Abstract
Objective
To develop a risk score for developing geographic atrophy (GA) involving easily obtainable information among patients with bilateral large drusen.
Design
Cohort study within a multicenter randomized clinical trial.
Participants
1052 participants with ≥10 large (>125 μ) drusen and visual acuity ≥20/40 in each eye.
Methods
In the Complications of Age-related Macular Degeneration (AMD) Prevention Trial (CAPT), one eye of each participant was randomly assigned to laser treatment and the contralateral eye was assigned to observation to evaluate whether laser treatment of drusen could prevent vision loss. Gradings by a reading center were used to identify three outcomes for GA: CAPT endpoint GA (total area of GA (>250 u) >1 disc area), GA (>175 u) involving the foveal center (CGA), and GA of any size and location (any GA). Four established risk factors (age, smoking status, presence of hypertension, Age-related Eye Disease Study (AREDS) simple severity scale score), both with and without a novel risk factor (night vision score), were used in assigning risk points. The risk scores were evaluated for the ability to discriminate and calibrate GA risk.
Main Outcome Measures
Development of endpoint GA, CGA and any GA.
Results
Among 942 CAPT participants who completed 5 years of follow-up and did not have any GA at baseline, 64 (6.8%) participants developed CAPT endpoint GA, 90 (9.6%) developed CGA, and 342 (34.4%) developed any GA. The 5-year incidence of endpoint GA in one or both eyes of a participant increased with the 15-point GA risk score, from 0.6% for <7 points to 15% for ≥12 points. The 5-factor risk score predicted development of GA moderately well with the area under the receiver operating characteristic curve (AUC) 0.76 (95% confidence interval: 0.71–0.81) for endpoint GA; 0.76 (0.71–0.80) for CGA and 0.68 (0.65–0.72) for any GA. Prediction from the risk score without the night vision score had lower AUCs (range: 0.67 to 0.72).
Conclusions
If validated in other patients, the GA risk scores will be useful for identifying high risk patients for clinical trials of prevention of GA and for clinical assessment of GA risk in early AMD patients.
INTRODUCTION
Age-related macular degeneration (AMD) is the leading cause of irreversible blindness in the developed world. Choroidal neovascularization (CNV) and geographic atrophy (GA) are two forms of end-stage AMD. GA is responsible for about 10% of the severe vision loss attributed to the AMD,1 and affects approximately 900,000 persons in the United States.2 Anti-vascular endothelial growth factor (Anti-VEGF) therapy has been proven to be highly effective in reducing the vision loss in patients with CNV.3–4 Although several agents to prevent the development or arrest the progression of GA are currently under investigation in clinical trials, none have yet been shown to be effective.
GA progresses gradually over time, and the causes of GA are largely unknown. However, data from large observational studies and clinical trial cohorts have consistently identified age, current smoking status, hypertension, drusen size or area, and pigmentary changes as risk factors.5–15 Recent investigations have identified genes associated with GA, including Complement Factor H, Complement Factor B, LOC387715 and Complement C3 variant.16–18 More recently, night vision as assessed by a 10-item questionnaire was found to be highly predictive of the development of GA, independent of other established risk factors.19
In this paper, we describe the development and evaluation of risk scores for the development of GA within 5 years based only on readily available risk factors. Risk scores are useful for both clinical research studies and individual patient care. Predictive summary scores were first introduced by the Framingham Heart Study Group for the 10-year risk of coronary heart disease,20 and have been applied to many disease areas, including the development of glaucoma for patients with ocular hypertension.21–23 Although a prediction model including ocular, environmental and genetic risk factors for advanced AMD (GA and CNV combined) has been developed recently,18 a risk score for GA alone has not been developed.
METHODS
Details of the design and methods of the clinical trial have been reported elsewhere;24–25 only major features related to this paper are described here. The Complications of Age-related Macular Degeneration Prevention Trial (CAPT) was a multi-center randomized clinical trial to evaluate low-intensity laser treatment of eyes with drusen for the prevention of vision loss from AMD in participants with bilateral large drusen. For each participant, one eye was randomized to laser treatment with the contralateral eye assigned to observation. The CAPT results showed that there was no statistical difference between treated and observed eyes on visual acuity loss, incidence of CNV, or incidence of endpoint GA.25
A total of 1,052 participants were enrolled into CAPT between May 1999 and March 2001 from 22 participating clinical centers. The institutional review board associated with each center approved the study protocol and written informed consent was obtained from each participant. Data management was compliant with Health Insurance Portability and Accountability Act (HIPAA)guidelines. The conduct of the clinical trial adhered to the tenets of the Declaration of Helsinki. CAPT eligibility criteria specified that each eye have ≥ 10 large drusen (≥ 125 μ in diameter). Neither eye was to have evidence of CNV, serous pigment epithelial detachment, GA within 500 microns of the foveal center or total area >1 MPS disc area (DA).
At the initial visit and annual visits thereafter, certified photographers adhering to a standardized protocol for field definition and image sequencing took stereoscopic, color fundus photographs on film and a fluorescein angiogram on film, with frames from each eye. Color photographs were also taken at 6 months. All photographic images were graded independently by two trained readers in the CAPT Reading Center who later openly discussed their discrepancies to arrive at consensus. The fundus features described in the baseline grading included number of drusen, largest drusen size, drusen area, drusen confluence, geographic atrophy, focal hyperpigmentation and RPE depigmentation.
Risk Factors Assessment
At initial visit, information regarding age, cigarette smoking status, current use of medication for hypertension was collected through questioning participants by use of a standardized questionnaire. Blood pressure (BP) was measured once while the participant was sitting. Hypertension was defined as reported current use of anti-hypertensive medications, or systolic BP ≥ 140 mmHg or diastolic BP ≥ 90 mmHg in participants not taking anti-hypertensive medications.
