Abstract
Background
Premenstrual dysphoric disorder (PMDD), a dysphoric form of premenstrual syndrome, is included as a diagnosis for further study in the DSM-IV-TR (APA, 2000). The present study investigated whether a marker of risk for Major Depressive Disorder (MDD), prefrontal brain asymmetry, also characterizes women with PMDD.
Methods
In a sample of 25 college women with PMDD symptomatology and 25 matched controls, resting frontal electroencephalographic (EEG) activity was assessed on four occasions within a two-week span.
Results
Across several frontal sites women with PMDD had relatively less left than right prefrontal brain activity, consistent with a diathesis-stress model for menstrual-related dysphoria.
Conclusions
The findings suggest an overlap in the risk profile for MDD and PMDD.
Keywords: EEG asymmetry, premenstrual dysphoria, risk, diathesis
INTRODUCTION
Premenstrual dysphoric disorder (PMDD) is a severe dysphoric form of premenstrual syndrome (PMS) that has an estimated prevalence of 3%–8% (Steiner, 2000) and an additional 19% identified as ‘near-threshold’ cases that failed to meet the strict PMDD criteria (Wittchen, 2002) and is included as a diagnosis for further study in the DSM-IV-TR (APA, 2000). PMDD is a complex, chronic, psychoneuroendocrine disorder that affects functioning and well-being and continues throughout the reproductive years. Like all premenstrual syndromes, PMDD is characterized by its symptom pattern being linked to the menstrual cycle, with pronounced symptoms in the late luteal phase, symptom remission during the menstrual flow, and a symptom-free period in the follicular phase of the cycle (Freeman, 2004). The DSM-IV diagnosis of PMDD requires at least five of 11 symptoms, including at least one of the mood symptoms, that are severe pre-menstrually and abate post-menstrually, and that markedly impair functioning. These 11 PMDD symptoms comprise depressed mood, anxiety/tension, mood swings, irritability/marked anger, decreased interest, difficulty concentrating, fatigue, appetite changes, sleep difficulties, feeling out of control and physical symptoms (APA, 2000).
The similarities in symptoms between PMDD and major depressive disorder (MDD) are evident, with a severe case of PMDD resembling a time-limited full depressive episode, as there are 7 overlapping symptomatic criteria (Yonkers et al., 1997). Women with PMDD are more likely to have had a history of depression (Endicott & Halbreich, 1988) and are more likely to develop a subsequent episode of MDD (Yonkers et al., 1997). The comorbidity between the two disorders is significant and is conservatively estimated across studies at 30% for current MDD (Endicott, 1994) or past MDD (Yonkers et al., 1997; Cohen et al., 2002; Pearlstein et al., 1990). Additionally, PMDD is associated with an increased risk of developing MDD, above and beyond a family history and a personal history of depression (Graze et al., 1990). Most women with MDD (83% current MDD; 57% past MDD) have premenstrual changes, including an increase in the severity and appearance of depressive symptoms and less control of suicidal impulses (Endicott & Halbreich, 1988). Whether such symptomatic similarities reflect similar underlying liabilities for the two conditions remains an empirical question.
One promising biological marker of risk for MDD is prefrontal brain asymmetry measured by resting electroencephalographic (EEG) activity, which can distinguish depressed individuals — those currently symptomatic and those in remission — from never-depressed individuals (e.g., Allen, Urry, Hitt, & Coan, 2004; Debener et al., 2000; Gotlib, Ranganath, and Rosenfeld, 1998; Henriques & Davidson, 1991; Stewart, Bismark, Towers, Coan, & Allen, in press). This pattern of asymmetry is characterized by relatively less left than right frontal activity, inferred by relatively greater left than right frontal alpha band power, since alpha is generally inversely related to other indices of active cortical processing (Allen, Coan, & Nazarian, 2004). Since the pattern of findings to date suggests that prefrontal brain asymmetry indexes a risk for depression and a variety of mood-related psychopathology (e.g., Allen, Urry, et al. 2004; Coan & Allen, 2004; Thibodeau, Jorgensen, & Kim, 2006), frontal EEG asymmetry may also be a marker of PMDD risk.
