Abstract
Objective
To evaluate changes in vascular and musculoskeletal involvement in subjects in the Scleroderma Lung Study (SLS), a multicenter, double-blind, randomized, controlled trial comparing placebo treatment to oral cyclophosphamide for 1 year in systemic sclerosis (SSc) patients with interstitial lung disease. Subjects were then followed off study agent for an additional 12 months.
Methods
The following parameters were noted at baseline and every 6 months for each patient: digital tip ulcers, other dermal ulcers, joint swelling, joint tenderness, large joint contractures, muscle tenderness, muscle weakness, oral aperture, hand extension, and fist closure.
Results
158 patients were enrolled from 13 centers in the United States; 79 were randomized to the cyclophosphamide group (CYC) and 79 to the placebo group. There were no differences in dermal ulcer and musculoskeletal measures between CYC and placebo groups at baseline, 12, and 24 months. Improvement in FVC % predicted was associated with improvement in Rodnan skin score (P<0.05) at 12 and 24 months, and with increased mean oral aperature at 24 months (P=0.005).
Conclusions
These data document the frequency and course of these vascular and musculoskeletal features over time, thus providing essential information for sample size calculations and magnitude of effect in future clinical trials. There was no treatment effect of cyclophosphamide on the vascular and musculoskeletal features described.
Keywords: Scleroderma, musculoskeletal, joints, vascular, randomized controlled trial, cyclophosphamide
The Scleroderma Lung Study (SLS) was a multicenter, randomized, double-blind, controlled trial to determine efficacy and safety of daily oral cyclophosphamide versus placebo for one year in systemic sclerosis (SSc) patients with interstitial lung disease, followed by one year of observation off study medication (1). This was the first and largest prospective, randomized controlled trial to demonstrate a small, but significant treatment benefit in percent predicted forced vital capacity (FVC) and in skin scores in SSc patients (1).
Dermal ulcers and musculoskeletal manifestations of SSc contribute to disability and are important factors in patients’ evaluation of quality of life (2). We therefore measured these parameters as secondary outcomes in the SLS study, arguing that an effective agent for pulmonary fibrosis and skin sclerosis might also be effective for other common disease manifestations. The following features were noted at baseline and every 6 months by the same trained observer for each patient: digital tip ulcers, other dermal ulcers, joint swelling, joint tenderness, large joint contractures, muscle tenderness, muscle weakness, oral aperture, maximal hand extension, and fist closure distance. These measures are the subject of this report.
METHODS
Subjects
Patients with SSc by the American College of Rheumatology definition(3) were enrolled if they met criteria described previously(1). Subjects had to have disease duration of 7 years or less, determined from the time of the first non-Raynaud’s phenomenon sign or symptom typical of SSc; had to have a percent predicted forced vital capacity < 85% and > 44% anda percent predicted diffusing capacity in liters of carbon monoxide (DLCO) of > 30%, dyspnea on moderate exertion, and findings on high resolution computed tomography of the chest (HRCT) and/or bronchoalveolar lavage (BAL) consistent with SSc-related alveolitis (namely, any ground glass opacificaton on HRCT and/or ≥3% neutrophils and/or ≥2% eosinophils in BAL fluid). A summary of the major exclusion criteria are as follows: active infection, pulmonary hypertension, significant renal compromise, other serious medical illnesses with a prognosis less than 2 years, and (for those of child-bearing potential) pregnancy, breast feeding, or unwillingness to use reliable contraception. The complete listing of inclusion and exclusion criteria can be found in reference 5. Subjects were classified as diffuse cutaneous SSc (dcSSc) if their skin thickening involved the trunk and/or the proximal extremities. Subjects were classified as limited cutaneous disease (lcSSc) if their skin thickening was restricted to the face and the extremities distal to the elbows and knees (4). A1l subjects provided written informed consent according to medical institutional review board guidelines at the participating sites.
Screening and Randomization
Screening methods have already been described in detail previously (5). Subjects meeting eligibility criteria were randomized by site to receive either oral cyclophosphamide (CYC, titrated up to 2 mg/kg as tolerated, Bristol-Myers Squibb, New York, NY) or identical-appearing placebo once daily for 12 months, followed by another year of follow-up off study medication.
Disallowed Medications
All medications with putative disease-modifying properties (e.g., minocycline, D-penicillamine, cyclosporine, azathioprine, methotrexate, colchicine, etc) were prohibited during and for at least 1 month prior to starting study medication. Corticosteroid use was limited to ≤ 10 mg of prednisone or equivalent per day.