The score on the AREDS simple severity scale at study enrollment was determined using the following definition.26 For each eye, one point was assigned for presence of large drusen and one point for presence of pigmentary changes. The points from the two eyes are added together to provide the score, which can range from 0 to 4.
At baseline, a 10-item night vision symptoms questionnaire (NVQ-10) was self-administered.19 The first 4 items are on a 5-point scale from “None” to “Stopped doing because of my eyesight” and ask about the difficulty in seeing moving subjects, reading street signs when driving at night, difficulty in seeing street signs as a passenger in the car at night, and difficulty with the oncoming headlights or streetlights when driving at night. The next 6 items are on a 4-point scale from “Not at all” to “Very” and ask about how bothered the participant is by: poor vision at night, problem in reading in dim light, a dark spot in the middle of vision in dim light, poor vision in dim lighting, problems adjusting to the dark when entering a theater, and trouble seeing the stars in the sky at night. For the night vision score, each item is scored between 100 (none or not at all) and 0 (stop doing because of eyesight, or very bothered). An overall NVQ-10 score for each participant was calculated based on the average score of 10 items. The score ranges from 0 to 100 with lower scores indicating worse night vision.
Geographic Atrophy Definitions
Readers in the CAPT Reading Center evaluated the annual follow-up fundus color photographs for the presence of GA, amount of GA (<0.028 DA (i.e., 250 μ in diameter), 0.028–1 DA, 1–2 DA, >2 DA), presence of a new area of GA, considering only the central area within 500 μ of foveal center, only the annulus from 500 to 1500 μ, and only the annulus from 1500 to 3000 μ, and whether the total area of GA within 3000 μ of foveal center was greater than 1 DA. GA was considered to be present when the color photographs showed an area of atrophy of the retinal pigment epithelium with 2 of the following 3 features: visible choroidal vessels, sharp edges, and a more or less circular shape. “CAPT Endpoint GA” was defined as development of a total of >1 DA of new, additional atrophy when all areas of GA (>250 μ in diameter) within 3000 μ of the foveal center were combined. Endpoint GA was used in CAPT to identify eyes that had progressed. CGA was defined as development of GA (>175 μ in diameter) involving the center of macula. CGA was used in AREDS to identify eyes that had progressed. Any GA was defined as the presence of any size GA (i.e., including areas <0.028 DA) within 3000 μ of the foveal center. Evaluation of GA was not performed after an eye developed CNV because the neovascular complex and subsequent scarring often occupied or obscured the retinal area most likely to develop GA.
Statistical Analysis
Analyses were restricted to 942 CAPT participants who completed 5-year follow-up, did not have any GA at baseline, and had information available on all the baseline risk factors.
The development of the risk score followed the approach used for the Framingham Study risk score.27 Specifically, a multivariate logistic regression model was fit to the data and included 5 risk factors as predictors: age (50–59, 60–69, 70–79, 80+), smoking status (never or former vs. current), hypertension status (no vs. yes), AREDS simple severity score (2, 3, 4), and night vision score (<60, 60–75, 75–85, >85). The outcome was the development of CAPT endpoint GA in one or both eyes (person-specific GA yes/no) during a 5-year follow-up period. Estimates of the regression coefficients corresponding to each level of a risk factor were obtained, and risk points were assigned for each level of a risk factor based on the value of the associated regression coefficient and the reference regression coefficient corresponding to one risk point. The risk score for a participant was determined as the total of risk points based on a participant’s risk factor profile. Because the night vision questionnaire is not commonly administered in clinical practice, another risk points system was developed by using the same methodology as described above but without the inclusion of the night vision score (i.e., only including age, smoking, hypertension, and AREDS simple scale score).
The performance of the derived risk score from the multivariate prediction model was evaluated based on the ability to distinguish high-risk participants from low-risk participants (discrimination) and on the agreement between the predicted risk associated with specific scores and the observed proportion developing the form of GA under consideration (calibration). Discrimination was summarized by the area under the receiver operating characteristic (ROC) curve (i.e., AUC, or c-statistic), yielded by the logistic regression model that used only the risk score as a predictor. The AUC ranges from 0.5 to 1, with 0.5 indicating no discriminative ability and 1 indicating perfect discriminative ability. An AUC greater than 0.9 is considered excellent, greater than 0.8 to 0.9 very good, 0.7 to 0.8 good, 0.6 to 0.7 average, and less than 0.6 poor.29 The 95% confidence interval (CI) of AUC was determined based on the bootstrap method involving 2000 samples.30 The difference in AUC from the risk score with vs. without consideration of the night vision score was assessed through comparison of correlated AUCs based on a bootstrap z-statistic approach.28
Calibration was assessed by the Brier score,31 a standardized summary measure of the mean squared differences between the observed person-specific GA outcome (0 for without GA and 1 for with GA) and the predicted probability of person-specific GA from the logistic regression model using the risk score as the only predictor. The Brier score ranges from 0 (predictions and observed outcomes match perfectly) to 1 (predictions and observed outcomes totally mismatch). Additionally, to help in the choice of scores for identifying high risk GA patients, we calculated the sensitivity and specificity associated with the various cutpoints of risk score.
The above assessments of the GA risk score were performed for CAPT endpoint GA, CGA and any GA in one or both eyes (i.e., person-specific) and in untreated eyes only. All data analyses were performed in SAS 9.1. (SAS Institute, Inc., Cary, NC).
