statistical significance.
Creation of stable HEK 293 cell lines expressing FGFR3/A391E
HEK 293 cells were transfected with plasmids encoding FGFR3/A391E. Twenty-four hours after transfection, culture medium was replaced by normal HEK 293 medium supplemented with 0.6 µg/ml Geneticin® (G418 from Invitrogen, CA). Two or three days later, the cells which grew to about 90% confluency were passed and seeded with high dilution ratio into a 150mm cell culture dish. The medium with antibiotics was replaced every two to three days until colonies of cells appeared around two weeks after the initial seeding.
Statistical analysis
Data were analyzed for significance using the Student t-test in Microsoft Excel to compare the measurements to a null hypothesis. The p-value cutoff for significance was determined using the Bonferroni correction for multiple comparisons. ANOVA was used to compare results for the four different TM domains.
Results and Discussions
Expression of Neu and FGFR3 transmembrane domains
PCR products encoding the transmembrane domains (with amino acid sequences shown in Figure 1A) were cloned into the pcDNA 3.1+ vector. All constructed TM plasmids contained a Vesicular Stomatitis Virus glycoprotein (VSV-G) tag (YTDIEMNRLGK) at the N-terminus (Figure 1B). The VSV-G tag comprises amino acids 501–511 of the Vesicular Stomatitis Virus glycoprotein and has been widely used as a tag in expression vectors [43, 44]. The engineered VSV-G tag allowed the detection of the TM domains on the cell surface using an anti-VSV-G antibody. The construct also contained the signal peptide of FGFR3 at the N-terminus, to direct the proteins to the plasma membrane.
Figure 1. Plasmids encoding Neu and FGFR3 TM domains.
(A) The amino acid sequences of FGFR3, FGFR3/A391E, Neu and Neu/V664E TM domains. The amino acids in the hydrophobic hydrocarbon core - embedded domain are underlined. The mutant amino acids (E) are shown in italic. (B) Structure of the constructed genes, containing the FGFR3 signal peptide at the N-terminus, a VSV-G tag for probing expression, and the TM domains.
In order to confirm the expression of the transmembrane domains in the cells and their localization on the cell membrane, HEK 293 and B104-1-1 cells were transfected with the four plasmids encoding the TM domains of FGFR3, FGFR3/A391E, Neu and Neu/V664E. Twenty-four hours after transfection, the membrane localization of the expressed proteins was probed using anti-VSV-G antibodies followed by fluorescently-tagged secondary antibodies. The confocal microscope images in Figure 2 show the staining patterns of the expressed TM domains in HEK 293 cells (A–D). All four TM domains could be detected on the cell surface. However, the cells transfected with the plasmids encoding FGFR3/WT TM domain and FGFR3/A391E TM domain showed lower percentage of stained cells compared with those cells transfected with the Neu/WT and Neu/V664E TM domain plasmids. Different versions of FGFR3 TM domains were designed (not shown here) containing extracellular and cytoplasmic sequences of different lengths, and they exhibited similar low propensities for membrane localization, significantly lower than the propensities of Neu and Neu/V664E TM domains. These results for HEK 293 cells were similar to results for B104-1-1 cells, with Neu and Neu/V664E TM domains more abundant on the cell surface than FGFR3 and FGFR3/A391E TM domain (not shown).
Figure 2.
Immunofluorescent staining of the TM domains in HEK 293 cells. (A). FGFR3 TM domain. B. FGFR3/A391E TM domain. (C). Neu TM domain. (D). Neu/V664E TM domain. Intact cells were stained with anti-VSV-G antibodies, followed by Alexa Fluor 647 goat anti rabbit IgG (H+L). Images were acquired with a Nikon confocal laser scanning microscope. Neu TM and Neu/V664E TM express at the cell surface with higher efficiency, only a small fraction of cells express FGFR3 and FGFR3/A391E TM domains in their plasma membrane. These results, along with results shown in Figure 3 suggest that the FGFR3 TM domains are not trafficked efficiently to the cell surface. Similar results were observed for B104-1-1 cells (not shown).