Baseline and Follow-up Measurements
Full descriptions of study outcome measurements have been described in detail previously (1). Serum creatine phosphokinase (CPK) was measured at baseline prior to initiation of therapy. Baseline and follow-up measurements relevant to this report included the following: (1) digital tip ulcers defined as ulcers distal to the distal interphalangeal (DIP) joint; (2) other dermal ulcers; (3) joint swelling noted at the wrists, elbows, knees and metacarpophalangeal (MCP) joints; (4) joint tenderness noted at the wrists, elbows, knees, and MCPs; (5) large joint contractures defined as loss of motion at the wrists, elbows and/or knees; (6) muscle tenderness on palpation recorded as either present or absent; (7) proximal muscle weakness on objective testing and recorded as present or absent; (8) oral aperture measured as the distance from the lower edge of the upper lip to upper edge of the lower lips when patients are asked maximally to open their mouth during three trials; (9) hand extension defined as the average distance from the most external point of the thumb to the most external point of the fifth finger during maximum active effort to extend the fingers during three trials; (10) fist closure defined as the average distance from the distal tip of the fourth finger to the distal palmar crease during three trials; (11) modified Rodnan skin score; (12) pulmonary function tests including FVC, DLCO, MIP (maximal inspiratory pressures), and MEP (maximal expiratory pressures); (13) serum hemoglobin; (14) Health Assessment Questionnaire- Disability Index (HAQ-DI); and (15) the 36-item Medical Outcomes Survey (SF-36). These measures were repeated at 6, 12, 18, and 24 months of the study.
Treatment with Cyclophosphamide or Placebo
Treatment with CYC (in 25-mg gelcaps) or placebo was initiated at 1 mg/kg/day PO (to the nearest 25 mg) and increased every month by one capsule until a maximum dose of 2 mg/kg/day PO or the highest tolerable dose. Methods of monitoring drug toxicity and criteria for discontinuing or adjusting study drug in response to drug-related toxicities have already been described (1). A Data Safety and Monitoring Board provided oversight of the study.
Statistical Analysis
Variables were expressed as mean, standard deviation, and frequencies. To evaluate baseline differences between CYC and placebo, the Student’s t-test and Wilcoxon Rank Sum test were applied to continuous and ordinal variables, while the chi-square and Fisher’s exact tests were applied to categorical variables. To evaluate treatment group differences in change within a variable from baseline to 12 months, the Student’s t-test or Wilcoxon Rank Sum test was used on change scores for continuous or ordinal variables (mean oral aperture, hand extension, fist closure), while the chi-square and Fisher’s exact tests were used for dichotomous variables (digital tip ulcers, other ulcers, swollen joints, tender joints, contractures, muscle tenderness, muscle weakness) after characterizing patients as having either improved, not changed, or worsened. A p-value 0.05 or less was considered statistically significant.
Longitudinal trend analyses were performed using linear mixed effects modeling over the entire period of study evaluation. Continuous variables were modeled as a linear function of time (months), the treatment group, and the interaction of time and treatment group, with patient as random intercept. For dichotomous variables such as digital tip ulcers mixed effects logistic regression was used. Likelihood-based measures of goodness-of-fit such as AIC (Akaike's information criterion) and BIC (Bayesian information criterion) were used to determine if additional random effects should be incorporated into the models. For certain variables displaying extreme non-normality such as swollen joint count, tender joint count, and fist closure, an alternative method of trend analysis was derived by fitting a least-squares line to each individual patient’s set of available points and determining the slope of each individual line. The Student’s t-test or Wilcoxon Rank Sum Test was then used on the individual slopes to make group comparisons.
Multivariate analysis was performed to determine if improvements in Rodnan skin score, swollen joint count, tender joint count, mean oral aperture, and fist closure were correlated with changes in internal organ involvement (FVC, DLCO, hemoglobin) and quality of life measures (HAQ-DI, SF-36) at 12 and 24 months. Best fit multiple linear regression based upon adjusted R2 was used to model baseline-to-12 month and baseline-to-24 month change scores of Rodnan skin score, swollen joint count, tender joint count, mean oral aperture, and fist closure as functions of baseline-to-12 month and baseline-to-24 month change scores of FVC, DLCO, hemoglobin, HAQ-DI, and SF-36 controlled for gender, age, and type of SSc.