Two small-scale studies have examined prefrontal brain asymmetry as a function of premenstrual dysphoric symptoms. Baehr, Rosenfeld, Miller, and Baehr (2004) observed two monthly cycles for five women diagnosed with PMDD and one monthly cycle for five non-PMDD control participants. Asymmetry scores did not differ for control or PMDD participants outside of the luteal phase, but scores for the PMDD group fell into the negative range (less left frontally active) during the luteal period while the control participants remained stable. In contrast, Accortt and Allen (2006) used a larger unmedicated sample, with carefully timed late luteal and follicular visits by women either high or low in self-reported premenstrual negative affect. Women reporting high pre-menstrual distress (n=12) exhibited significantly less relative left frontal-temporal activity at rest than women low in pre-menstrual distress (n=11), regardless of the phase of cycle (follicular versus luteal). These pilot studies suggested the possibility of a common diathesis for both major depression and premenstrual dysphoria (Accortt & Allen, 2006).
The present study investigated whether a putative endophenotype of risk for depression, resting frontal EEG asymmetry, is present among those with PMDD symptomatology in a large sample that, unlike the pilot study (Accortt & Allen, 2006), includes individuals with MDD and dysthymia. This allows the current study to examine whether frontal EEG asymmetry is sensitive to PMDD above and beyond a history of MDD. Participants with current PMDD symptomatology are hypothesized to show relatively less left frontal activity than those without PMDD (regardless of MDD diagnosis), providing a strong test of the role of resting frontal EEG asymmetry as a risk factor for menstrual-related dysphoria.
METHODS
Participant Selection
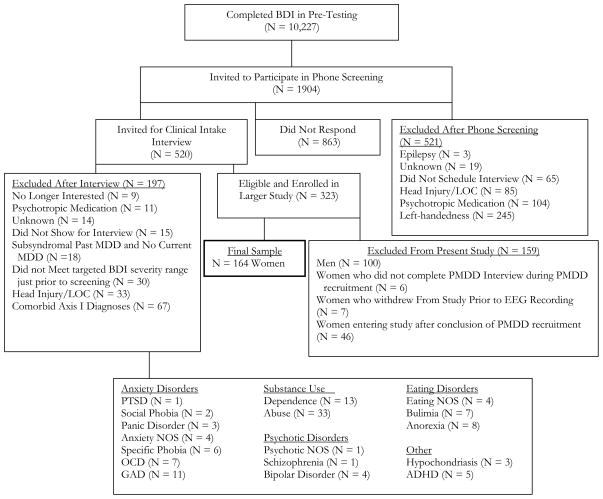
The sample included women with and without PMDD symptomatology who were participants in a larger study of EEG asymmetry and risk for MDD (Stewart et al., in press). Figure 1 provides a detailed flow chart summarizing study recruitment across a four year period. During an initial telephone screening, subjects met the following inclusion criteria: 1) free of current prescription and/or use of psychotropic medication and not under the influence of psychoactive substances; 2) no current psychological disorder other than PMDD, MDD, or dysthymia; 3) no history of mania or psychosis; 4) absence of disorders affecting the central nervous system (e.g., previous head injury resulting in the loss of consciousness); and 5) strong right-handedness as indicated by a score of 36 or greater on the 39-point inventory of Chapman and Chapman (1987). Eligible participants were invited to the laboratory for an intake interview.
Figure 1.
Note: BDI = Beck Depression Inventory. LOC = Loss of consciousness. MDD = major depressive disorder. PMDD = pre-menstrual dysphoric disorder. PTSD = posttraumatic stress disorder. NOS = Not otherwise specified. OCD = obsessive compulsive disorder. GAD = generalized anxiety disorder. ADHD = attention deficit hyperactivity disorder.
During the intake interview, participants were assessed by a graduate-level clinician with the Hamilton Depression Rating Scale (HRSD; Hamilton, 1967), the Structured Clinical Interview for the DSM-IV (SCID; First et al., 1994) and with a custom PMDD interview1. The custom PMDD interview involved asking each woman a series of questions concerning PMDD symptoms, and was created from the DSM-IV-TR PMDD criteria, and modeled after the SCID in terms of format. Women who met PMDD criteria were asked to complete a daily symptom rating checklist (Endicott et al., 2006) for two consecutive cycles. All women included in the study were asked about their current phase of cycle, by counting backwards from the first day of the next anticipated menses, and about their use of oral contraceptives in order to determine cycle timing. Inter-rater reliability data of the custom PMDD interview are available from an ongoing study with an independent set of 25 recorded interviews that were rated by two independent trained raters. Perfect agreement was obtained (Kappa = 1.0) in this sample of 10 PMDD+ and 15 PMDD− women.