RESULTS
Of the 942 CAPT participants included in the analysis, mean age (SD) at study entry was 71 (7.5) years old, with a range of 50 to 90 years, 5% were current smokers, 64% had hypertension. Because of the CAPT eligibility criteria, all participants had large drusen in each eye, thus none of the participants had an AREDS severity score of 0 or 1. Twenty-one percent had an AREDS score of 2, and more than half (56%) had a score of 4 (Table 1). The mean (SD) night vision score was 70 (20), with a range of 3 to 100. During 5-years of follow-up, 64 (6.8%) participants developed CAPT endpoint GA, 90 (9.6%) developed CGA, and 324 (34.4%) developed any GA in one or both eyes.
Table 1.
Risk factors for the development of endpoint geographic atrophy in one or both eyes within 5 years of follow-up in multivariate logistic regression models with and without night vision score
| Multivariate Model with inclusion of night vision score | Multivariate model without inclusion of night vision score | ||||||
|---|---|---|---|---|---|---|---|
| Risk Factors | N | GA by 5 years n (%) | Regression coefficient (SE) | Odds ratio (95% CI) | Regression Coefficient (SE) | Odds ratio (95% CI) | Risk points* |
| Intercept | −6.29 (1.02) | −5.57 (0.93) | |||||
| AREDS simple scale | P<0.0001 | P<0.0001 | |||||
| 2 | 199 | 3 (1.51) | Reference | Reference | 0 | ||
| 3 | 214 | 11 (5.14) | 1.38 (0.66) | 3.98 (1.08–14.6) | 1.28 (0.66) | 3.59 (0.99–13.1) | 4 |
| 4 | 529 | 50 (9.45) | 1.95 (0.60) | 7.03 (2.15–23.0) | 1.93 (0.60) | 6.88 (2.12–22.4) | 5 |
| Night vision score | P=0.0003 | ||||||
| > 85 | 231 | 7 (3.03) | Reference | 0 | |||
| 75.1 to 85 | 211 | 8 (3.79) | 0.20 (0.53) | 1.23 (0.44–3.48) | 1 | ||
| 60.1 to 75 | 237 | 19 (8.02) | 1.05 (0.46) | 2.87 (1.17–7.04) | 3 | ||
| ≤ 60 | 263 | 30 (11.4) | 1.48 (0.44) | 4.37 (1.85–10.3) | 4 | ||
| Age (Years) | P=0.08 | P=0.09 | |||||
| 50–59 | 87 | 2 (2.30) | Reference | Reference | 0 | ||
| 60–69 | 271 | 24 (8.86) | 1.50 (0.76) | 4.48 (1.02–19.8) | 1.50 (0.81) | 4.13 (0.95–18.0) | 1 |
| 70–79 | 492 | 30 (6.10) | 0.97 (0.75) | 2.63 (0.60–11.5) | 1.03 (0.75) | 2.79 (0.65–12.0) | 3 |
| ≥ 80 | 92 | 8 (8.70) | 1.23 (0.82) | 3.43 (0.69–17.2) | 1.42 (0.75) | 4.49 (0.91–22.1) | 4 |
| Smoking Status | P=0.49 | P=0.39 | |||||
| Never/former | 892 | 59 (6.61) | Reference | Reference | 0 | ||
| Current | 50 | 5 (10.0) | 0.36 (0.52) | 1.43 (0.52–3.99) | 0.46 (0.51) | 1.58 (0.59–4.27) | 1 |
| Hypertension Status | P=0.20 | P=0.29 | |||||
| No | 338 | 18 (5.33) | Reference | Reference | 0 | ||
| Yes | 604 | 46 (7.62) | 0.37 (0.29) | 1.45 (0.81–2.61) | 0.30 (0.29) | 1.36 (0.76–2.41) | 1 |
The regression coefficient 0.36 is considered as one risk point.
AREDS = Age-related Eye Disease Study; GA = Geographic atrophy; SE = Standard error; CI = Confidence interval.
Risk Score Development with 5 Factors
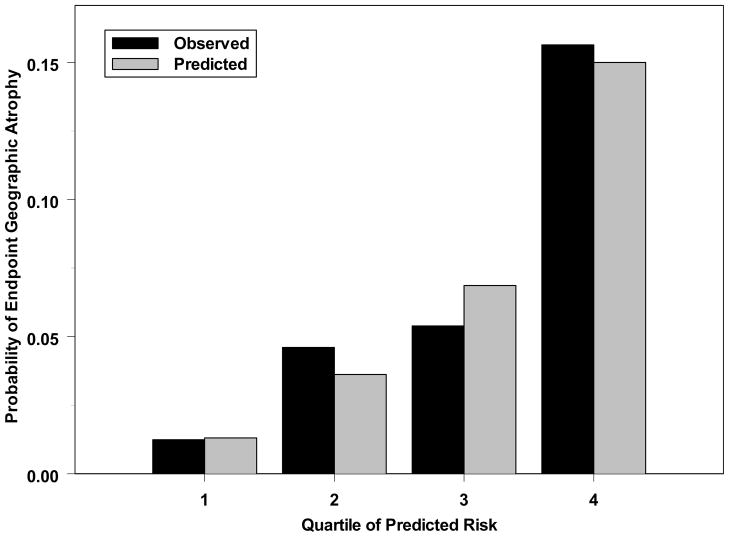
In the multivariate analysis of all 5 risk factors (Table 1, middle columns), a higher AREDS severity score was significantly associated with increased risk of endpoint GA (Odds Ratio (OR)=7.03 for severity score of 4 vs. 2, p<0.0001), and a decreased night vision score was associated with an increased risk of GA (OR=4.37 for 4th quartile vs. 1st quartile, p=0.0003). Increased age was marginally associated with increased risk of GA (p=0.08). Current smoking (p=0.49) and hypertension (p=0.20) were not statistically significantly associated with endpoint GA in this group of participants. However, because increased age, cigarette smoking, and hypertension have been identified as risk factors for GA in several other studies,7,9,11–15 we retained them in the prediction model for developing the risk score. The final prediction model including all 5 factors predicted the risk of endpoint GA moderately well with an AUC of 0.77 (95% CI: 0.71–0.83), and calibrated well as evaluated by the Hosmer-Lemeshow test which showed no significant difference (p=0.33) between observed and expected number of patients with endpoint GA (Figure 1, available at http://aaojournal.org).
