Abstract
To exploit the full potential of human pluripotent stem cells for regenerative medicine, developmental biology, and drug discovery, defined culture conditions are needed. Media of known composition that maintain human embryonic stem (hES) cells have been developed, but finding chemically-defined, robust substrata has proved difficult. We employed an array of self-assembled monolayers to identify peptide surfaces that sustain pluripotent stem cell self-renewal. The effective substrates display heparin-binding peptides, which can interact with cell surface glycosaminoglycans, and can be used with a defined medium to culture hES cells for more than 3 months. The resulting cells maintain a normal karyotype and display high levels of pluripotency markers. The peptides are able to support growth of multiple (eight) pluripotent cell lines on a variety of scaffolds. Our results indicate that synthetic substrates that recognize cell surface glycans can facilitate the long-term culture of pluripotent stem cells.
Human pluripotent stem cells have the remarkable capacity to both self-renew indefinitely and differentiate into many different cell types1–3. Progress in developing defined conditions for human embryonic stem (hES) cell propagation has resulted from elucidating the roles of soluble factors4–6. In contrast, it has proved challenging to identify defined substrata for this purpose. The typical mixtures employed to date are composed of extracellular matrix (ECM) proteins from animal or human sources4,7. The batch-to-batch inconsistency that accompanies the use of complex mixtures complicates hES cell propagation. Moreover, the use of animal-derived ECM proteins exposes cells to potentially hazardous pathogens and allows for the transfer of immunogenic epitopes8. Individual ECM proteins9–11 have been employed as substrates for hES cell self-renewal, but even single ECM proteins have multiple domains that can engage a variety of cell-surface receptors. As a result, it is difficult to elucidate the key interactions that give rise to reproducible culture conditions.
We sought to replace the substrata used for hES cell culture with a fully defined, synthetic alternative. A synthetic surface could minimize the exposure of pluripotent cells to hazardous contaminants and yield a more homogenous cell culture. Moreover, it could illuminate the minimum requirements for adhesion and self-renewal. Although synthetic substrata have been described12–20, none have been shown to be effective for the long-term culture of both hES cells and iPS cells. Most of these substrata were either used in combination with a conditioned medium12–14 or were tested with only a limited number of cell lines17–20. Additionally, the mechanisms by which these substrates function are largely unknown. Here we describe a unique synthetic surface that engages cell surface glycosaminoglycans and supports the long-term propagation of multiple hES and induced pluripotent stem (iPS) cell lines in fully defined conditions.
RESULTS
Peptide substrates for hES cell adhesion
To seek a substrate with the desired activity, we employed a defined surface array21 composed of peptide-substituted alkanethiol conjugates, which form self-assembled monolayers (SAMs) on gold22. SAMs can present a variety of ligands with control over surface density, making them valuable probes of the consequences of engaging cell surface ligands23. Previous work employed surface arrays that present peptides derived from laminin at high densities12 or cell-binding peptides identified by phage display18. Those screens yielded synthetic surfaces capable of supporting the short-term propagation of hES cells. To identify a synthetic surface that would be effective for long-term propagation, we expanded our array screen12,21 to assess surfaces that bear bioactive peptide sequences reported to bind to diverse cell surface receptors (Supplementary Table 1). We did not limit our screen to known protein-binding sequences (e.g. the integrin ligand RGD24) but also included peptides that can interact with glycosaminoglycans. Because cell adhesion is influenced not just by the identity of the adhesive epitopes, but by their density on the substrate, and whether they are present alone or in combination21,25, we used the array to present bioactive peptides alone, in different combinations, and at varying surface densities. In total, we screened over 500 unique surfaces based on 18 bioactive peptides.
The surfaces were first evaluated for their abilities to support hES cell adhesion. Cells (H1 or H9) that had been cultured on Matrigel were dissociated and applied to the array in a protein-free basal medium, to minimize the adsorption of proteins from the medium. After 1 hour, the basal medium was replaced with defined mTeSR medium4 supplemented with Y-27632, an inhibitor of rho-associated kinase (ROCK). This small molecule aids the survival of dissociated hES cells26. Using these conditions, hES cells were propagated on the array for 6 days and then fixed and stained for the markers of pluripotency Oct-4 and SSEA-4 (Fig. 1a). In this way, we identified several surfaces that could sustain cell adhesion over the course of the experiment. Adhesion to the SAMs required the ROCK inhibitor, even after colonies had been established (Supplementary Fig. 1).
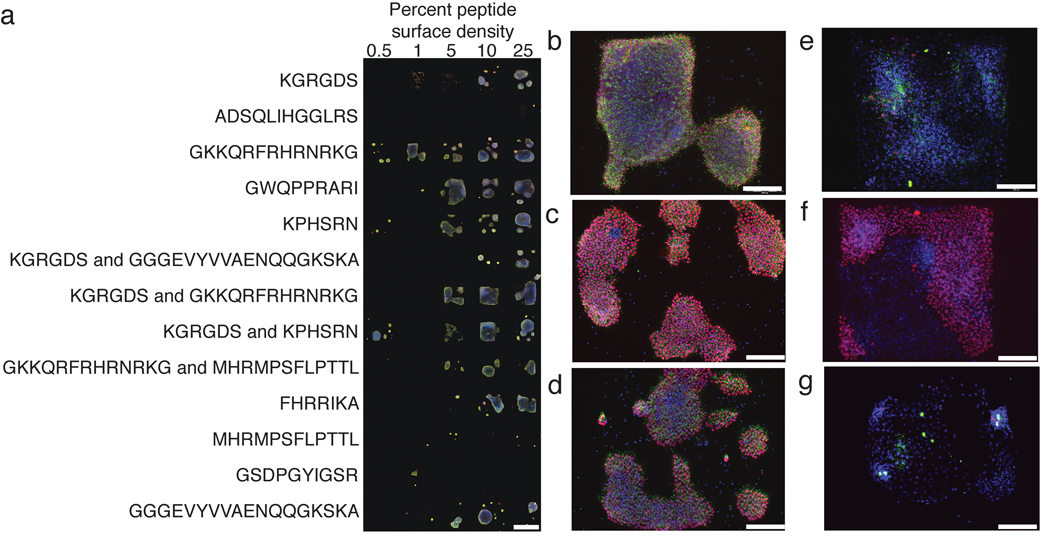
Figure 1.