Participant Grouping
The final sample of 164 women consisted of university undergraduate students age 18 to 25 (M = 18.9 years ± 1.2 SD). Demographic information is provided in Table 1. Full DSM-IV PMDD criteria were met by 8% of the sample, comparable to the 6% estimated prevalence among 14 to 24 year old females (Wittchen, 2002). An additional 7% met all DSM-IV-TR PMDD criteria (including the impairment criterion), except for symptoms present “most of the week”. Thus 15% of the sample were included in the PMDD symptomatology group. A total of 30 women endorsed sufficient pre menstrual symptoms during the intake interview to be invited to complete the daily PMDD symptom diary (Daily Record of Severity of Problems, DRSP). Due to poor compliance, however, the diary data were unable to provide conclusive evidence of 2 consecutive cycles with late luteal phase mood worsening. Partial diary data disconfirmed at least one cycle of late luteal mood worsening in 5 participants all of whom were excluded from the PMDD classification. Among the remaining 25 interview-identified participants, 13 met DSM-IV-TR criteria during the interview, endorsing five or more of the DSM-IV-TR items with symptoms that lasted four or more days and produced impairment. The other 12 participants endorsed five or more of the DSM-IV-TR items during the interview, with symptoms that caused clinically significant impairment, but that lasted fewer than four days. All 25 women, by virtue of having significant symptoms that resulted in impairments in function were thus included in the PMDD symptomatology group. A group of 25 control subjects without PMDD were selected from the pool of 164 women and matched for depression classification2 with participants in the PMDD symptomatology group. The 25 PMDD positive cases endorsed an average of 6.72 PMDD symptoms and the 25 controls endorsed an average of 1.00 PMDD symptoms.
Table 1.
Demographic Information
| Entire Sample | Matched Controls for EEG | PMDD Symptomatology | |
|---|---|---|---|
| N subjects | 164 | 25 | 25 |
| Mean Age | 18.8 +/− 1.2 | 18.8 +/− 0.62 | 18.9 +/− 1.4 |
| % HBC | 36.6 % | 32 % | 40 % |
| N with Dysthymia | 11 | 1 | 2 |
| N with lifetime MDD | 35 | 10 | 9 |
| Mean HRSD | 8.0 +/− 6.9 | 9.6 +/− 8.3 | 12.2 +/− 7.0 |
| Mean # PMDD | 2.2 +/− 2.6 | 1.0 +/− 1.6 | 6.8 +/− 1.7 |
| Symptoms * | |||
| Race Percentage | |||
| Caucasian | 73.8 % | 76 % | 84 % |
| Other | 9.1 % | 16 % | |
| Asian | 5.5 % | 4 % | 4 % |
| Black | 3.7 % | 4 % | 8 % |
| Am. Indian | 3.7 % | 4 % | |
| More than one | 0.6 % | ||
| No Response | 3.7 % | ||
p<0.01 for the comparison of the matched controls and participants with PMDD symptomatology. Unless marked with an asterisk, no significant differences between these groups were found.
Note: PMDD= Pre-menstrual dysphoric disorder. MDD= Major Depressive Disorder. HBC= Hormonal Birth Control. HRSD = Hamilton Rating Scale for Depression. Means are shown +/− standard deviations.
Physiological Recording and Data Reduction
Participants made four post-intake visits to the laboratory for EEG assessments within a two-week period3. A 64-channel electrode cap with additional electrodes to monitor eye movements was used to acquire scalp potentials. Impedances at all sites were less than 10K ohms. Signals were recorded with a SynAmps2 amplifier. All EEG sites were referenced online to a reference lead posterior to Cz, recorded with digital differential amplifiers (bandpass DC to 200 Hz), and digitized continuously at 1000 Hz. Resting EEG was for two eight minute periods, separated by about 20 minutes, each in blocks with eyes-open (O) and with eyes-closed (C), in one of two counterbalanced orders (OCCOCOOC or COOCOCCO).