Figure 1.
Comparison of observed and predicted 5-year incidence of endpoint geographic atrophy based on multivariate prediction model involving 5 risk factors. The x-axis is the predicted probability of risk divided into 4 groups of approximately 235 participants each. There is no significant difference between predicted and observed risk of geographic atrophy (p=0.33).
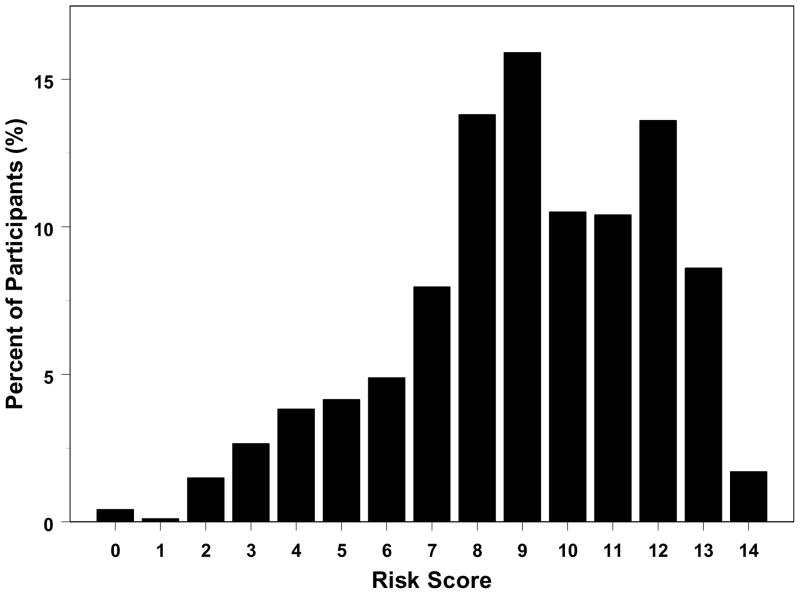
Based on the regression coefficients from the multivariate logistic regression model, risk points were assigned to each level of a risk factor (last column of Table 1). We considered a participant aged 50–59 years, AREDS severity score of 2, night vision score >85, not currently smoking, and without hypertension as having the referent risk factor profile. Participants with this risk profile were assigned 0 risk point. We arbitrarily assigned the regression coefficient of 0.36 associated with current smoking as equivalent to one risk point and divided each regression coefficient associated with different levels of the risk factors by 0.36 to determine the number of risk points (rounded to one digit). The risk score is the sum of the risk points from each of the 5 risk factors and can range from 0 to 15. The distribution of risk score for CAPT participants is shown in Figure 2 (available at http://aaojournal.org). None of the participants had the maximum risk score of 15, 44 (4.67%) had a risk score of less than 4, while the majority (81%) of participants had a risk score of 7–13.
Figure 2.
The distribution of geographic atrophy risk score (involving 5 risk factors) of participants (N=942).
The 5 factor risk score is strongly predictive of CAPT endpoint GA (Table 2). The 5-year incidence of endpoint GA increased with GA risk score: 0.6% for ≤ 6 points, 3% for 7–8 points, 4% for 9 points, 6% for 10 points, 11% for 11 points, and 15% for ≥12 points. The AUC for endpoint GA is 0.76 (95% CI: 0.71–0.81), indicating good prediction power. The risk score from all 5 risk factors has statistically significantly higher prediction power than the risk score from other subsets of risk factors (AUC differences 0.03 to 0.13, all p<0.03, Table 3). When used alone, the AREDS simple scale score and the night vision score have similar predictive capability. Also, models that include age, smoking status, and hypertension have similar predictive capability whether the AREDS simple scale score or the night vision score is included in the model.
Table 2.
Prediction of 5-year risk of geographic atrophy by risk score involving 5 risk factors (N=942 patients)
| Endpoint GA | CGA | Any size GA | |||||
|---|---|---|---|---|---|---|---|
| GA risk score | N | In either eye (n=64) | In untreated eye (n=45) | In either eye (n=90) | In untreated eye (n=68) | In either eye (n=324) | In untreated eye (n=214) |
| 0–6 | 165 | 1 (0.60) | 0 (0.00) | 2 (1.21) | 1 (0.61) | 21 (12.7) | 13 (7.88) |
| 7–8 | 205 | 7 (3.41) | 4 (1.95) | 5 (2.44) | 4 (1.95) | 57 (27.8) | 34 (16.6) |
| 9 | 150 | 6 (4.00) | 3 (2.00) | 12 (8.00) | 9 (6.00) | 48 (32.0) | 35 (23.3) |
| 10 | 99 | 6 (6.06) | 4 (4.04) | 11 (11.1) | 7 (7.07) | 34 (34.3) | 19 (19.2) |
| 11 | 98 | 11 (11.2) | 8 (8.16) | 17 (17.4) | 12 (12.2) | 45 (45.9) | 29 (29.6) |
| 12 | 128 | 20 (15.6) | 15 (11.7) | 21 (16.4) | 15 (11.7) | 61 (47.7) | 45 (35.2) |
| >12 | 97 | 13 (13.4) | 11 (11.3) | 22 (22.7) | 20 (20.6) | 58 (59.8) | 39 (40.2) |
| AUC (95% CI*) | 0.76 (0.71–0.81) | 0.79 (0.75–0.84) | 0.76 (0.71–0.80) | 0.77 (0.72–0.81) | 0.68 (0.65–0.72) | 0.68 (0.64–0.72) | |
AUC = area under the receiver operating characteristic curve; GA = geographic atrophy; CGA = GA involving the foveal center; CI = Confidence interval.