The poor expression of FGFR3 TM domains on the cell surface was unexpected because all four plasmids were very similar, with identical signal peptide sequences and TM domains of very similar hydrophobicity. Thus, a question arised if the lower FGFR3 TM domain concentration on the cell surface was due to overall lower expression in cells or due to impeded trafficking of FGFR3 TM domains to the cell surface. To answer this question, we assessed the expression of the four TM domains in B104-1-1 and HEK 293 cells using Western blotting. In Figure 3, we see bands at about 6 kDa that are reactive to the VSV-G tag. The FGFR3/A391E and Neu/V664E TM domain bands appeared at lower apparent molecular weights than the wild-type bands, probably due to the negative charge of the glutamic acid in FGFR3/A391E and Neu/V664E. We see that while the expression of FGFR3/A391E TM domain was lower than that of FGFR3/WT domain, there was no significant difference in the expression levels of FGFR3/A391E, Neu/WT and Neu/V664E TM domains. Thus, the observed difference in immunostaining patterns in Figure 2 was not due to differences in overall expression. Instead, it appears that the FGFR3 TM domains were not being trafficked efficiently to the cell surface.
Figure 3.
Western Blot analysis of the expression of the TM domains in B104-1 cells (A) and HEK 293 cells (B). Cells were transfected with the same amount (1 µg of plasmid per well) of pcDNA3.1+ plasmids encoding the TM domain of FGFR3 (lane 1), FGFR3/A391E (lane 2), Neu (lane 3) and Neu /V664E (lane 4). Cells were lysed 24 hours after transfection. The proteins were separated by SDS-PAGE gel and detected with anti-VSV-G antibodies. The expression of the four TM constructs is comparable.
Dimerization of transmembrane domains in cellular membranes
The overall goal of this study was to assess the potential inhibitory effects of the TM domains on the activation of Neu/V664E and FGFR3/A391E. While these peptides dimerize in model systems [9, 12, 13, 17], their dimerization in mammalian membranes has not been demonstrated directly. Therefore, we first sought to prove that the transmembrane domains have a propensity to dimerize in cellular membranes using chemical cross-linking, a technique that is widely used to assess dimerization propensities [22, 45, 46]. We used a membrane permeable chemical cross-linker, Ethylene glycol succinimidyl succinate (EGS), to cross-link the TM domains within all the cellular membranes, not only the plasma membrane [42]. Figure 4 shows the Western blot result from such a cross-linking experiment. Two different bands were observed on the gel. One band appeared at about 6 kDa, corresponding to the monomeric TM domains, and another appeared at approximately 12 kDa, corresponding to the dimeric TM domains. These results suggest that the four TM domains can dimerize in cells. We quantified the intensities of the monomeric and dimeric bands, calculating the ratios R=(dimer)/(monomer)2 for the wild-types and the mutants. To correctly quantify both the weak dimeric bands and the strong monomeric bands, we exposed the film for a short period of time, quantified the monomeric bands, re-exposed the film for a longer time, and quantified the dimeric bands. Thus, the band intensities were within the so-called linear range, proportional to the protein concentrations. Detailed discussion of how to ensure that the data are in the linear range is given in the Supplement to our previous paper [42]. The effect of the mutations was quantified as RNeu/V664E/RNeu=1.7 and RFGFR3/A391E/RFGFR3=1.5. Thus, the mutations increase the cross-linking propensities of the two TM domains.
Figure 4.
Western blot analysis of the TM domains after chemical cross-linking. B104-1-1 cells were transfected with plasmids encoding the TM domains of FGFR3 (lane 1), FGFR3/A391E (lane 2), Neu (lane 3) and Neu/V664E (lane 4). Twenty-four hours after transfection, cells were subjected to cross-linking, followed by lysis and Western blotting. Dimeric bands were observed for all constructs. The cross-linker used was Ethylene glycol succinimidyl succinate (EGS), a membrane permeable linker that cross-links proteins within all cellular membranes [42].