Strategy for the identification of peptide-substituted surfaces for hES cell adhesion and survival. (a) The image shows H9 cells bound to a representative array presenting bioactive peptides in different combinations and at distinct surface densities. Surface densities are reported as the percentage of peptide-substituted alkanethiol in the mixed SAM. Cells were fixed, immunostained for Oct-4 (red) or SSEA-4 (green), and counterstained with DAPI (blue). (b–g) Representative higher magnification images of cells (H1, c–d, and H9, b, e–g) on arrays. (b–d) Cells were propagated on surfaces presenting heparin-binding peptides (b) GKKQRFRHRNRKG from vitronectin, (c) GWQPPRARI from fibronection, or (d) FHRRIKA from bone sialoprotein, or on surfaces presenting (e) the fibroblast growth factor receptor binding peptide (GGGEVYVVAENQQGKSKA) and the integrin binding peptide KGRGDS, (f) KGRGDS and another bioactive peptide derived from fibronectin, KPHSRN, and (g) laminin-derived GSDPGYIGSR. Scale bars represent 1 mm, (a), and 200 µm, (b–g).
Examination of the effective surfaces revealed some intriguing trends. Although surfaces substituted with the integrin ligand RGD routinely supported hES cell binding, their ability to maintain hES cell markers was inconsistent (Fig. 1e,f). Another integrin-binding peptide GSDPGYIGSR also could mediate hES cell adhesion, but the resulting cells exhibit marked decreases in Oct-4 and SSEA-4 levels (Fig. 1g). In contrast, surfaces presenting heparin-binding peptides (GKKQRFRHRNRKG, FHRRIKA, and GWQPPARARI) consistently mediated hES cell adhesion and allowed for hES cell propagation. Specifically, cells cultured on these surfaces for 6 days maintained high levels of Oct-4 and SSEA-4 (Fig. 1b–d). Surfaces displaying the heparin-binding peptide GKKQRFRHRNRKG27 derived from vitronectin not only supported adhesion but did so at the lowest peptide substitution levels (Fig. 1a,b). Other peptide-substituted surfaces were either unable support hES cell adhesion or did so inconsistently (Supplementary Table 1). The data from our array screens led us to conclude that surfaces presenting heparin-binding peptides are effective at supporting short term hES cell survival.
Adhesion through cell-surface glycosaminoglycans
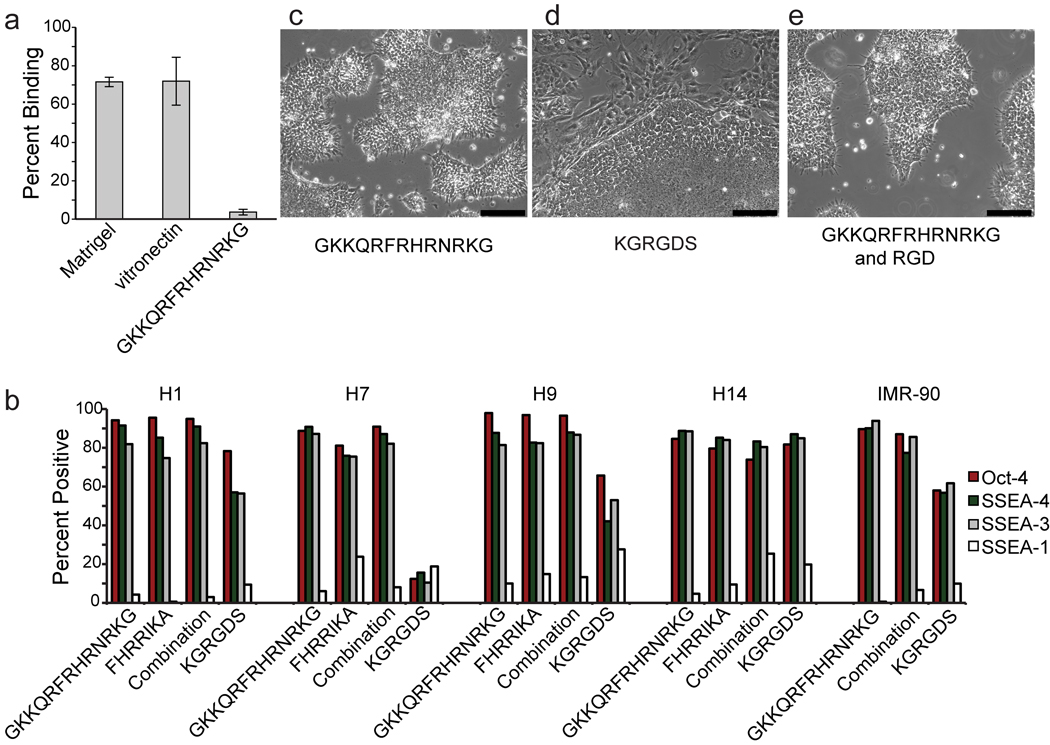
We anticipated that surfaces displaying heparin-binding peptides would promote cell adhesion and spreading by interacting with cell-surface glycosaminoglycans (GAGs). GAGs have a variety of roles; they participate in receptor—signaling complexes, cell— cell recognition and adhesion, and cell—matrix interactions28. To test whether GAGs participate in hES cell binding to the heparin-binding peptide-substituted surfaces, we exposed cells to the enzyme chondroitinase ABC. Although this enzyme catalyzes the hydrolysis of only a subset of GAGs, cells exposed to it exhibit decreased binding to the synthetic surfaces presenting GKKQRFRHRNRKG (Supplementary Fig. 2). Soluble heparin, which should compete with cell surface GAGs, also inhibited binding (Fig. 2a). In addition to completely blocking cell interactions to the synthetic GKKQRFRHRNRKG-presenting surfaces, heparin mitigated cell adhesion to Matrigel and vitronectin, suggesting that these substrates also bind to hES cells through GAG interactions (Fig. 2a). These data suggest that the GAGs found on the surface of hES cells can play critical roles in mediating attachment to the substratum. Additionally, they highlight that a substratum consisting of even a single ECM protein, like vitronectin, supports cell adhesion through multiple interactions. Finally, they reveal that synthetic surfaces presenting a heparin-binding peptide can enable cell adhesion through a single binding epitope.