Based on the bootstrap of 2000 samples.
Table 3.
Discrimination capability of alternative logistic regression models for development of geographic atrophy
| Model | Risk factors included for risk score calculation | AUC (95% CI)* | P-value for comparison to model 1 |
|---|---|---|---|
| 1 | Age, smoking status, hypertension, AREDS simple scale score, night vision score | 0.76 (0.71 – 0.81) | |
| 2 | Age, smoking status, hypertension, AREDS simple scale score | 0.67 (0.62 – 0.72) | P<0.001 |
| 3 | Age, smoking status, hypertension, Night vision score | 0.69 (0.63 – 0.75) | P<0.001 |
| 4 | AREDS simple scale score, night vision score | 0.73 (0.68 – 0.79) | P=0.03 |
| 5 | AREDS simple scale score only | 0.63 (0.58 – 0.69) | P<0.001 |
| 6 | Night vision score only | 0.65 (0.59 – 0.71) | P<0.001 |
AUC = area under the receiver operating characteristic curve; CI = Confidence interval; AREDS=Age-related Eye Disease Study.
AUC was determined based on the c-statistic from the logistic regression model. 95% confidence interval was calculated based on bootstrap of 2000 samples.
Despite the fact that the risk score was developed for prediction of CAPT endpoint GA, the risk score is also strongly predictive of the two other types of GA. The risk score for CGA has an AUC of 0.76 (0.71–0.80). The risk score is less predictive of any GA, with an AUC of 0.68 (0.65–0.72). When the risk score was applied to untreated eyes only, similar predictive capability was obtained (Table 2).
The sensitivity and specificity corresponding to the various cutpoints for the 5 factor risk score are shown in Table 4 (available at http://aaojournal.org) for each type of GA. Using a cutpoint of 9 or above to define high risk provides sensitivity and specificity combinations of 88% and 41% for endpoint GA, 92% and 43% for CGA, and 76% and 47% for any GA. Higher specificity with lower sensitivity can be obtained by using a cutpoint of 8 or below.
Table 4.
The sensitivity and specificity for detecting different types of geographic atrophy using various cutpoints of the geographic atrophy risk score involving 5 risk factors
| Endpoint GA (n=64 cases) | Central GA (n=90 cases) | GA of any size (n=324 cases) | ||||
|---|---|---|---|---|---|---|
| Cutpoint as high risk | Sensitivity | Specificity | Sensitivity | Specificity | Sensitivity | Specificity |
| ≥ 4 | 100 | 5.01 | 100 | 5.16 | 99.1 | 6.63 |
| ≥ 5 | 98.4 | 9.00 | 98.9 | 9.27 | 97.5 | 11.7 |
| ≥ 6 | 98.4 | 13.4 | 97.8 | 13.7 | 96.0 | 17.2 |
| ≥ 7 | 98.4 | 18.7 | 97.8 | 19.1 | 93.5 | 23.3 |
| ≥ 8 | 89.1 | 26.5 | 93.3 | 27.5 | 86.4 | 31.7 |
| ≥ 9 | 87.5 | 41.2 | 92.2 | 42.6 | 75.9 | 47.3 |
| ≥ 10 | 78.1 | 57.6 | 78.9 | 58.8 | 61.1 | 63.8 |
| ≥ 11 | 68.8 | 68.2 | 66.7 | 69.1 | 50.6 | 74.3 |
| ≥ 12 | 51.6 | 78.1 | 47.8 | 78.6 | 36.7 | 82.9 |
| ≥ 13 | 20.3 | 90.4 | 24.4 | 91.2 | 17.9 | 93.7 |
| ≥ 14 | 3.12 | 98.4 | 4.44 | 98.6 | 3.40 | 99.2 |
GA = geographic atrophy.
The risk score is shown to be well calibrated for endpoint GA and CGA. The Brier score is close to 0 (0.06 for endpoint GA, 0.08 for CGA, and 0.21 for any GA), indicating predictions by risk score and observed GA outcomes match moderately well.
Risk Score Development without Inclusion of Night Vision Score
A multivariate logistic regression model was fit that only included age, smoking, hypertension, and AREDS simple scale as predictors. Because the regression coefficients for each of risk factors were almost the same (with the exception of the intercept term) as those from the multivariate prediction model that included all 5 risk factors (Table 1), the risk points corresponding to each level of a risk factor remained the same. The total risk score from the 4 risk factors ranges from 0 to 11, with the majority (80%) having a risk score of 5 or above (Table 5) (available at http://aaojournal.org).
Table 5.