Inhibition of Neu/V664E phosphorylation in stable B104-1-1 cells
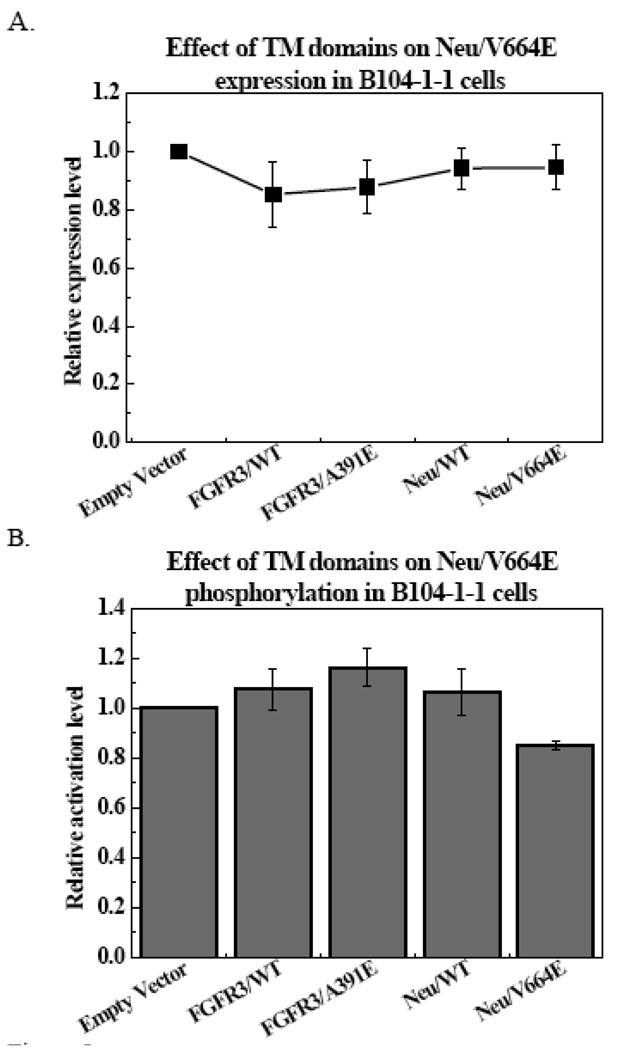
B104-1-1 cells, which stably express Neu/V664E, were used to investigate the effect of the TM domains on the activation of Neu/V664E [47]. As shown in our previous work, the activation of RTKs is dependent on their expression level [42]. Thus, phosphorylation levels in the presence and in the absence of the TM domains can be compared only when the expression levels of the receptors are similar, necessitating the use of cells that stably express the receptor. We first verified that the expression level of Neu/V664E in the stable line is not affected by the expression of the TM constructs. Figure 5A shows the effect of the expression of the TM domains on Neu/V664E expression. These are the results from six independent experiments for each TM domain. We used the Student t-test in Microsoft Excel to compare the measurements to a null hypothesis of one (no effect of the TM domains). The p-values were 0.33, 0.073, 0.23 and 0.87 for the TM domains of FGFR3, FGFR3/A391E, Neu, and Neu/V664E, respectively. These large p-values demonstrated that the transient expression of the TM domains in B104-1-1 stable cells did not have a statistically significant effect on the expression level of Neu/V664E.
Figure 5.
Effect of the TM domains on the expression and phosphorylation of Neu/V664E in stable cells. Results shown are average of six sets of independent experiments. Values in cells transfected with empty pcDNA 3.1 vectors are assigned as one. The phosphorylation of Neu/V664E in stable cell lines can be specifically inhibited by the TM domain of Neu/V664E.
Figure 5B shows the phosphorylation of Neu/V664E in the presence of the four TM domains, together with the standard errors. These are the averages of six independent experiments. Again, we used the Student t-test to compare the measurements to a null hypothesis of one (no inhibition by the TM domains). The p-values obtained were 0.43, 0.083, 0.52 and 0.0006 for the TM domains of FGFR3, FGFR3/A391E, Neu, and Neu/V664E, respectively. To evaluate the significance, we used the Bonferroni correction for multiple comparisons, such that the p-value cutoff for significance was 0.013, not 0.05. Thus, only the Neu/V664E TM domain exhibited a statistically significant effect, reducing the phosphorylation of Neu/V664E.
Next, we compared the effects of the four TM domains to each other using ANOVA. We found a significant p-value of 0.041, demonstrating that the effects of the four TM domains are not the same, with the Neu/V664E TM domain data set exhibiting the largest difference. The ANOVA analysis further confirmed the conclusion that Neu/V664E specifically reduced the phosphorylation of Neu/V664E. In contrast, the changes in the phosphorylation due to the presence of FGFR3, FGFR3/A391E and Neu TM domains were not statistically significant. Importantly, our results showed that Neu/V664E could be inhibited by Neu/V664E TM domain, but not by Neu TM domain. This finding is indicative of specific inhibition, and suggests that heterodimers of Neu and Neu/V664E form with lower probability than Neu/V664E homodimers.