Each EEG record was visually screened to remove epochs with movement and muscle artifacts. A computer-based blink rejection algorithm then rejected any epoch with activity greater than ± 75 microvolts in amplitude in the vertical ocular channel, and an artifact rejection algorithm rejected segments with large fast deviations in amplitude in any channel (e.g., DC shifts and spikes) that may have been missed by human inspection. Data were re-referenced to three reference montages: 1) computer averaged mastoids (LM; “linked” mastoids); 2) the average of active EEG sites (AR; average reference); and, 3) current source density (CSD, using algorithms from Kayser & Tenke, 2006 and based on the spherical spline approach summarized by Perrin, Pernier, Bertrand, & Echallier, 1989, 1990).
Each one-minute EEG block was divided into 117 2.048 second epochs that overlapped by 1.5 seconds. A Fast Fourier Transform (FFT) was applied to all artifact-free epochs, after the data was weighted with a Hamming window that tapered the distal 50% of each epoch. The power spectra of these epochs were then averaged across all eight minutes. Average power in the 8–13 Hz band was taken as an index of alpha power. Finally, an asymmetry score was computed by taking the difference of natural log transformed scores for all sites that had symmetrical left and right locations: ln[right]-ln[left]), with higher values on this index putatively reflecting relatively greater left activity (i.e. relatively greater right alpha; Allen, Nazarian, et al., 2004). Asymmetry scores for the two resting sessions within-day were averaged within day to provide the estimate of brain activity for each lab visit. Thus, separate asymmetry scores for each day for four days for each of three reference montages were used in analyses, resulting in 12 asymmetry scores per participant at each homologous pair. Analyses for the present study were performed on a specific subset of those pairs (F2-F1, F4-F3, F6-F5, F8-F7) that correspond to regions commonly studied throughout the asymmetry literature (pairs F4-F3 and F8-F7; see review by Coan & Allen, 2004) as well as pairs of channels that neighbor pairs F4-F3 and F8-F7.
RESULTS
To assess whether EEG asymmetry is a marker of premenstrual dysphoria, and is independent of MDD history, asymmetry scores for each of four frontal regions were examined using a full factorial mixed linear model (SAS 9.2). PMDD status and lifetime MDD status were between-subject factors, and day (4), reference (3: AR, CSD, LM) and channel (F2-F1, F4-F3, F6-F5, F8-F7) were repeated factors. Specific effects of interest were the main effect of PMDD status, the main effect of lifetime MDD status, and their interaction. Total alpha (8–13 Hz) results are reported here4. Cohen’s d is reported to indicate effect size between groups.
Results (see Figure 2) indicate that a main effect of PMDD emerged (F(1, 46) = 15.0, p < .001, d = 1.10), showing that PMDD+ participants displayed relatively less left frontal activity than PMDD- participants, replicating previous work (Accortt & Allen, 2006) and consistent with previous findings in MDD women. In addition, a main effect of lifetime MDD emerged (F(1, 46) = 9.6, p < .01, d = .88), indicating that women with lifetime MDD (M = .013, SE = .006) also displayed lower relative left frontal activity than women without MDD (M = .046, SE = .009).
Figure 2.
Frontal EEG alpha asymmetry at four frontal regions as a function of PMDD status (25 PMDD+, 25 PMDD−). Error bars depict standard error.
Furthermore, interactions for PMDD by lifetime MDD (F(1, 46) = 4.3, p < .05), reference by PMDD by lifetime MDD (F(2, 92) = 4.1, p < .05), PMDD by lifetime MDD by channel (F(3, 138) = 3.7, p < .05) emerged and were qualified by a PMDD by lifetime MDD by reference by channel interaction (F(6, 276) = 3.0, p < .01), and follow-up mixed models run for each reference separately indicated that the main effect of PMDD was the only significant effect that emerged for AR (F(1, 46) = 6.9, p < .05) and LM (F(1, 46) = 10.4, p < .01) reference montages. For the CSD reference, however, in addition to the significant PMDD effect (F(1, 46) = 4.2, p < .05), significant interactions were found for the PMDD by lifetime MDD interaction (F(1, 46) = 5.5, p < .05) and PMDD by MDD by channel interaction (F(3, 138) = 4.1, p < .01). This interaction was driven by F2-F1, as at all channels except F2-F1 for PMDD+ participants, lifetime MDD+ was linked to relatively less left frontal activity than lifetime MDD−, whereas only F2-F1 showed this pattern in PMDD− participants (all p < .05).