Prediction of 5-year risk of geographic atrophy by risk score without consideration of night vision score (N=942 patients)
| GA risk score | N | Endpoint GA | Central GA | GA anywhere | |||
|---|---|---|---|---|---|---|---|
| In either eye (n=64) | In untreated eye (n=45) | In either eye (n=90) | In untreated eye (n=68) | In either eye (n=324) | In untreated eye (n=214) | ||
| <5 | 193 | 3 (1.55) | 2 (1.04) | 2 (1.04) | 1 (0.52) | 24 (12.4) | 12 (6.22) |
| 5–6 | 100 | 3 (3.00) | 1 (1.00) | 4 (4.00) | 3 (3.00) | 23 (23.0) | 16 (16.0) |
| 7 | 114 | 7 (6.14) | 5 (4.39) | 9 (7.89) | 7 (6.14) | 32 (28.1) | 17 (14.9) |
| 8 | 270 | 26 (9.63) | 16 (5.93) | 27 (10.0) | 19 (7.04) | 116 (43.0) | 78 (28.9) |
| 9 | 224 | 19 (8.48) | 16 (7.14) | 39 (17.4) | 32 (14.3) | 107 (47.8) | 77 (34.4) |
| 10 | 41 | 6 (14.6) | 5 (12.2) | 9 (22.0) | 6 (14.6) | 22 (53.7) | 14 (34.2) |
| AUC (95% CI*) | 0.67 (0.62–0.72) | 0.69 (0.64–0.75) | 0.71 (0.66–0.76) | 0.72 (0.67–0.77) | 0.68 (0.64–0.71) | 0.68 (0.64–0.72) | |
AUC = area under the receiver operating characteristic curve; GA = geographic atrophy; CI = Confidence interval.
Based on the bootstrap of 2000 sample.
The predictions of GA by risk score without consideration of night vision scores are summarized in Table 5 (available at http://aaojournal.org). The 5-year incidence of GA increased with risk score for each of the types of GA considered. The AUC for endpoint GA decreased by 0.09 (p<0.001) relative to the risk score that included night vision score (Table 3). When CGA was considered, the AUC decreased by 0.05 (p=0.04); however, there was no decrease in AUC for any GA. Using a cutpoint of 7 or above to define high risk provides sensitivity and specificity combinations of 91% and 33% for endpoint GA, 93% and 34% for CGA, and 86% and 40% for any GA.
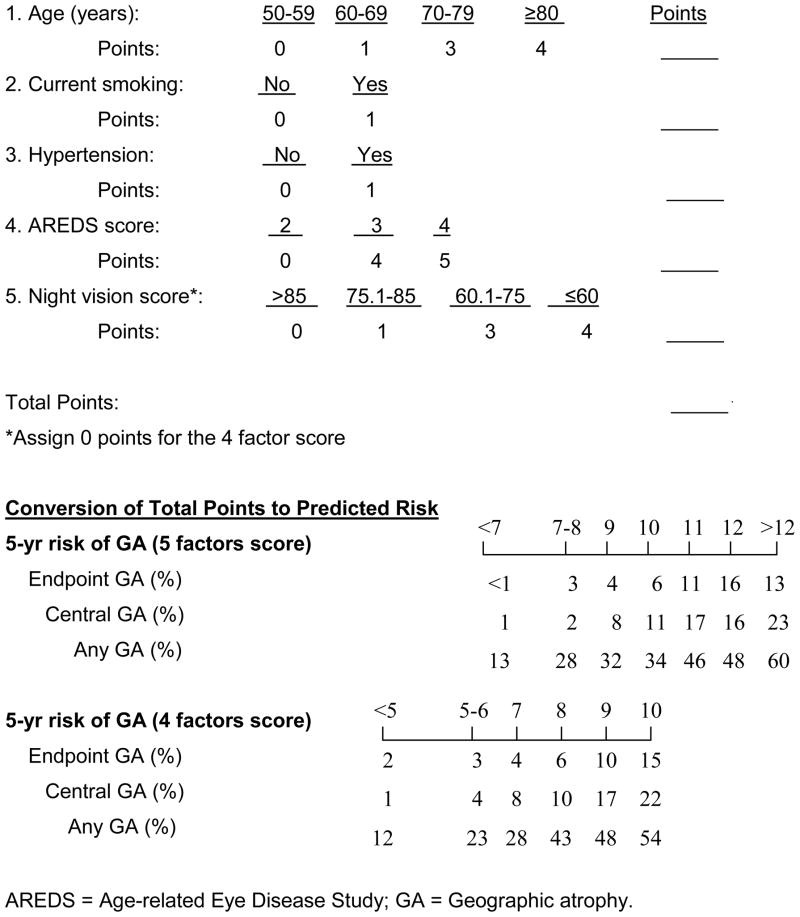
Computation of Risk Scores and the Predicted Risk of GA
To facilitate the use of the risk scores, we developed a worksheet (Figure 3). The total number of risk points and the associated predicted risk for each type of GA can be found in the lower panel. As an example, a 75-years old patient having bilateral large drusen and depigmentation in only the right eye (i.e., AREDS simple scale score of 3), currently smoking, taking anti-hypertensive drugs, and a having night vision score of 65, has a total 5 factor risk score of 3+4+1+1+3=12. This corresponds to a predicted 5-year incidence of 16% each for endpoint GA and CGA, and 48% for any GA. If night vision score is not available, the total points from the four factor scoring is 3+4+1+1=9, and the corresponding predicted 5-year incidence is 10% for endpoint GA, 17% for CGA, and 48% for any GA.
Figure 3.
Worksheet to calculate the risk score and corresponding 5-year probability of developing various types of geographic atrophy.
DISCUSSION
We developed a 15-point GA risk score from five easily accessible risk factors that predicts moderately well the 5-year risk of endpoint GA, CGA and any GA (c-statistic: 0.68–0.79). This predictive power is similar to the predictive power of the Framingham risk score for coronary heart disease (c-statistic: 0.63–0.83),20 similar to the recently developed risk score for glaucoma (c-statistic: 0.68–0.73) 21–22 and also similar to the prediction of advanced AMD using demographic and environmental variables (c-statistic: 0.73–0.76).18 When the score is computed without consideration of night vision there is a decrease in prediction power (c-statistic: 0.67–0.72).