For the longitudinal and multivariate analyses, swollen joint count and tender joint count were included as continuous variables. Statistical software Stata 10 was used for the analysis (Stata Corporation, College Station, TX).
RESULTS
Table 1 shows the baseline demographic, musculoskeletal, and clinical characteristics of the patients including age, sex, duration of disease, digital-tip and other dermal ulcers, musculoskeletal findings, pulmonary function test results (including FVC, DLCO), HAQ-DI, SF-36, and medications. As expected, the majority of subjects were female with a mean age of 48.5 years and average disease duration of 3.1 years from the first non-Raynaud’s phenomenon symptom characteristic of SSc. Approximately two-thirds of subjects (59.5%) had diffuse cutaneous disease. There was no significant difference in these parameters between those randomized to cyclophosphamide and those to placebo except for the HAQ-DI which was significantly higher in the CYC group than the placebo group (p<0.02) (Table 1). Musculoskeletal manifestations of SSc were evenly divided between those randomized to CYC and those to placebo. Given the small percentage of subjects with swollen or tender joints, the median score for swollen and tender joint counts was 0. Median swollen and tender joint counts and interquartile range (IQR) of patients who had a positive joint count are noted in Table 1.
Table 1.
Baseline Characteristics
| All (n=158) | CYC (n=79) | Placebo (n=79) | P value† | |
|---|---|---|---|---|
| Age (yrs), mean (SD) | 48.5 (12.2) | 48.9 (12.2) | 48.2 (12.4) | 0.72 |
| Female, N (%) | 111 (70.3) | 60 (75.6) | 51 (64.6) | 0.15 |
| Duration (yrs), mean (SD) | 3.1 (2.1) | 3.05 (2.3) | 3.2 (1.9) | 0.69 |
| dcSSc, N (%) | 94 (59.5) | 49 (62) | 45 (57) | 0.70 |
| Digital Tip Ulcers, N (%) | 12 (7.6) | 5 (6.3) | 7 (8.9) | 0.55 |
| Non-digital Ulcers, N (%) | 17 (10.8) | 6 (7.6) | 11 (13.9) | 0.23 |
| Joint Swelling, N (%) | 37 (23.3) | 18 (22.8) | 19 (23.8) | 0.99 |
| Swollen Joint Count, median (IQR) a | 2 (2–3) | 2 (1.5–2) | 2 (2–4) | 0.16 |
| Joint Tenderness, N (%) | 47 (29.9) | 20 (25.6) | 27 (34.2) | 0.26 |
| Tender Joint Count, median (IQR) b | 3 (1–6) | 3 (1.5–6) | 6 (1–7) | 0.97 |
| Large Joint Contractures, N (%) | 74 (46.5) | 38 (47.5) | 36 (45.6) | 0.57 |
| Muscle Tenderness, N (%) | 26 (16.4) | 13 (16.5) | 13 (16.3) | 0.97 |
| Muscle Weakness, N (%) | 20 (12.7) | 9 (11.4) | 11 (13.9) | 0.68 |
| Oral Aperture (mm), mean (SD) | 45.1 (8.5) | 44.9 (8.6) | 45.4 (8.5) | 0.69 |
| Hand Extension (mm), mean (SD) | 175 (34.8) | 169.9 (37.6) | 180.3 (31) | 0.06 |
| Fist Closure (mm), mean (SD) | 12.1 (19.5) | 15 (24) | 9.1 (13.1) | 0.06 |
| CPK (U/L), mean (SD) | 135.3 (155.2) | 144.8 (191.2) | 125.7 (108.3) | 0.57 |
| Hemoglobin (g/dL), mean (SD) | 12.8 (1.6) | 12.9 (1.4) | 12.6 (1.8) | 0.15 |
| FVC (% predicted), mean (SD) | 68.1 (12.1) | 68.5 (12.9) | 67.8 (11.3) | 0.73 |
| DLCO (% predicted), mean (SD) | 47.4 (14.1) | 47.6 (14.3) | 47.2 (14) | 0.85 |
| MIP (cm, H2O), mean (SD) | 88.6 (30.8) | 85.7 (27.1) | 92.7 (34.3) | 0.22 |
| MEP (cm, H2O), mean (SD) | 91.8 (36.2) | 88.9 (35.2) | 95.6 (38.4) | 0.32 |
| Skin Score, mean (SD) | 14.7 (10.9) | 14 (10.5) | 15.4 (11.3) | 0.44 |
| HAQ-DI, mean (SD) | 0.83 (0.69) | 0.96 (0.67) | 0.69 (0.69) | 0.02 |
| SF-36 PCSa, mean (SD) | 33.5 (10.8) | 32.9 (10.9) | 34.4 (10.9) | 0.38 |
| SF-36 MCSb, mean (SD) | 49.8 (10.6) | 48.8 (10.4) | 50.7 (10.7) | 0.30 |
| NSAID usage, N (%) | 60 (38.0) | 32 (40.5) | 28 (35.4) | 0.512 |
| Pain medication usage, N (%) | 49 (31.0) | 29 (36.7) | 20 (25.3) | 0.122 |
| Glucocorticoid usage, N (%) | 42 (26.6) | 19 (24.1) | 23 (29.1) | 0.471 |
Patients with positive swollen joint count
Patients with positive tender joint count
PCS (Physical component score)
MCS (Mental component score)
CYC versus Placebo
Auto-antibodies were drawn at baseline for 108 of the subjects (51 CYC, 57 placebo). Anti-Scl 70 antibodies were positive in 33.3% of the subjects and anti-RNA polymerase III antibodies were found in 15.7% of the subjects. There was no difference in presence of auto-antibodies between CYC and placebo groups.