Figure 2.
Surfaces displaying heparin-binding peptides support hES cell adhesion and self-renewal. (a) The plot shows percentage of cells binding to the indicated surfaces as measured by a luminescence assay. Percentages of cell binding represent the average ratio of the mean luminescence of cell lysates plated in the presence of heparin versus those without heparin. Error bars, s.d. (n=3). (b) The plots show the fraction of cells expressing the indicated markers after culture for 3 passages in mTeSR + Ri on surfaces presenting the indicated peptides. (c – e) Micrographs show the morphology of cells cultured on the indicated surfaces. Scale bars, 200 µm.
Growth rates of hES cells
We compared the growth of hES cells cultured on surfaces presenting synthetic peptides to that on more complex surfaces. First, we used a colorimetric assay that assesses cell number to generate growth curves for hES cells (H9 and H13) cultured on Matrigel, vitronectin, and streptavidin-modified surfaces presenting biotinylated-GKKQRFRHRNRKG. The results for Matrigel and surfaces presenting GKKQRFRHRNRKG were similar (Supplementary Figs. 3a and 3b). Second, we monitored cell division using a fluorescence assay; again, the data indicate that cells cultured on synthetic surfaces presenting the heparin-binding peptide GKKQRFRHRNRKG or a combination of GKKQRFRHRNRKG and KRGDS propagate at a rate similar to those cultured on Matrigel (Supplementary Fig. 3c). In contrast, with vitronectin-coated or KRGDS-presenting surfaces, fewer cell divisions occurred (Supplementary Fig. 3c). We also compared our surface to one coated with polylysine, because polylysine had been reported to support hES cell propagation in a defined medium for multiple passages15. Our results indicate that cells cultured on polylysine-coated surfaces do not readily divide (Supplementary Fig. 3c). We conclude that, for hES cell proliferation, surfaces presenting GKKQRFRHRNRKG are comparable to Matrigel and superior to polysine-coated surfaces.
Analysis of stem cell markers
We examined whether surfaces bearing heparin-binding peptides could support hES cell (H1, H7, H9, H14) self-renewal over multiple passages. Cells were transferred from Matrigel to two different surfaces, each of which presents a different heparin-binding peptide. After 3 passages or 21 days, the cells maintained high levels of markers of pluripotency (Fig. 2b) and grew in the compact colonies characteristic of undifferentiated pluripotent stem cells (Fig. 2c). Similar results were obtained for iPS cells (IMR-90-1) cultured on GKKQRFRHRNRKG-presenting surfaces (Fig. 2b). For comparison, we analyzed hES cells grown on a surface displaying the RGD sequence. When the KRGDS sequence was used on SAMs at densities comparable to those employed for the heparin-binding surfaces (3 pmol/mm2), the cell population was heterogeneous (Fig. 2d and Supplementary Fig. 4a–c). Comparison of this population to cells grown on Matrigel or the heparin-binding peptides revealed that the RGD-substituted surfaces afford fewer cells that display markers of pluripotency (Fig. 2b). Overall, cells cultured on RGD-substituted SAMs exhibit a variety of morphologies and differentiate into all three embryonic germ layers as determined by immunostaining (Supplementary Fig. 4). These data are consistent with those from our array screen in which RGD-presenting surfaces were inferior substrata for pluripotent stem cell propagation.
To determine whether RGD-displaying SAMs induce differentiation, we cultured cells on synthetic surfaces presenting a combination of RGD and GKKQRFRHRNRKG. Cells cultured on these surfaces maintained high levels of markers of pluripotency and grew in the compact colonies characteristic of undifferentiated pluripotent stem cells (Fig. 2b,e). These data indicate that RGD-substituted surfaces do not induce differentiation, but rather, at least for the KRGDS peptide, that they are inadequate substrates for maintaining a homogenous population of undifferentiated pluripotent cells.
Long-term culture of human pluripotent cells
A major challenge for defined culture conditions is long-term pluripotent stem cell propagation. To test whether our surface can meet this challenge, we cultured human pluripotent stem cells (H9, H13, H14 and DF19-97T) on SAMs presenting the heparin-peptide GKKQRFRHRNRKG for 2–3 months. We used surfaces displaying this peptide because they provide strong, consistent attachment and promote colony spreading at low surface densities. Human pluripotent stem cells cultured long-term continued to grow as compact colonies and maintained markers of pluripotency (Fig. 3a–c).