To our knowledge, this is the first study to develop risk scores specifically for predicting GA rather than CNV and GA combined. Data from CAPT is especially well-suited for developing a model for GA because participants had substantial drusen burden (each eye have at least 10 large drusen (≥ 125 μ)), were followed prospectively at least 5 years, and had yearly color photographs taken by certified photographers with interpretation at a central reading center. The long-term follow-up of these high risk participants provided sufficient GA cases to develop a valid prediction model and derive risk scores from the resulting prediction model. Appropriate prediction models require at least 10 cases per predictor,32 and our prediction model includes more than 12 GA cases per risk factor.
The absence of patients with AREDS simple scores of 0 and 1 in the CAPT population is a theoretical weakness in our development of GA risk scores. However, examination of the AREDS data on progression to central GA revealed that the 5-year risk for participants with AREDS score of 0 was 0.0% [0/1446] and was 0.5% [3/635] for participants with a score of 1.26 Thus, the only patients with any substantial risk of developing GA are those with an AREDS simple score of 2 or more.
The GA risk scores we developed may improve the design and analysis of clinical trials to prevent GA. Progression from drusen to GA takes years,33 and only a small percentage of AMD patients will develop GA even among those starting with bilateral large drusen (6.8% for endpoint GA in CAPT participants, and 6% for CGA in AREDS participants).7 Smaller sample sizes and/or shorter follow-up periods may be used if trials include only higher risk patients. Statistical analyses may be more precise if the baseline risk score is used as a covariate. In addition, enrolling the highest risk patients decreases the risk-benefit ratio in clinical trials. The night vision questionnaire may be used when screening patients to more finely stratify patients by risk of developing GA than is possible with knowledge of only age, cigarette smoking, hypertension, presence of large drusen or pigmentation changes.
The GA risk scores also provide an easy way for ophthalmologists to estimate the 5-year risk of developing GA among their AMD patients. These estimates may help in explaining the implications of newly detected signs of early AMD to patients.
Our risk score was developed from readily available risk factors, and it does not consider other risk factors that are more difficult to obtain, specifically the genetic risk factors. Complement Factor H, Complement Factor B, LOC387715 and complement C3 variant were recently found to be associated with risk of GA.16–18 Including these genetic risk factors and other risk factors (such as dietary or supplemental antioxidant intake) in the risk score development may improve its predictive power for GA. Seddon et al recently developed a comprehensive predictive model for advanced AMD (CNV and GA combined) based on both genetic, demographic and environmental variables, and found that the AUC (c-statistic) improved from 0.73 to 0.83 when genetic data was included in the prediction model.18
Despite the fact that our risk scores were developed to predict endpoint GA, they performed well for predicting CGA and any GA. In addition, very similar discrimination was obtained when it was applied to the untreated eye of CAPT participants. However, before it is taken for use in clinical practice and research, external validation 34 needs to be established by applying it to other independent AMD cohorts, such as by applying the risk score without consideration of night vision score to the AREDS datasets.
In summary, the GA risk scores developed from the CAPT data discriminated several levels of risk and provided accurate estimates of risk for the CAPT participants. If the discrimination and accuracy are validated in other independent groups of patients, they will provide useful tools for identifying high risk patients for clinical trials for prevention of GA and for GA risk assessment of AMD patients.
Acknowledgments
Supported by grants EY012211, EY012261, and EY012279, from the National Eye Institute, National Institutes of Health, Department of Health and Human Services.
Footnotes
Presented in part at the meeting of the Association for Research and Vision in Ophthalmology in Fort Lauderdale, Florida on May 4, 2009.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sarks JP, Sarks SH, Killingsworth MC. Evolution of geographic atrophy of the retinal pigment epithelium. Eye (Lond) 1988;2:552–77. doi: 10.1038/eye.1988.106. [DOI] [PubMed] [Google Scholar]
- 2.Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–72. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 3.Brown DM, Kaiser PK, Michels M, et al. ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–44. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 4.Rosenfeld PJ, Brown DM, Heier JS, et al. ANCHOR Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 5.Smith W, Assink J, Klein R, et al. Risk factors for age-related macular degeneration: pooled findings from three continents. Ophthalmology. 2001;108:697–704. doi: 10.1016/s0161-6420(00)00580-7. [DOI] [PubMed] [Google Scholar]
- 6.Cong RH, Zhou B, Su QM, et al. Smoking and the risk of age-related macular degeneration: a meta-analysis. Ann Epidemiol. 2008;18:647–56. doi: 10.1016/j.annepidem.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Age-Related Eye Disease Study Research Group. Risk factors for the incidence of advanced age-related macular degeneration in the Age-Related Eye Disease Study (AREDS): AREDS report no. 19. Ophthalmology. 2005;112:533–9. doi: 10.1016/j.ophtha.2004.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS report no. 17. Arch Ophthalmol. 2005;123:1484–98. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Complications of Age-related Macular Degeneration Prevention Trial (CAPT) Research Group. Risk factors for choroidal neovascularization and geographic atrophy in the Complications of Age-related Macular Degeneration Prevention Trial. Ophthalmology. 2008;115:1474–9. doi: 10.1016/j.ophtha.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Ying GS, Maguire MG, Alexander J, et al. Description of the Age-Related Eye Disease Study 9-step severity scale applied to participants in the Complications of Age-related Prevention Trial. Arch Ophthalmol. 2009;127:1147–51. doi: 10.1001/archophthalmol.2009.189. [DOI] [PubMed] [Google Scholar]