Cyclophosphamide versus Placebo
Analysis of ulcers and musculoskeletal manifestations of systemic sclerosis showed no difference between cyclophosphamide and placebo arms after 12 months of treatment (Table 2). Analysis of the subjects off treatment medication at 18 and 24 months also showed no significant differences in dermal ulcers or musculoskeletal measures between the cyclophosphamide and placebo groups.
Table 2.
Change from baseline in dermal ulcer and musculoskeletal measurements at 12 months
| CYC | Placebo | ||||||
|---|---|---|---|---|---|---|---|
|
Baseline (N=79) |
12 months (N=69) |
Delta |
Baseline (N=79) |
12 months (N=63) |
Delta |
P value* |
|
| Digital Tip Ulcers, N (%) |
7 (8.9) | 9 (13.0) | +4.1 | 5 (6.3) | 11 (17.5) | +11.2 | 0.23 |
| Other Dermal Ulcers, N (%) |
11 (13.9) | 6 (8.7) | −5.2 | 6 (7.6) | 9 (14.3) | +6.7 | 0.64 |
| Joint Swelling, N (%) |
19 (23.8) | 4 (5.8) | −18.0 | 18 (22.8) | 7 (11.3) | −11.5 | 0.75 |
| Joint Tenderness, N (%) |
27 (34.2) | 20 (29.0) | −5.2 | 20 (25.6) | 18 (29.0) | +3.4 | 0.35 |
| Large Joint Contractures, N (%) |
36 (45.6) | 31 (45.6) | 0.0 | 38 (47.5) | 34 (54.8) | +7.3 | 0.13 |
| Muscle Tenderness, N (%) |
13 (16.3) | 10 (14.5) | −1.8 | 13 (16.5) | 7 (11.3) | −5.2 | 0.25 |
| Muscle Weakness, N (%) |
11 (13.9) | 4 (5.8) | −8.1 | 9 (11.4) | 5 (8.1) | −3.3 | 0.92 |
| Oral Aperature (mm), mean +/− SD |
44.9 (8.6) | 46.5 (8.2) | 1.0 (4.3) |