These experiments, however, could not elucidate the effect of FGFR3 and FGFR3/A391E TM domains on the phosphorylation of Neu/V664E. These peptides were not trafficked to the plasma membranes of B104-11 cells efficiently, and thus the observed lack of inhibition could be due to the low concentration of the FGFR3 TM domains in the plasma membrane, rather than the lack of specific interactions between them and Neu/V664E.
In Figure 5, the average effect of Neu/V664E TM domain on Neu/V664E phosphorylation was about 15% reduction (the largest reduction observed in an experiment was about 20%). Thus, the effect was modest. Yet, it may be important for biological function. For instance, the A391E mutation was found to increase the activation of a chimeric Neu/FGFR3 receptor by no more than 40%, as compared to wild-type (1). Yet, this mutation is pathogenic none the less. Thus, an inhibitory effect of 15–20 % may be non-trivial.
Inhibition of FGFR3/A391E phosphorylation in stable cells
A stable cell line expressing FGFR3/A391E was created using the protocol described in Methods. The four TM domains were transiently expressed in these cells. 24 hours after transfection, the cells were starved for another 24 hours, and then lysed and analyzed using Western blotting. The expression and the activation of FGFR3/A391E were probed with anti-N-FGFR3 (Santa Cruz Technology) and anti-p-FGFR3 (Cell Signaling Technology) antibodies, respectively. The expression of the TM domains was probed with anti-VSV-G antibodies (Sigma-Aldrich). In Figure 6A, we show the effect of the four TM domains on FGFR3/A391E expression. The statistical analysis for significance revealed that none of the TM domains affected the expression of FGFR3/A391E. In Figure 6B, we show the effect of the TM domains on FGFR3/A391E phosphorylation. The statistical analysis, using the same approaches employed to analyze the data in Figure 4, demonstrated that none of the TM domains, including the TM domain of FGFR3/A391E, decreased the phosphorylation of FGFR3/A391E significantly. This, however, may be due to the fact that FGFR3 TM domains are not trafficked efficiently to the plasma membrane of HEK 293 cells.
Figure 6.
Effect of the TM domains on the expression and phosphorylation of FGFR3/A391E in stable cells. Results shown here are average of three sets of independent experiments. Values in cells transfected with empty pcDNA 3.1 vectors are assigned as one. None of the TM domains could decrease the phosphorylation of FGFR3/A391E.
Importantly, Neu/V664E TM domain is efficiently trafficked to the cell membrane and does not reduce the phosphorylation of FGFR3/A391E. Thus, Neu/V664E TM domain does not interact with the unrelated FGFR3/A391E despite its ability to form hydrogen bonds with the backbone of a neighboring helix. This finding further confirms that the inhibitory activity of Neu/V664E TM domain is highly specific. Overall, the results that we presented here suggest that TM domain peptides could be exploited in the future for the development of specific inhibitors of mutant RTKs. However, the inhibitory effect that we observed was rather modest, and thus the search for potent inhibitors may require optimization of RTK TM domain sequences to increase their dimerization propensities, without affecting the specificity of their interactions.
Conclusion
In this study, we explored whether the phosphorylation of two pathogenic RTK mutants, Neu/V664E and FGFR3/A391E, could be reduced due to interactions of these receptors with their isolated TM domains. The results pertaining to FGFR3/A391E TM domain were inconclusive, since it was not trafficked efficiently to the cell membrane. However, we demonstrated that Neu/V664E TM domain can specifically inhibit the phosphorylation of Neu/V664E, whereas the wild-type Neu TM domain cannot. Furthermore, the TM domain of Neu/V664E did not engage in promiscuous interactions with FGFR3/A391E. Overall, this study suggests that the interactions between RTK TM domains are specific despite the common structural motives, and thus could be exploited in the development of specific inhibitors of mutant RTKs. However, the inhibitory effect is modest, and optimization of the RTK TM sequences may be needed in order to develop better RTK inhibitors.