DISCUSSION
Replicating and extending previous studies, PMDD was characterized by a pattern of resting brain asymmetry like that seen in depression. Moreover, the present findings are consistent with previous findings derived from a sample of women with high levels of menstrual-related dysphoria (Accortt & Allen, 2006). Women with PMDD symptomatology had relatively less left than right prefrontal brain activity, consistent with a diathesis-stress model for menstrual-related dysphoria. Thus, in addition to growing evidence suggesting that relatively less left prefrontal brain activity is a risk factor for MDD, our current findings suggest that it may also serve as a diathesis for a broader range of dysphoric mood including that of PMDD symptomatology. The present study additionally improves upon previous work (Accortt & Allen, 2006) by examining a larger sample, using a reliable structured clinical interview based upon the DSM-IV criteria to confirm PMDD classifications (as opposed to the Menstrual Distress Questionnaire, Moos 1968), and including women diagnosed with MDD and dysthymia. Our findings suggest that the relationship of PMDD status and prefrontal brain asymmetry cannot be solely accounted for by comorbid MDD. The age range of our cohort is 18–25, which does not span a majority of the period of risk for either MDD or PMDD, yet studies find that premenstrual symptoms worsen with age (Hartlage et al., 2004; Steiner, 2000). The present study is an early onset sample, in terms of PMDD and MDD, and may thus be expected to have more lifetime mood instability and more lifetime episodes than those with later onset.
Results of the present study are consistent with the notion that a shared risk profile may exist for women with hormonal related depression and MDD. The underlying risk, as indexed by prefrontal brain asymmetry, may serve to potentiate mood volatility in the face of stressors (both hormonal and psychosocial), leading to an increased likelihood of MDD or PMDD. Such episodes may sensitize depressed women to pre-menstrual exacerbation of their MDD symptoms, and repeated depressive episodes may act as a “kindling” mechanism in which increasingly severe depression occurs with little provocation (Post & Ballenger, 1981). The provocation, in this case, may be fluctuations of hormones and neurotransmitters related to the menstrual cycle (Breaux et al., 2000; Kendler et al., 2001).
Strengths, Limitations, and Future Directions
A limitation of the present study was that endocrinological measures of each woman’s menstrual cycle were not collected. On the other hand, the present study did not seek to access EEG asymmetry at each phase of the menstrual cycle. Moreover, our previous study that was designed to specifically address cycle-related changes in prefrontal brain asymmetry found stability of asymmetry across phases of the menstrual cycle, rendering the absence of endocrinological measures less critical for the aim of the present study. A second limitation was that two months of diary data were not available to properly confirm a PMDD DSM-IV-TR diagnosis, although a daily web diary was set up to assess this information. In the absence of financial incentive for regular completion, however, women did not consistently fill out the diary every day for two months. Lastly, although we collected data regarding use of oral contraceptives (OC), we did not collect data on the specific composition of OC nor of other hormonal based therapies. Because synthetic estrogen and progestin as opposed to progestin-only OCs may influence depressive and physical symptoms in depressed women (Young et al., 2007), future work should gather information about the specific composition of the hormonal birth control (HBC) used in research samples.
This study found that a putative marker of risk for depression, frontal EEG asymmetry, is also sensitive to PMDD symptomatology in women with and without MDD or dysthymia. It remains to be determined whether it can also prospectively identify those women at risk for the future development of PMDD. Ideally, frontal EEG asymmetry could help us learn more about the etiology of depression and hormonal-related depression specifically, and test whether they may share etiological factors.
Acknowledgments
This research was supported in part by grants from the National Institutes of Health (R01-MH066902) and the National Alliance for Research on Schizophrenia and Depression (NARSAD) to John Allen. The authors wish to thank Eliza Fergerson, Jamie Velo, Dara Halpern, Craig Santerre, Amanda Brody, Jay Hegde and myriad research assistants for their help on this project.