45.4 (8.5) | 46.1 (8.2) | 0.7 (4.7) |
0.34 |
| Hand Extension (mm), mean +/− SD |
169.9 (37.6) |
172.3 (32.5) |
2.8 (29.5) |
180.3 (31.0) |
179.1 (29.1) |
−0.9 (21.4) |
0.30 |
| Fist Closure (mm), mean +/− SD |
14.9 (24.0) |
13.1 (19.7) |
−2.4 (22.8) |
9.1 (13.1) | 11.6 (19.5) |
1.4 (17.9) |
0.44 |
CYC vs Placebo at 12 months
Longitudinal analysis
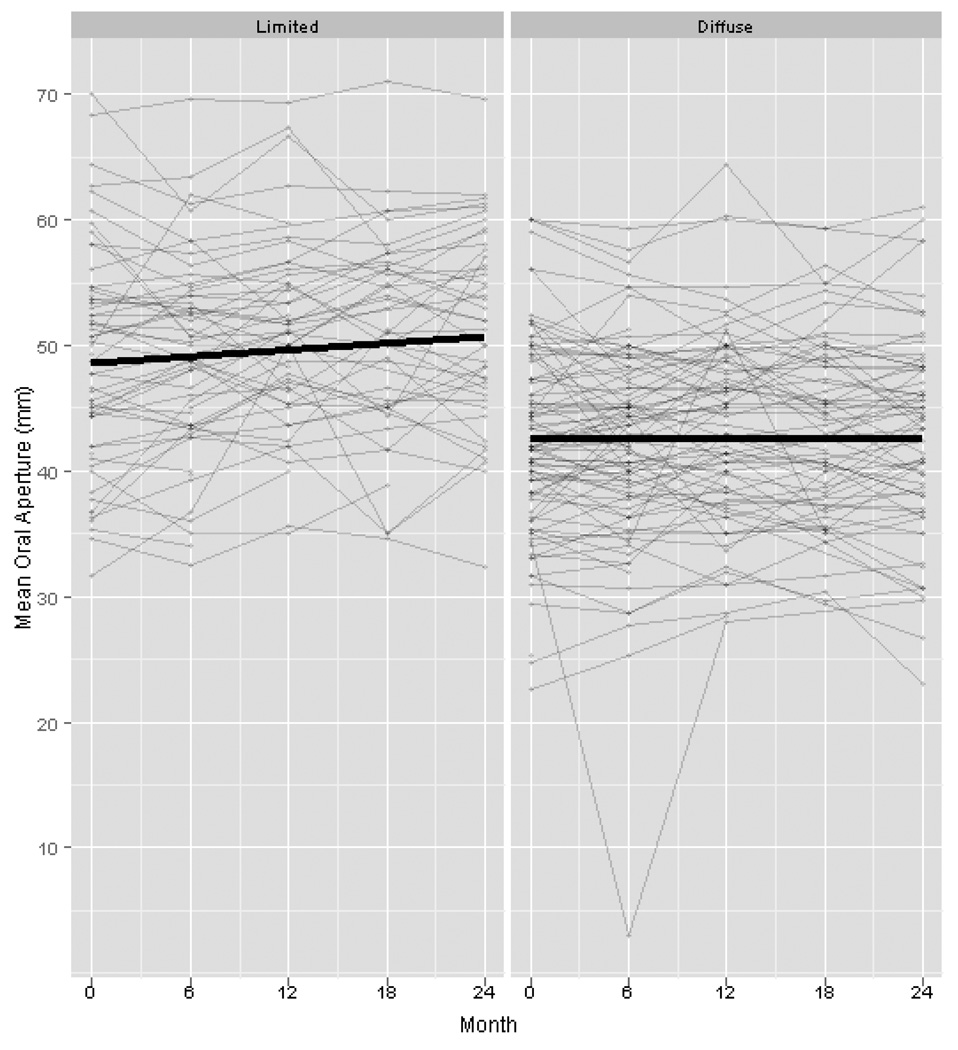
Longitudinal analysis of the data demonstrated an overall increased risk of developing digital tip ulcers over time regardless of treatment or extent of skin involvement. SSc subjects enrolled in this study had a 5% greater odds of developing digital tip ulcers per month throughout length of the study (OR 1.05, P<0.001). There was no correlation between duration of disease and the occurrence of digital ulcers (P=0.70). There was a decline in swollen joint count over the 2 years of the study regardless of treatment (P=0.002) and there was a trend of decreased tender joint count (P=0.07) over time. There was no significant difference in the rate of decline in swollen or tender joint counts between the CYC and placebo groups. Mean oral aperture also improved over time in the study participants as a whole (P=0.02), and the improvement in oral aperture was greater in the lcSSc patients than dcSSc (Figure 1, P=0.007). A trend of improvement was also noted for fist closure regardless of treatment assignment over the 24 months (P=0.08).
Figure 1.