- 11.Tan JS, Mitchell P, Kifley A, et al. Smoking and the long-term incidence of age-related macular degeneration: the Blue Mountains Eye Study. Arch Ophthalmol. 2007;125:1089–95. doi: 10.1001/archopht.125.8.1089. [DOI] [PubMed] [Google Scholar]
- 12.Tomany SC, Wang JJ, van Leeuwen R, et al. Risk factors for incident age-related macular degeneration: pooled findings from 3 continents. Ophthalmology. 2004;111:1280–7. doi: 10.1016/j.ophtha.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Klein R, Klein BE, Tomany SC, Cruickshanks KJ. The association of cardiovascular disease with the long-term incidence of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 2003;110:1273–80. doi: 10.1016/S0161-6420(03)00599-2. [DOI] [PubMed] [Google Scholar]
- 14.Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004;137:486–95. doi: 10.1016/j.ajo.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 15.Klein R, Knudtson MD, Cruickshanks KJ, Klein BE. Further observations on the association between smoking and the long-term incidence and progression of age-related macular degeneration: the Beaver Dam Eye Study. Arch Ophthalmol. 2008;126:115–21. doi: 10.1001/archopht.126.1.115. [DOI] [PubMed] [Google Scholar]
- 16.Seddon JM, Francis PJ, George S, et al. Association of CFH Y402H and LOC387715 A69S with progression of age-related macular degeneration. JAMA. 2007;297:1793–800. doi: 10.1001/jama.297.16.1793. [DOI] [PubMed] [Google Scholar]
- 17.Yates JR, Sepp T, Matharu BK, et al. Genetic Factors in AMD Study Group. Complement C3 variant and the risk of age-related macular degeneration. N Engl J Med. 2007;357:553–61. doi: 10.1056/NEJMoa072618. [DOI] [PubMed] [Google Scholar]
- 18.Seddon JM, Reynolds R, Maller J, et al. Prediction model for prevalence and incidence of age-related macular degeneration based on genetic, demographic and environmental variables. Invest Ophthalmol Vis Sci. 2009;50:2044–53. doi: 10.1167/iovs.08-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ying GS, Maguire MG, Liu CC, Antoszyk AN Complications of Age-related Macular Degeneration Prevention Trial Research Group. Night vision symptoms and progression of age-related macular degeneration (AMD) in the Complications of Age-related Macular Degeneration Prevention Trial. Ophthalmology. 2008;115:1876–82. doi: 10.1016/j.ophtha.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Agostino RB, Sr, Grundy S, Sullivan LM, et al. CHD Risk Prediction Group. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–7. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 21.Medeiros FA, Weinreb RN, Sample PA, et al. Validation of a predictive model to estimate the risk of conversion from ocular hypertension to glaucoma. Arch Ophthalmol. 2005;123:1351–60. doi: 10.1001/archopht.123.10.1351. [DOI] [PubMed] [Google Scholar]
- 22.Ocular Hypertension Treatment Study Group, European Glaucoma Prevention Study Group. Validated prediction model for the development of primary open-angle glaucoma in individuals with ocular hypertension. Ophthalmology. 2007;114:10–9. doi: 10.1016/j.ophtha.2006.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medeiros FA, Weinreb RN. Predictive models to estimate the risk of glaucoma development and progression. Prog Brain Res. 2008;173:15–24. doi: 10.1016/S0079-6123(08)01102-3. [DOI] [PubMed] [Google Scholar]
- 24.Complications of Age-Related Macular Degeneration Prevention Trial Research Group. The Complications of Age-Related Macular Degeneration Prevention Trial (CAPT): rationale, design and methodology. Clin Trials. 2004;1:91–107. doi: 10.1191/1740774504cn007xx. [DOI] [PubMed] [Google Scholar]
- 25.Complications of Age-Related Macular Degeneration Prevention Trial Research Group. Laser treatment in patients with bilateral large drusen: the Complications of Age-Related Macular Degeneration Prevention Trial. Ophthalmology. 2006;113:1974–86. doi: 10.1016/j.ophtha.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Age-Related Eye Disease Study Research Group. A simplified severity scale for age-related macular degeneration: AREDS report no. 18. Arch Ophthalmol. 2005;123:1570–4. doi: 10.1001/archopht.123.11.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sullivan LM, Massaro JM, D’Agostino RB., Sr Presentation of multivariate data for clinical use: the Framingham Study risk score functions. Stat Med. 2004;23:1631–60. doi: 10.1002/sim.1742. [DOI] [PubMed] [Google Scholar]
- 28.Margolis DJ, Bilker W, Boston R, et al. Statistical characteristics of area under the receiver operating characteristic curve for a simple prognostic model using traditional and bootstrapped approaches. J Clin Epidemiol. 2002;55:518–24. doi: 10.1016/s0895-4356(01)00512-1. [DOI] [PubMed] [Google Scholar]
- 29.Choi B. Slopes of a receiver operating characteristic curve and likelihood ratios for a diagnostic test. Am J Epidemiol. 1998;148:1127–32. doi: 10.1093/oxfordjournals.aje.a009592. [DOI] [PubMed] [Google Scholar]
- 30.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Vol. 57 London: Chapman and Hall; 1993. pp. 168–177. Monographs on Statistics and Applied Probability. [Google Scholar]
- 31.Brier GW. Verification of weather forecasts expressed in terms of probability. Mon Weather Rev. 1950;78:1–3. [Google Scholar]
- 32.Harrell FE, Jr, Lee KL, Mark DB. Multivariate prognostic models: issues in developing models, evaluating assumption and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 33.Klein ML, Ferris FL, III, Armstrong J, et al. AREDS Research Group. Retinal precursors and the development of geographic atrophy in age-related macular degeneration. Ophthalmology. 2008;115:1026–31. doi: 10.1016/j.ophtha.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 34.Altman DG, Royston P. What do we mean by validating a prognostic model? Stat Med. 2000;19:453–73. doi: 10.1002/(sici)1097-0258(20000229)19:4<453::aid-sim350>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]