Footnotes
A copy of the interview is available by contacting the authors.
In terms of matching, although there were 2 women in the PMDD symptomatology classification who met criteria for dysthymia status, there was only 1 individual without a PMDD diagnosis with dysthymia; thus the other PMDD participant was matched with a woman meeting criteria for MDD status instead.
Three women had sessions that extended beyond two weeks, but all sessions for these women occurred within 18 days.
Examining these same sites in an identical model using lower alpha (8–10.5 Hz) as the dependent variable, the PMDD main effect (F(1, 46) = 24.2, p < .0001, d = 1.39) emerged, replicating patterns demonstrated in total alpha results.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Accortt EE, Allen JJB. Frontal EEG Asymmetry and Premenstrual Dysphoric Symptomatology. Journal of Abnormal Psychology. 2006;115:179–184. doi: 10.1037/0021-843X.115.1.179. [DOI] [PubMed] [Google Scholar]
- Allen JJB, Coan JA, Nazarian M. Issues and assumptions on the road from raw signals to metrics of frontal EEG asymmetry in emotion. Biological Psychology. 2004;67:183–218. doi: 10.1016/j.biopsycho.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Allen JJB, Urry HL, Hitt SK, Coan JA. The stability of resting frontal electroencephalographic asymmetry in depression. Psychophysiology. 2004;41(2):269–80. doi: 10.1111/j.1469-8986.2003.00149.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th edition, text revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Angst J, Sellaro R, Merikangas KR, Endicott J. The epidemiology of perimenstrual psychological symptoms. Acta Psychiatr Scand. 2001;104(2):110–6. doi: 10.1034/j.1600-0447.2001.00412.x. [DOI] [PubMed] [Google Scholar]
- Baehr E, Rosenfeld JP, Miller, Baehr R. Premenstrual dysphoric disorder and changes in frontal alpha asymmetry. Int J Psychophysiol. 2004;52(2):159–67. doi: 10.1016/j.ijpsycho.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Bhatia SC, Bhatia SK. Diagnosis and treatment of premenstrual dysphoric disorder. Am Fam Physician. 2003;66:1239–48. [PubMed] [Google Scholar]
- Breaux C, Hartlage S, Gehlert S. Relationships of premenstrual dysphoric disorder to major depression and anxiety disorders: a re-examination. Journal of Psychosom Obstet Gynaecol. 2000;21(1):17–24. doi: 10.3109/01674820009075604. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. The measurement of handedness. Brain Cogn. 1987;6(2):175–83. doi: 10.1016/0278-2626(87)90118-7. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJ. Frontal EEG asymmetry as a moderator and mediator of emotion. Biol Psychol. 2004;67(1–2):7–49. doi: 10.1016/j.biopsycho.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Cohen LS, Miner C, Brown EW, et al. Premenstrual daily fluoxetine for premenstrual dysphoric disorder: A placebo-controlled, clinical trial using computerized diaries. Obstetrics Gynecolology. 2002;100:435–44. doi: 10.1016/s0029-7844(02)02166-x. [DOI] [PubMed] [Google Scholar]
- Contreras CM, Marvan ML, Alcala-Herrera V, Yeyha A. Relations between anxiety, psychophysiological variables and menstrual cycle in healthy women. Bol Estud Med Biol. 1989;37(1–2):50–6. [PubMed] [Google Scholar]
- Davidson RJ, Fox NA. Frontal brain asymmetry predicts infants’ response to maternal separation. Journal of Abnormal Psychology. 1989;98:127–131. doi: 10.1037//0021-843x.98.2.127. [DOI] [PubMed] [Google Scholar]
- Di Giulio G, Reissing ED. Premenstrual dysphoric disorder: prevalence, diagnostic considerations, and controversies. Journal of Psychosomatic Obstet Gynaecol. 2006;27(4):201–10. doi: 10.1080/01674820600747269. [DOI] [PubMed] [Google Scholar]