Figure 3.
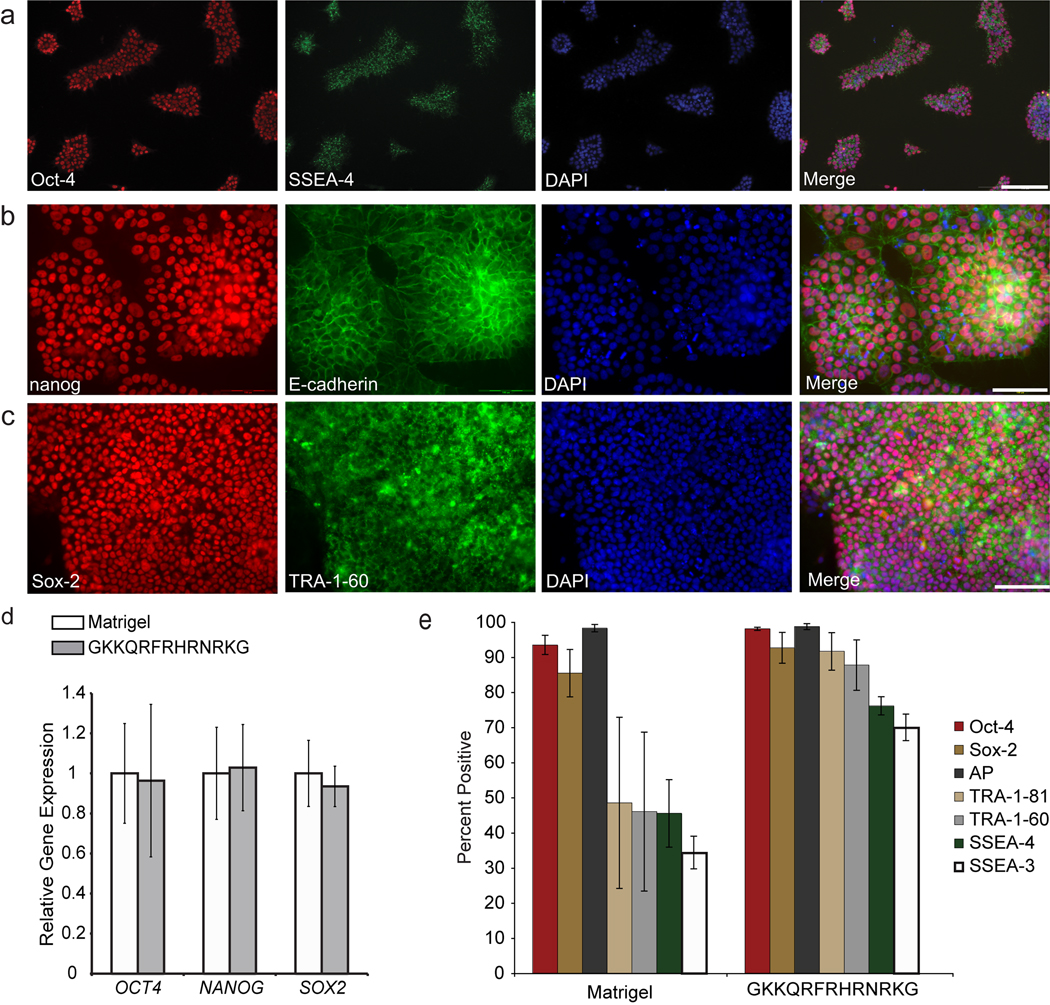
Synthetic surfaces support the long-term culture of pluripotent stem cells cells. (a) hES cells (H9) cultured for three months in mTeSR + Ri on the synthetic surface were immunostained for Oct-4 (red) and SSEA-4 (green). Blue indicates DAPI stain in all cases. Scale bar, 200 µm. (b–c) Induced pluripotent stem cells (DF19-97T) cultured for 2.5 months in mTeSR + Ri on the synthetic surface were immunostained for nanog (red) and E-cadherin (green) (b), or immunostained for Sox2 (red) and TRA-1-60 (green) (c). Scale bars, 100 µm. (d) The plot shows relative gene expression levels of the indicated genes as measured by real-time quantitative PCR analysis of hES cells (H13) cultured long-term (14 passages) in mTeSR + Ri on a surface displaying the peptide GKKQRFRHRNRKG and cells cultured concurrently in mTeSR on Matrigel. Error bars, s.d. (n=3). (e) Plots show the percentage of cells (H9) positive for the indicated markers as measured by flow cytometry after three months (17 passages). Cells were cultured in mTeSR + Ri on the synthetic surface or in mTeSR on Matrigel. Data represent the average of three consecutive passages ± standard deviation.
We compared cells cultured on our surfaces to those propagated on Matrigel by examining the expression of genes associated with pluripotency and differentiation. Flow cytometry analysis of cells (H9 and H14) cultured for three months (17 passages) indicate that the cells maintain high levels of markers both integral to and associated with pluripotent stem cells. They also possess low levels of the marker of differentiation SSEA-1 (Fig. 3d and Supplementary Fig. 5). Consistent with these results, real-time quantitative PCR (RT-qPCR) for cells (H13) cultured for 2 months on a synthetic surface indicates that the relative expression levels of key transcription factors required for pluripotency are similar to those of cells cultured on Matrigel (Fig. 3e). Additionally, a gene expression profile of 84 genes associated with pluripotency or early differentiation events was conducted on H9 cells that had been propagated for 3 months on the synthetic surface or on Matrigel (Supplementary Fig. 6a). The cells maintain similar expression levels of genes associated with pluripotency in both culture conditions. Most genes with expression levels differing by ≥4-fold are associated with differentiation, and their levels were lower for the cells cultured on our synthetic surface (Supplementary Fig. 6a,b). Human pluripotent stem cells (hES cell lines H1, H7, H9, H13, H14 and iPS cell line IMR-90) cultured for 1–3 months on our synthetic surface maintained a normal karyotype (Supplementary Fig. 7). These data demonstrate that our chemically-defined, synthetic surface can be used to propagate homogenous populations of pluripotent stem cells.