Change in mean oral aperature in limited vs diffuse SSc over 24 months
Correlation of Skin and musculoskeletal changes to internal organ involvement
The unadjusted analysis of change in skin and musculoskeletal findings to FVC and DLCO are presented in Table 3. The adjusted best fit models for change in skin and musculoskeletal findings to change in FVC and DLCO are included in Table 4. Improvement in Rodnan skin score at 12 and 24 months was correlated with increase in FVC after controlling for treatment assignment, SSc type (diffuse vs limited), and change in HAQ-DI. Every 1% improvement in FVC corresponded to a decrease in Rodnan skin score of 0.17 (95% CI 0.03–0.3) at 12 months and 0.11 (95% CI 0.001–0.22) at 24 months. Improvement in mean oral aperature was associated with an increase in %predicted FVC at 24 months (β=0.12, 95% CI 0.04–0.21). Increase in hemoglobin was associated with an improvement in swollen joint count at 24 months (β= −0.03, P=0.036), but not at 12 months. Changes in tender joint count and fist closure were not associated with changes in FVC, DLCO, HAQ-DI, SF-36, or hemoglobin. There was no correlation between change in skeletal muscle weakness and change in respiratory muscle strength as measured by MIP or MEP.
Table 3.
Simple linear regression of change in skin and musculoskeletal findings with change in FVC and DLCO
| Change in FVC (%) | Change in DLCO (%) | |||
|---|---|---|---|---|
| 12 Months | 24 Months | 12 Months | 24 Months | |
| Skin Score | −0.22 (0.001) | −0.13 (0.03) | −0.07 (0.25) | −0.11 (0.03) |
| Swollen Joint Count | −0.001 (0.49) | −0.002 (0.16) | <.001 (0.94) | 0 (0.82) |
| Tender Joint Count | −0.003 (0.33) | 0.001 (0.84) | −0.002 (0.46) | 0.003 (0.36) |
| Mean Oral Aperature (mm) | 0.18 (0.001) | 0.13 (0.003) | 0.05 (0.33) | 0.11 (0.003) |
| Fist Closure (mm) | 0.05 (0.82) | −0.24 (0.35) | −0.08 (0.69) | 0.03 (0.89) |
beta coefficient (P-value)
Table 4.
Best fit adjusted model of change in skin and musculoskeletal findings with change in FVC and DLCO
| Change in FVC (%) | Change in DLCO (%) | |||
|---|---|---|---|---|
| 12 Months | 24 Months | 12 Months | 24 Months | |
| Skin Score | −0.17 (0.02) | −0.11 (0.046) | NS | NS |
| Swollen Joint Count | NS | NS | NS | NS |
| Tender Joint Count | NS | NS | NS | 0.005 (0.08) |
| Mean Oral Aperature (mm) | NS | 0.12 (0.005) | NS | NS |
| Fist Closure (mm) | NS | NS | NS | NS |
beta coefficient (P-value)
NS= non-significant
DISCUSSION
The primary goal of the Scleroderma Lung Study was to determine the safety and efficacy of daily oral cyclophosphamide, compared with placebo, in a cohort of early SSc patients with evidence of mild to moderate interstitial lung disease. So as not to miss responses in other clinical aspects, the SLS investigators measured a number of secondary outcomes in order to identify changes in additional disease features, particularly musculoskeletal features, that might respond to immunosuppression. As noted earlier(1), the study was able to demonstrate that 12 months of oral cyclophosphamide had a modest but statistically significant benefit in lung disease, skin involvement, and quality of life measures. In this analysis, no improvement in musculoskeletal features was noted with use of oral cyclophosphamide in the SLS study. However, there are several factors to take into consideration when making conclusions about these data.
Digital tip ulcers are a vascular event believed to be due primarily to ischemic changes from a non-inflammatory occlusive vasculopathy. It may not be surprising that no difference was seen between the CYC and the placebo groups in the course of this study. However, due to the large impact these ulcers have on function it was thought worthwhile to include this feature as a secondary outcome. We noted a relatively low prevalence of digital tip ulcers at the start of our study (less than 8%), but it climbed to 22% at the end of 2 years. The prevalence of digital tip ulcers from other studies has ranged from 17.4 to 54% (6, 7). The lower prevalence of digital tip ulcers seen at the start of our study is unlikely to be secondary to the duration of disease as we found no correlation between disease duration and incidence of digital tip ulcers in our patients.
Non-digital tip ulcers were significantly more common among diffuse disease subjects compared to limited (15–20%% versus 2–7%). These ulcers arise over pressure points and are believed to be the result of minor trauma in skin with an inadequate blood supply to support healing. The modest improvement in skin score (1) did not translate into fewer of these ulcers over time, but a more effective anti-fibrotic agent might be more successful. This study may have been underpowered to identify a modest benefit, but certainly there was little evidence to suggest improvement.