Acknowledgement
Supported by NIH GM068619
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schlessinger J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell. 2002;110:669–672. doi: 10.1016/s0092-8674(02)00966-2. [DOI] [PubMed] [Google Scholar]
- 2.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Schlessinger J. Common and distinct elements in cellular signaling via EGF and FGF receptors. Science. 2004;306:1506–1507. doi: 10.1126/science.1105396. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Gureasko J, Shen K, Cole PA, Kuriyan J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell. 2006;125:1137–1149. doi: 10.1016/j.cell.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Kashles O, Szapary D, Bellot F, Ullrich A, Schlessinger J, Schmidt A. Ligand-induced stimulation of epidermal growth factor receptor mutants with altered transmembrane regions. Proc Natl Acad Sci U S A. 1988;85:9567–9571. doi: 10.1073/pnas.85.24.9567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carpenter CD, Ingraham HA, Cochet C, Walton GM, Lazar CS, Sowadski JM, Rosenfeld MG, Gill GN. Structural analysis of the transmembrane domain of the epidermal growth factor receptor. J Biol Chem. 1991;266:5750–5755. [PubMed] [Google Scholar]
- 7.Petti LM, Irusta PM, DiMaio D. Oncogenic activation of the PDGF beta receptor by the transmembrane domain of p185neu*. Oncogene. 1998;16:843–851. doi: 10.1038/sj.onc.1201590. [DOI] [PubMed] [Google Scholar]
- 8.Cheatham B, Shoelson SE, Yamada K, Goncalves E, Kahn CR. Substitution of the erbB-2 oncoprotein transmembrane domain activates the insulin receptor and modulates the action of insulin and insulin-receptor substrate 1. Proc Natl Acad Sci U S A. 1993;90:7336–7340. doi: 10.1073/pnas.90.15.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mendrola JM, Berger MB, King MC, Lemmon MA. The single transmembrane domains of ErbB receptors self-associate in cell membranes. J Biol Chem. 2002;277:4704–4712. doi: 10.1074/jbc.M108681200. [DOI] [PubMed] [Google Scholar]
- 10.Artemenko EO, Egorova NS, Arseniev AS, Feofanov AV. Transmembrane domain of EphA1 receptor forms dimers in membrane-like environment. Biochim Biophys Acta. 2008;1778:2361–2367. doi: 10.1016/j.bbamem.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Merzlyakov M, Cohen T, Shai Y, Hristova K. Energetics of ErbB1 transmembrane domain dimerization in lipid bilayers. Biophys J. 2009;96:4622–4630. doi: 10.1016/j.bpj.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li E, You M, Hristova K. FGFR3 dimer stabilization due to a single amino acid pathogenic mutation. J Mol Biol. 2006;356:600–612. doi: 10.1016/j.jmb.2005.11.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finger C, Escher C, Schneider D. The single transmembrane domains of human receptor tyrosine kinases encode self-interactions. Sci Signal. 2009;2:ra56. doi: 10.1126/scisignal.2000547. [DOI] [PubMed] [Google Scholar]
- 14.Langosch D, Brosig B, Kolmar H, Fritz HJ. Dimerisation of the glycophorin A transmembrane segment in membranes probed with the ToxR transcription activator. J Mol Biol. 1996;263:525–530. doi: 10.1006/jmbi.1996.0595. [DOI] [PubMed] [Google Scholar]
- 15.Ruan W, Becker V, Klingmuller U, Langosch D. The interface between self-assembling erythropoietin receptor transmembrane segments corresponds to a membrane-spanning leucine zipper. J Biol Chem. 2004;279:3273–3279. doi: 10.1074/jbc.M309311200. [DOI] [PubMed] [Google Scholar]
- 16.Gerber D, Sal-Man N, Shai Y. Two motifs within a transmembrane domain, one for homodimerization and the other for heterodimerization. J Biol Chem. 2004;279:21177–21182. doi: 10.1074/jbc.M400847200. [DOI] [PubMed] [Google Scholar]