Differentiation of human pluripotent cells
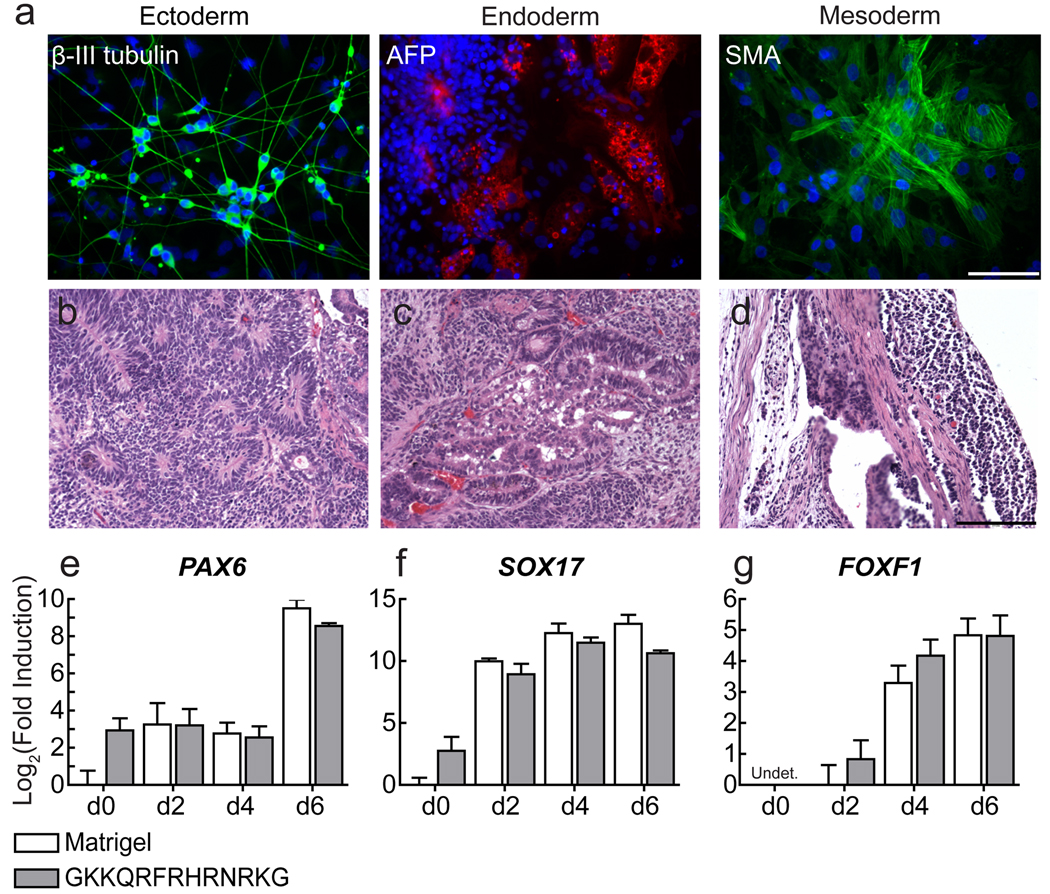
To evaluate further the pluripotency of the cells that had been cultured on the synthetic surfaces, we assessed their ability to differentiate. When placed in suspension culture, pluripotent stem cells form embryoid bodies that undergo differentiation into derivatives of all three embryonic germ layers. We subjected the H1, H7, H9, H13, H14 hES cell lines and the IMR-90 and DF19-97T iPS cell lines cultured on the synthetic substrate for 1–3 months to this differentiation protocol. All lines differentiated into a heterogenous population containing cells that stain positive for markers of ectoderm, endoderm, and mesoderm (Fig. 4a and Supplementary Fig. 8a–f). Additionally, H1 and H9 cell lines cultured on a synthetic surface for 6 and 19 passages, respectively, formed teratomas (Fig. 4b–d and Supplementary Fig. 8g).
Figure 4.
Pluripotent stem cells cells grown on synthetic surfaces maintain their ability to differentiate. (a) Micrographs show in vitro differentiation of hiPS cells (DF19-97T) maintained in mTeSR + Ri on surfaces presenting the heparin-binding peptide GKKQRFRHRNRKG for 3 months. Differentiated cells were stained for markers of ectoderm (green), endoderm (red), and mesoderm (green) and counterstained with DAPI (blue). Scale bar, 100 µm. (b–d) Micrographs show teratoma formation by H9 cells maintained on SAMs presenting the heparin-binding peptide GKKQRFRHRNRKG for 3 months in mTeSR + Ri. Teratomas contained a mixture of tissues resembling the (b) neural tube, (c) the gut, and (d) the mesenchyme. (Scale bars, 200 µm). (e–g) The plots show the fold induction (relative gene expression levels) of lineage specific genes representing (e) ectoderm, (f) endoderm, and (g) mesoderm after directed differentiation of hES cells (H14) cultured long-term (17 passages) on surfaces presenting GKKQRFRHRNRKG in mTeSR + Ri, compared to Matrigel. Expression was analyzed via RT-qPCR, error bars, s.d. (n = 3).