- Debener S, Beauducel A, Nessler D, Brocke B, Heilemann H, Kayser J. Is resting anterior EEG alpha asymmetry a trait marker for depression? Findings for healthy adults and clinically depressed patients. Neuropsychobiology. 2000;41:31–37. doi: 10.1159/000026630. [DOI] [PubMed] [Google Scholar]
- Endicott J. Differential diagnosis and comorbidity. In: Gold JH, Severino SK, editors. Premenstrual Dysphoria: Women’s Reality. APA Press; Washington, DC: 1994. [Google Scholar]
- Endicott J, Amsterdam J, Eriksson E, Frank E, Freeman E, Hirschfeld R, Ling F, Parry B, Pearlstein T, Rosenbaum J, Rubinow D, Schmidt P, Severino S, Steiner M, Stewart DE, Thys-Jacobs S. Is premenstrual dysphoric disorder a distinct clinical entity? J Womens Health Gend Based Medicine. 1999;8(5):663–79. doi: 10.1089/jwh.1.1999.8.663. [DOI] [PubMed] [Google Scholar]
- Endicott J, Halbreich U. Clinical significance of premenstrual dysphoric changes. J Clin Psychiatry. 1988;49:486–489. [PubMed] [Google Scholar]
- Endicott J, Nee J, Harrison W. Daily Record of Severity of Problems (DRSP): reliability and validity. Arch Womens Ment Health. 2006;9(1):41–9. doi: 10.1007/s00737-005-0103-y. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon MG, Williams JBW. Unpublished manuscript. New York: Biometrics Research Department, Ney York State Psychiatric Institute; 1994. The Structured Clinical Interview for DSMIV. [Google Scholar]
- Fox NA, Davidson RJ. Patterns of brain electrical activity during facial signs of emotion in 10 month-old infants. Develomental Psychology. 1988;24:230–246. [Google Scholar]
- Freeman EW. Luteal Phase Administration of Agents for the Treatment of Premenstrual Dysphoric Disorder. CNS Drugs. 2004;18(7):453–468. doi: 10.2165/00023210-200418070-00004. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Ranganath C, Rosenfeld JP. Frontal EEG alpha asymmetry, depression, and cognitive functioning. Cognition & Emotion. 1998;12:449–478. [Google Scholar]
- Graze KK, Nee J, Endicott J. Premenstrual depression predicts future major depressive disorder. Acta Psychiatr Scand. 1990;81(2):201–5. doi: 10.1111/j.1600-0447.1990.tb06479.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. British Journal of Social & Clinical Psychology. 1967;6(4):278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Hartlage SA, Brandenburg DL, Kravitz HM. Premenstrual Exacerbation of Depressive Disorders In a Community-Based Sample in the United States. Psychosomatic Medicine. 2004;66:698–706. doi: 10.1097/01.psy.0000138131.92408.b9. [DOI] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Left frontal hypoactivation in depression. Journal of Abnormal Psychology. 1991;100:535–545. doi: 10.1037//0021-843x.100.4.535. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Thornton LM, Gardner CO. Genetic Risk, Number of Previous Depressive Episodes, and Stressful Life Events in Predicting Onset of Major Depression. Am J Psychiatry. 2001;158:582–586. doi: 10.1176/appi.ajp.158.4.582. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, MS, Jin R, Merikangas KR, Walters EE. Lifetime Prevalence and Age-of-Onset Distributions of DSM-IV Disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Landen M, Eriksson E. How does premenstrual dysphoric disorder relate to depression and anxiety disorders? Depression & Anxiety. 2003;17(3):122–9. doi: 10.1002/da.10089. [DOI] [PubMed] [Google Scholar]
- Miller A, Fox NA, Cohn JF, Forbes EE, Sherrill JT, Kovacs M. Regional Patterns of Brain Activity in Adults With a History of Childhood-Onset Depression: Gender Differences and Clinical Variability. Am J Psychiatry. 2002;159:934–940. doi: 10.1176/appi.ajp.159.6.934. [DOI] [PubMed] [Google Scholar]
- Moos RH. The development of a menstrual distress questionnaire. Psychosomatic Medicine. 1968;30:853–867. doi: 10.1097/00006842-196811000-00006. [DOI] [PubMed] [Google Scholar]