- 17.Escher C, Cymer F, Schneider D. Two GxxxG-like motifs facilitate promiscuous interactions of the human ErbB transmembrane domains. J Mol Biol. 2009;389:10–16. doi: 10.1016/j.jmb.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 18.You M, Li E, Wimley WC, Hristova K. Forster resonance energy transfer in liposomes: measurements of transmembrane helix dimerization in the native bilayer environment. Anal Biochem. 2005;340:154–164. doi: 10.1016/j.ab.2005.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merzlyakov M, You M, Li E, Hristova K. Transmembrane helix heterodimerization in lipid bilayers: probing the energetics behind autosomal dominant growth disorders. J Mol Biol. 2006;358:1–7. doi: 10.1016/j.jmb.2006.01.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merzlyakov M, Hristova K. Forster resonance energy transfer measurements of transmembrane helix dimerization energetics. Methods Enzymol. 2008;450:107–127. doi: 10.1016/S0076-6879(08)03406-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li E, You M, Hristova K. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and forster resonance energy transfer suggest weak interactions between fibroblast growth factor receptor 3 (FGFR3) transmembrane domains in the absence of extracellular domains and ligands. Biochemistry. 2005;44:352–360. doi: 10.1021/bi048480k. [DOI] [PubMed] [Google Scholar]
- 22.Weiner DB, Liu J, Cohen JA, Williams WV, Greene MI. A point mutation in the neu oncogene mimics ligand induction of receptor aggregation. Nature. 1989;339:230–231. doi: 10.1038/339230a0. [DOI] [PubMed] [Google Scholar]
- 23.Webster MK, Donoghue DJ. Constitutive activation of fibroblast growth factor receptor 3 by the transmembrane domain point mutation found in achondroplasia. Embo J. 1996;15:520–527. [PMC free article] [PubMed] [Google Scholar]
- 24.Meyers GA, Orlow SJ, Munro IR, Przylepa KA, Jabs EW. Fibroblast growth factor receptor 3 (FGFR3) transmembrane mutation in Crouzon syndrome with acanthosis nigricans. Nat Genet. 1995;11:462–464. doi: 10.1038/ng1295-462. [DOI] [PubMed] [Google Scholar]
- 25.Li E, Hristova K. Role of receptor tyrosine kinase transmembrane domains in cell signaling and human pathologies. Biochemistry. 2006;45:6241–6251. doi: 10.1021/bi060609y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li E, Hristova K. Receptor tyrosine kinase transmembrane domains: Function, dimer structure and dimerization energetics. Cell Adh Migr. 2010;4:249–254. doi: 10.4161/cam.4.2.10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennasroune A, Fickova M, Gardin A, Dirrig-Grosch S, Aunis D, Cremel G, Hubert P. Transmembrane peptides as inhibitors of ErbB receptor signaling. Mol Biol Cell. 2004;15:3464–3474. doi: 10.1091/mbc.E03-10-0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roth L, Nasarre C, Dirrig-Grosch S, Aunis D, Cremel G, Hubert P, Bagnard D. Transmembrane domain interactions control biological functions of neuropilin-1. Mol Biol Cell. 2008;19:646–654. doi: 10.1091/mbc.E07-06-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lofts FJ, Hurst HC, Sternberg MJ, Gullick WJ. Specific short transmembrane sequences can inhibit transformation by the mutant neu growth factor receptor in vitro and in vivo. Oncogene. 1993;8:2813–2820. [PubMed] [Google Scholar]
- 30.Bennasroune A, Gardin A, Auzan C, Clauser E, Dirrig-Grosch S, Meira M, Appert-Collin A, Aunis D, Cremel G, Hubert P. Inhibition by transmembrane peptides of chimeric insulin receptors. Cell Mol Life Sci. 2005;62:2124–2131. doi: 10.1007/s00018-005-5226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lemmon MA, Engelman DM. Specificity and promiscuity in membrane helix interactions. Q Rev Biophys. 1994;27:157–218. doi: 10.1017/s0033583500004522. [DOI] [PubMed] [Google Scholar]
- 32.Sternberg MJ, Gullick WJ. A sequence motif in the transmembrane region of growth factor receptors with tyrosine kinase activity mediates dimerization. Protein Eng. 1990;3:245–248. doi: 10.1093/protein/3.4.245. [DOI] [PubMed] [Google Scholar]