The aforementioned investigations reveal that cells cultured on our synthetic surfaces can differentiate, but they do not address whether these culture conditions render them less prone to do so. To explore this issue, we took advantage of the procedures developed to differentiate human pluripotent cells to specific lineages in culture. We applied three differentiation protocols to hES cells (H14) that had been propagated for 3 months on either GKKQRFRHRNRKG or Matrigel. To monitor the rate of differentiation, RT-qPCR was performed to determine the relative expression levels of lineage-specific genes. For each germ layer, cells cultured long-term on GKKQRFRHRNRKG expressed lineage-specific genes at a level and timescale similar to those obtained for cells cultured on Matrigel (Fig. 4e–g and Supplementary Fig. 9). Together the data indicate that pluripotent cells propagated on the synthetic surface possess the differentiation potential of those cultured on Matrigel.
Portability of heparin-binding peptides
Our results demonstrate that peptide-substituted surfaces can be used for routine culture of pluripotent stem cells. One advantage of synthetic peptides is that they can be incorporated into a wide variety of materials. To determine the portability of the heparin-binding peptides, we tested whether displaying GKKQRFRHRNRKG on alternative scaffolds could yield materials that support cell adhesion and growth. To this end, we chemically conjugated the peptide CGKKQRFRHRNRKG to glass coverslips functionalized with bromoacetamide groups. The resulting material supported excellent cell attachment and allowed for colony spreading (Supplementary Fig. 10a–b). Because of their availability and ease of modification, we additionally tested streptavidin-coated surfaces, which can be decorated with biotinylated peptides. When biotinylated heparin-binding peptides were presented, the resulting surfaces support iPS and hES cell adhesion (Supplementary Fig. 10c–d) and propagation (data not shown). As with the SAMs, these surfaces required that the culture medium be supplemented with the ROCK inhibitor.
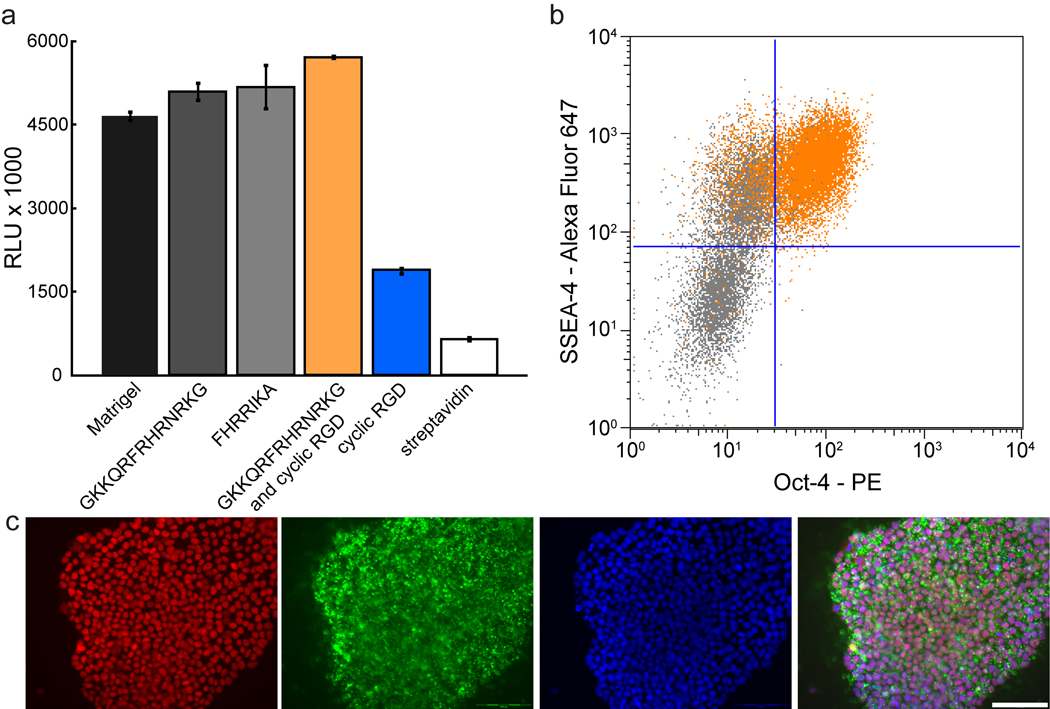
We also examined a combination surface. When a streptavidin-coated surface was modified with the GAG-binding peptide GKKQRFRHRNRKG and cyclic RGD20, the result was a robust substrate for hES cell adhesion (Fig. 5a). Notably, after colonies had formed on this surface, the ROCK inhibitor could be removed from the culture medium. Human ES cells (H9, H14, and SA02) propagated on streptavidin-coated surfaces modified with GKKQRFRHRNRKG and the cyclic RGD peptides for 1–2 months maintained high levels of pluripotency markers and a stable karyotype in the absence of ROCK inhibitor (Fig. 5b–c, Supplementary Fig. 6c–d, and Supplementary Fig. 7g–h). In contrast, streptavidin-coated surfaces displaying biotinylated cyclic RGD alone did not support hES cell adhesion in the absence of ROCKi (Supplementary Fig. 11b–h), and were inconsistent in their ability to maintain Oct-4 levels over multiple passages even in the presence of ROCKi (Supplementary Fig. 11i). Additional investigations are needed to elucidate how simultaneous GAG and integrin engagement influences pluripotent stem cell propagation.
Figure 5.