Previous controlled trials in rheumatoid arthritis have found that cyclophosphamide was beneficial and improved synovitis (8–10). However, our study did not find a significant benefit of CYC in swollen joint count. This study was likely underpowered to detect a significant improvement in swollen joint count given the small number of patients with active swollen joints enrolled in the SLS (23% at baseline and 13% at the end of the study.) The prevalence of arthritis in SSc described in the literature is relatively low and ranges from 3.9–19% (6, 11–13). The small percentage of SSc patients with active swollen joints may make it difficult to find meaningful differences in clinical trials.
The relatively small proportion of subjects with muscle weakness (12.7% overall at baseline) and muscle tenderness (16.4% at baseline) suggests that collecting data on these features may not be of use in future clinical trials. The frequency of muscle weakness in this study is consistent with the frequency reported in large SSc cohorts (11, 14). Furthermore, muscle disease in SSc is quite heterogeneous and in 1 series, only 3/23 patients with myopathy had classic inflammatory muscle disease requiring treatment with immunosuppression (15). There was also no significant correlation between change in skeletal muscle weakness and measures of respiratory muscle strength (MIP and MEP). It can be argued from these results, that muscle pain and weakness are not relevant study measures in SSc studies and probably had little confounding effect on the measures of lung physiology (i.e., FVC, TLC).
As noted earlier, both large joint contractures and small hand-joint contractures result in considerable disability in SSc (2). The proportion of subjects with large joint contractures did not change in the course of this trial and no treatment effect was noted. This emphasizes two points, the first being that these contractures tend to occur early in the disease process and the second that once present they are persistent. Small hand-joint contractures, as measured by the ability of subjects to form a fist, did seem to improve over the 24 months but was independent of treatment assignment.
We found that an increase in % predicted FVC was associated with an improvement in Rodnan skin score. This is consistent with previous findings that skin score is associated with FVC (16, 17). Mean oral aperture showed an improvement over the 2 years of the study, but again there were no noted benefits of CYC over placebo. We found a small association between change in mean oral aperture and change in FVC. This is the first report of an association between change in oral aperature and FVC, and will have to be confirmed in future studies. There was an association in change in hemoglobin and decreased synovitis, but the findings were not clinically significant (β coefficient −0.03). Changes in other musculoskeletal findings (tender joint count and fist closure) were not associated with change in internal organ involvement.
In conclusion, this group of relatively early (mean disease duration of 3.1 years) SSc subjects with interstitial lung disease did not demonstrate a response to cyclophosphamide in terms of dermal ulcers or musculoskeletal manifestations. Some of this lack of response can be attributed to the fact that the study population was not selected on any of these features, particularly on features such as swollen joint count, muscle weakness and dermal ulcers that were present in a relatively small number.
However, the careful 24 month followup of these subjects provides insight into the frequency and course of these features over time and will contribute to the better design of future trials.
Acknowledgments
Grant supporters: American College of Rheumatology, Scleroderma Foundation, Cure Scleroderma Foundation
Dr. Khanna was supported by a National Institutes of Health Award (NIAMS K23 AR053858-03) and the Scleroderma Foundation (New Investigator Award).
Dr. Furst receives funding from Abbott, Actelion, Amgen, BMS, BiogenIdec, Centocor, Corrona, Genentech, Gilead, GSK, Human Genome sciences, Merck, Nitec, Novartis, Roche, UCB, Wyeth, Xoma
REFERENCES
- 1.Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354(25):2655–2666. doi: 10.1056/NEJMoa055120. [DOI] [PubMed] [Google Scholar]
- 2.Malcarne VL, Hansdottir I, McKinney A, Upchurch R, Greenbergs HL, Henstorf GH, et al. Medical signs and symptoms associated with disability, pain, and psychosocial adjustment in systemic sclerosis. J Rheumatol. 2007;34(2):359–367. [PubMed] [Google Scholar]