- Pearlstein TB, Frank E, Rivera-Tovar A, Thoft JS, Jacobs E, Mieczkowski TA. Prevalence of axis I and axis II disorders in women with late luteal phase dysphoric disorder. J Affect Disorders. 1990;20(2):129–34. doi: 10.1016/0165-0327(90)90126-s. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalography and clinical. Neurophysiology. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Corrigenda. Electroencephalography and clinical. Neurophysiology. 1990;76:565–566. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Post RM, Ballenger JC. Kindling models for the progressive development of psychopathology: Sensitization to electrical, pharmacological, and psychological stimuli. In: van Praag HM, van Praag HM, Lader MH, Rafaelsen OJ, Sachar EJ, editors. Experimental and Clinical Psychiatry. 4. Vol. 1. New York: Marcel Dekker; 1981. pp. 609–51. [Google Scholar]
- Steffens DC, Krishnan KR, Helms MJ. Are SSRIs better than TCAs? Comparison of SSRIs and TCAs: a meta-analysis. Depress Anxiety. 1997;6(1):10–8. doi: 10.1002/(sici)1520-6394(1997)6:1<10::aid-da2>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Steiner M, Steinberg S, Stewart D. Fluoxetine in the treatment of premenstrual dysphoria. New England Journal of Medicine. 1995;332:1529–34. doi: 10.1056/NEJM199506083322301. [DOI] [PubMed] [Google Scholar]
- Steiner M. Premenstrual syndrome and premenstrual dysphoric disorder: Guidelines for management. Journal of Psychiatry Neuroscience. 2000;25:459–68. [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, Bismark AW, Towers DN, Coan JA, Allen JJB. Resting frontal EEG asymmetry as an endophenotype for depression risk: Sex-specific patterns of frontal brain asymmetry. Journal of Abnormal Psychology. doi: 10.1037/a0019196. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeau R, Jorgensen RS, Kim S. Depression, anxiety, and resting frontal EEG asymmetry: A meta-analytic review. Journal of Abnormal Psychology. 2006;115:715–729. doi: 10.1037/0021-843X.115.4.715. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Davidson RJ, Wheeler RE, Kinney L. Psychometric properties of resting anterior EEG asymmetry: Temporal stability and internal consistency. Psychophysiology. 1992;29:576–592. doi: 10.1111/j.1469-8986.1992.tb02034.x. [DOI] [PubMed] [Google Scholar]
- Wheeler RE, Davidson RJ, Tomarken AJ. Frontal brain asymmetry and emotional reactivity: A biological substrate of affective style. Psychophysiology. 1993;30:82–89. doi: 10.1111/j.1469-8986.1993.tb03207.x. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Becker E, Leib R. Prevalence, incidence and stability of premenstrual dysphoric disorder in the community. Psychological Medicine. 2002;32(1):119–32. doi: 10.1017/s0033291701004925. [DOI] [PubMed] [Google Scholar]
- Wood SH, Mortola JF, Chan YF. Treatment of premenstrual syndrome with fluoxetine: a double-blind, placebo-controlled, crossover study. Obstetrics & Gynecololgy. 1992;80:339–44. [PubMed] [Google Scholar]
- Yonkers KA, Halbreich U, Freeman E. Symptomatic improvement of premenstrual dysphoric disorder with sertraline treatment: a randomized controlled trial. Journal of the American Medical Association. 1997;278:983–8. [PubMed] [Google Scholar]
- Yonkers KA. Management strategies for PMS/PMDD. Journal of Family Practice suppl. 2004:S15–S20. [PubMed] [Google Scholar]
- Young SA, Hurt PH, Benedek DM. Treatment of premenstrual dysphoric disorder with sertraline during the luteal phase: a randomized, double-blind, placebo-controlled cross-over trial. Journal of Clinical Psychiatry. 1998;59:76–80. doi: 10.4088/jcp.v59n0206. [DOI] [PubMed] [Google Scholar]
- Young EA, Kornstein SG, Harvey AT, Wisniewski SR, Barkin J, Fava M, Trivedi MH, Rush AJ. Influences of hormone-based contraception on depressive symptoms in premenopausal women with major depression. Psychoneuroendocrinology. 2007;32(7):843–53. doi: 10.1016/j.psyneuen.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]