Streptavidin-coated surfaces presenting heparin-binding peptides support robust adhesion and self-renewal. (a) The plot shows hES cell (H9) adhesion to the indicated surfaces as measured by a luminescence assay. Error bars, s.d. (n = 3). (b) Flow cytometric analysis of hES cells (H14, orange dot plots) cultured for one month (10 passages) on a combination of GKKQRFRHRNRKG and cyclic RGD in mTeSR alone, showing expression of Oct-4 (X-axis) and SSEA-4 (Y-axis). Data from partially differentiated cells also are shown (gray dot plots). (c) The micrographs show hES cells (H9) cultured in mTeSR for two months (17 passages) on a combination of GKKQRFRHRNRKG and cyclic RGD and immunostained for Oct-4 (red), SSEA-4 (green) and counterstained with DAPI (blue). Scale bar, 100 µm.
DISCUSSION
Elucidating the molecular mechanisms underlying the proliferation and differentiation of human pluripotent stem cells is vital for their use in fundamental biological investigations and cell-based therapies. Our results underscore the utility of the surface array strategy for identifying active surfaces and for providing insights into cell surface characteristics that can be exploited for long-term culture. In our hands, the most effective peptide substrates for pluripotent stem cell adhesion and propagation are those that can bind anionic polysaccharides like heparin, supporting a model in which the synthetic surfaces act by engaging the glycosaminoglycans on pluripotent cells. That these simple synthetic substrata can replace Matrigel for pluripotent cell culture is consistent with the proposition that embryonic stem cells have an innate capacity for self-renewal and do not require a rich milieu of external signals29.
Still, our data indicate that adhesion is not the sole prerequisite for self-renewal. We and others14,16,20 have found that materials decorated with RGD-containing peptides vary in their ability to support hES cell self-renewal despite supporting adhesion. Many previous investigations to develop substrata for hES cell culture have focused on the importance of integrins binding to ECM proteins10,11,19. The RGD substituted material that is most successful at maintaining hES proliferation requires high densities of the integrin epitope KGGNGEPRGDTYRAY or KGGPQVTRGDVFTMP16. Such a high level of substitution could yield surfaces that manifest their activities through bulk properties rather than their ability to engage a particular type of cell surface receptor. Alternatively, the more complex sequence may engage specific integrins or other crucial cell surface receptors that the shorter RGD sequences cannot. The origins of the varying outcomes obtained with different surfaces that present RGD-containing peptides remains to be elucidated.
Though integrin ligands alone were not effective to maintain long term pluripotent stem cell culture on our surfaces, we did find that they could augment the activity of the glycosaminoglycan binding substrates. Adhesion of pluripotent cells to surfaces presenting a heparin-binding peptide alone require ROCK inhibition even after the formation of colonies. In contrast, streptavidin-coated surfaces presenting a combination of the high-affinity cyclic RGD and GKKQRFRHRNRKG did not require ROCK inhibitor after colonies had formed.
The heparin-binding peptides described here can be incorporated into a wide variety of materials25,30 for culturing pluripotent human cells. Because GKKQRFRHRNRKG need only be present at a low surface density to serve as a substrate, additional signals or cell binding epitopes can easily be incorporated to study their effects on self-renewal and differentiation. Moreover, the active surfaces in our array screen are portable. For instance, peptides presented on strepavidin-coated surfaces provide a defined, modular, and facile method for propagating pluripotent stem cells that is inexpensive and readily accessible. Surfaces presenting the heparin-binding peptide derived from vitronectin are the most robust of all the synthetic surfaces we have tested to date12,18,21. In summary, our fully defined culture system has both practical and fundamental advantages for pluripotent stem cell culture.
METHODS
Methods and any associated references are available in the online version of the paper at http://www.nature.com/naturemethods/.
Methods
Cell Culture
Human ES cell lines (H1, H7, H9, H13, H14, and SA02) and iPS cell lines (IMR90-1 and the vector-free DF19-97T) were maintained on Matrigel-coated plates using the mTeSR1 medium (StemCell Technologies). Cells were maintained at 37 °C/5% CO2. They were passaged manually every 5–6 days after treatment with the protease dispase (Gibco, 2 mg/mL) for 5–6 minutes31. Human ES cells grown on synthetic surfaces were maintained in mTeSR1 medium supplemented with ROCK inhibitor Y-27632 (Calbiochem, 5 ⎧M) or H-1152 (Alexis, 0.5 ⎧M). Cells were passaged manually every 5–7 days after treatment with an enzyme-free, Hanks’-based cell dissociation buffer (Sigma) for 10–15 minutes. Cells were seeded onto new surfaces at 25,000 cells/cm2.
Differentiation Assays
Embryoid bodies were formed in poly(2-hydroxyethyl methacrylate) (Sigma) coated flasks (Greiner Bio-One), and cultured in medium consisting of Iscove’s modified Dulbecco’s medium (Gibco), 15% fetal bovine serum (Gibco), 1% non-essential amino acids (Gibco), and 0.1 mM β-mercaptoethanol (Gibco). Teratomas were generated as described32. A detailed protocol can be obtained from the WiCell research institute at www.wicell.org. This protocol was approved by the University of Wisconsin—Madison Research Animal Resources Center (RARC) and was performed by personnel listed on the Stem Cell Research Oversight Committee (SCRO) protocol after completing the RARC animal handling course and the appropriate animal facilities orientation. For the directed differentiation assays, cells were dissociated with enzyme-free, Hanks’-based cell dissociation buffer (Sigma) and plated onto new Matrigel surfaces at 17,500–30,000 cells/cm2 in mTeSR1 supplemented with 5 ⎧M Y-27632. The basal differentiation medium (RDM) consisted of Advanced RPMI 1640 supplemented with B27, Penicillin-Streptomycin, and Glutamax (Invitrogen). For mesoderm differentiation, cells were treated with RDM supplemented with 100 ng/mL Activin A (R&D Systems) for 1 day followed by RDM supplemented with 10 ng/mL BMP4 (R&D Systems) for 5 days33. For endoderm differentiation, cells were treated with RDM supplemented with 100 ng/mL Activin A for 6 days34. For ectoderm differentiation, cells were treated with RDM supplemented with 10 ⎧M SB421542 (Tocris) and 500 ng/mL Noggin (R&D Systems) for 6 days35.