- 3.Preliminary criteria for the classification of systemic sclerosis (scleroderma) Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23(5):581–590. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 4.Medsger TJ. Classification, Prognosis. In: Clements PJFD, editor. Systemic Sclerosis. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 17–28. [Google Scholar]
- 5.Tashkin DP, Elashoff R, Clements PJ, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354:2655–2666. doi: 10.1056/NEJMoa055120. Supplement to: In: 2006 http://content.nejm.org/cgi/data/354/25/2655/DC1/1. [DOI] [PubMed] [Google Scholar]
- 6.Ferri C, Valentini G, Cozzi F, Sebastiani M, Michelassi C, La Montagna G, et al. Systemic sclerosis: demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine (Baltimore) 2002;81(2):139–153. doi: 10.1097/00005792-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Nihtyanova SI, Brough GM, Black CM, Denton CP. Clinical burden of digital vasculopathy in limited and diffuse cutaneous systemic sclerosis. Ann Rheum Dis. 2008;67(1):120–123. doi: 10.1136/ard.2007.072686. [DOI] [PubMed] [Google Scholar]
- 8.Currey HL, Harris J, Mason RM, Woodland J, Beveridge T, Roberts CJ, et al. Comparison of azathioprine, cyclophosphamide, and gold in treatment of rheumatoid arthritis. Br Med J. 1974;3(5934):763–766. doi: 10.1136/bmj.3.5934.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fosdick WM, Parsons JL, Hill DF. Long-term cyclophosphamide therapy in rheumatoid arthritis. Arthritis Rheum. 1968;11(2):151–161. doi: 10.1002/art.1780110205. [DOI] [PubMed] [Google Scholar]
- 10.Townes AS, Sowa JM, Shulman LE. Controlled trial of cyclophosphamide in rheumatoid arthritis. Arthritis Rheum. 1976;19(3):563–573. doi: 10.1002/art.1780190308. [DOI] [PubMed] [Google Scholar]
- 11.Della Rossa A, Valentini G, Bombardieri S, Bencivelli W, Silman AJ, D'Angelo S, et al. European multicentre study to define disease activity criteria for systemic sclerosis. I. Clinical and epidemiological features of 290 patients from 19 centres. Ann Rheum Dis. 2001;60(6):585–591. doi: 10.1136/ard.60.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ostojic P, Damjanov N. Different clinical features in patients with limited and diffuse cutaneous systemic sclerosis. Clin Rheumatol. 2006;25(4):453–457. doi: 10.1007/s10067-005-0041-0. [DOI] [PubMed] [Google Scholar]
- 13.Scussel-Lonzetti L, Joyal F, Raynauld JP, Roussin A, Rich E, Goulet JR, et al. Predicting mortality in systemic sclerosis: analysis of a cohort of 309 French Canadian patients with emphasis on features at diagnosis as predictive factors for survival. Medicine (Baltimore) 2002;81(2):154–167. doi: 10.1097/00005792-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Mimura Y, Ihn H, Jinnin M, Asano Y, Yamane K, Tamaki K. Clinical and laboratory features of scleroderma patients developing skeletal myopathy. Clin Rheumatol. 2005;24(2):99–102. doi: 10.1007/s10067-004-0975-7. [DOI] [PubMed] [Google Scholar]
- 15.Clements PJ, Furst DE, Campion DS, Bohan A, Harris R, Levy J, et al. Muscle disease in progressive systemic sclerosis: diagnostic and therapeutic considerations. Arthritis Rheum. 1978;21(1):62–71. doi: 10.1002/art.1780210111. [DOI] [PubMed] [Google Scholar]
- 16.McNearney TA, Reveille JD, Fischbach M, Friedman AW, Lisse JR, Goel N, et al. Pulmonary involvement in systemic sclerosis: associations with genetic, serologic, sociodemographic, and behavioral factors. Arthritis Rheum. 2007;57(2):318–326. doi: 10.1002/art.22532. [DOI] [PubMed] [Google Scholar]
- 17.Morelli S, Barbieri C, Sgreccia A, Ferrante L, Pittoni V, Conti F, et al. Relationship between cutaneous and pulmonary involvement in systemic sclerosis. J Rheumatol. 1997;24(1):81–85. [PubMed] [Google Scholar]
- 18.Hanitsch LG, Burmester GR, Witt C, Hunzelmann N, Genth E, Krieg T, et al. Skin sclerosis is only of limited value to identify SSc patients with severe manifestations--an analysis of a distinct patient subgroup of the German Systemic Sclerosis Network (DNSS) Register. Rheumatology (Oxford) 2009;48(1):70–73. doi: 10.1093/rheumatology/ken408. [DOI] [PubMed] [Google Scholar]
- 19.La Montagna G, Sodano A, Capurro V, Malesci D, Valentini G. The arthropathy of systemic sclerosis: a 12 month prospective clinical and imaging study. Skeletal Radiol. 2005;34(1):35–41. doi: 10.1007/s00256-004-0830-6. [DOI] [PubMed] [Google Scholar]