- 33.Linggi B, Carpenter G. ErbB receptors: new insights on mechanisms and biology. Trends Cell Biol. 2006;16:649–656. doi: 10.1016/j.tcb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 34.Drevs J, Medinger M, Schmidt-Gersbach C, Weber R, Unger C. Receptor tyrosine kinases: the main targets for new anticancer therapy. Curr Drug Targets. 2003;4:113–121. doi: 10.2174/1389450033346885. [DOI] [PubMed] [Google Scholar]
- 35.Harari D, Yarden Y. Molecular mechanisms underlying ErbB2/HER2 action in breast cancer. Oncogene. 2000;19:6102–6114. doi: 10.1038/sj.onc.1203973. [DOI] [PubMed] [Google Scholar]
- 36.Vajo Z, Francomano CA, Wilkin DJ. The molecular and genetic basis of fibroblast growth factor receptor 3 disorders: the achondroplasia family of skeletal dysplasias, Muenke craniosynostosis, and Crouzon syndrome with acanthosis nigricans. Endocr Rev. 2000;21:23–39. doi: 10.1210/edrv.21.1.0387. [DOI] [PubMed] [Google Scholar]
- 37.L'Hote CG, Knowles MA. Cell responses to FGFR3 signalling: growth, differentiation and apoptosis. Exp Cell Res. 2005;304:417–431. doi: 10.1016/j.yexcr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 38.van Rhijn BW, van Tilborg AA, Lurkin I, Bonaventure J, de Vries A, Thiery JP, van der Kwast TH, Zwarthoff EC, Radvanyi F. Novel fibroblast growth factor receptor 3 (FGFR3) mutations in bladder cancer previously identified in non-lethal skeletal disorders. Eur J Hum Genet. 2002;10:819–824. doi: 10.1038/sj.ejhg.5200883. [DOI] [PubMed] [Google Scholar]
- 39.Aviezer D, Golembo M, Yayon A. Fibroblast growth factor receptor-3 as a therapeutic target for Achondroplasia--genetic short limbed dwarfism. Curr Drug Targets. 2003;4:353–365. doi: 10.2174/1389450033490993. [DOI] [PubMed] [Google Scholar]
- 40.Bargmann CI, Hung MC, Weinberg RA. Multiple independent activations of the neu oncogene by a point mutation altering the transmembrane domain of p185. Cell. 1986;45:649–657. doi: 10.1016/0092-8674(86)90779-8. [DOI] [PubMed] [Google Scholar]
- 41.Bargmann CI, Weinberg RA. Oncogenic activation of the neu-encoded receptor protein by point mutation and deletion. Embo J. 1988;7:2043–2052. doi: 10.1002/j.1460-2075.1988.tb03044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He L, Hristova K. Pathogenic activation of receptor tyrosine kinases in mammalian membranes. J Mol Biol. 2008;384:1130–1142. doi: 10.1016/j.jmb.2008.10.036. [DOI] [PubMed] [Google Scholar]
- 43.Chuan YC, Iglesias-Gato D, Fernandez-Perez L, Cedazo-Minguez A, Pang ST, Norstedt G, Pousette A, Flores-Morales A. Ezrin mediates c-Myc actions in prostate cancer cell invasion. Oncogene. 2009 doi: 10.1038/onc.2009.442. [DOI] [PubMed] [Google Scholar]
- 44.Raats JM, Hof D. Recombinant antibody expression vectors enabling double and triple immunostaining of tissue culture cells using monoclonal antibodies. Eur J Cell Biol. 2005;84:517–521. doi: 10.1016/j.ejcb.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 45.Fanger BO, Stephens JE, Staros JV. High-yield trapping of EGF-induced receptor dimers by chemical cross-linking. Faseb J. 1989;3:71–75. doi: 10.1096/fasebj.3.1.2783412. [DOI] [PubMed] [Google Scholar]
- 46.Monsonego-Ornan E, Adar R, Feferman T, Segev O, Yayon A. The transmembrane mutation G380R in fibroblast growth factor receptor 3 uncouples ligand-mediated receptor activation from down-regulation. Mol Cell Biol. 2000;20:516–522. doi: 10.1128/mcb.20.2.516-522.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kokai Y, Myers JN, Wada T, Brown VI, LeVea CM, Davis JG, Dobashi K, Greene MI. Synergistic interaction of p185c-neu and the EGF receptor leads to transformation of rodent fibroblasts. Cell. 1989;58:287–292. doi: 10.1016/0092-8674(89)90843-x. [DOI] [PubMed] [Google Scholar]