Fabrication of Peptide-presenting Surfaces
Peptide-ATs were synthesized as described previously12,21. Briefly, bioactive peptides were synthesized on PAL-polystyrene resin (Applied Biosystems) via standard Fmoc chemistry using an automated peptide synthesizer. Trityl-protected alkanethiol with a carboxyl group was coupled to the N-terminus of the peptides on the solid support. Peptide-ATs were cleaved off the resin using 92.5% TFA/2.5% triisopropylsilane/2.5% ethalenedithiol/2.5% H2O. The resulting material was precipitated in ice cold ether and purified by HPLC. The glucamine-AT and the perfluorinated-AT were synthesized as reported21,36. Peptide-ATs and glucamine-AT were dissolved at a 1 mM concentration in 30% DMSO/H2O. Perfluorinated-AT was dissolved at a 1 mM concentration in ethanol. Gold-coated glass slides (250 Å Gold, 10 Å chromium-coated slides, 22 mm square, 0.16 mm thick) were purchased from EMF Corporation. Arrays were prepared as described previously12. When larger areas of peptide-AT SAMs were needed, whole chips presenting the same SAM were fabricated by sandwiching solutions of peptide-AT/glucamine-AT between two gold-coated slides. SAMs were allowed to form in humidity chambers for 24 h before use.
To fabricate peptide-displaying glass slides, glass surfaces were functionalized with amino groups using an established protocol 37. The resulting dried surfaces were treated with a mixture of 50 mM bromoacetic anhydride and 50 mM triethylamine in anhydrous DMF for 4 hours. The slides were washed with water and exposed overnight to the cell adhesive peptide CGKKQRFRHRNRKG, which can undergo reaction via its N-terminal cysteine residue with the bromoacetamide-substituted surface. Any remaining bromoacetamide groups were capped by treatment with 50 mM cysteine in PBS (pH 8.5) for 2 hours.
To display the peptide on polystyrene, non-tissue culture treated plates (Falcon) were coated with 10 ⎧g/mL streptavidin in Hanks’ balanced salt solution (Gibco). Wells were washed with Hanks’ balanced salt solution (Gibco) and then coated with 5 ⎧M biotin-Ahx-GKKQRFRHRNRKG (Biomatik), biotin-LC-GRGDS (Anaspec), or cyclo RGD-d-FK-PEG-PEG-Biotin (Peptides International) in Hanks’ balanced salt solution. Surfaces presenting a 7:3 ratio of biotin-Ahx-GKKQRFRHRNRKG to RGD-d-FK-PEG-PEG-Biotin were used to propagate hES cells without ROCK inhibitor after the formation of colonies.
Microscopy and immunostaining
Images were collected with a Hamamatsu digital camera mounted onto an Olympus IX81 microscope. Primary antibodies used in this study included Oct-4 (R&D Systems, 1:400), nanog (R&D Systems, 1:100), Sox-2 (R&D Systems, 1:250), SSEA-4 (Santa Cruz Biotechnology, 1:400), TRA-1-60 (Santa Cruz Biotechnology, 1:500), β-III tubulin (R&D Systems, 1:3000), nestin (1:3000), α-fetoprotein (Sigma, 1:250), FoxA2 (R&D Systems, 1:100), α-smooth muscle actin (Sigma, 1:1000), and fatty acid binding protein 4 (R&D Systems, 1:250). Cells were fixed with PBS containing 4% formaldehyde and 0.15% picric acid for 20 minutes at 37 °C, and then permeabilized and blocked with PBS containing 0.1% Triton X-100 and 2% BSA. All antibodies were incubated in blocking buffer overnight at 4 °C, except for the antibodies against β-III tubulin, nestin, α-smooth muscle actin, which were incubated for 1 hour at room temperature. Secondary staining was performed with Alexa Fluor 488-, rhodamine-, or Alexa Fluor 594-conjugated antibodies (Invitrogen, 1:1000), which were diluted in blocking buffer and exposed to cells for 1 hour at room temperature. Cells were counterstained with 4’,6-diamidino-2-phenylindole, dilactate (DAPI, Invitrogen). Peptide array mosaics were generated using the AnalySIS acquisition software. Image overlays were generated using ImageJ software.
Flow Cytometry
Human ES cells were dissociated with 0.05% trypsin-EDTA with 2% chicken serum (Gibco). Surface marker staining was performed in PBS containing 2% BSA (w/v) at 4 °C for 30 minutes with the following antibodies; alkaline phosphatase allophycocyanin conjugated (R&D Systems), Tra 1-60 Alexa Fluor 488 conjugated (BD), Tra 1-81 Alexa Fluor 647 conjugated (BD), SSEA-4 Alexa Fluor 647 conjugated (BD), SSEA-3 phycoerythrin (PE) conjugated (BD), SSEA-1 PE conjugated (R&D Systems). Antibody exposure was followed by 1 wash and then a 30 min fixation with 2% formaldehyde/PBS at room temperature. For internal marker staining, hES cells were fixed with 2% formaldehyde/PBS at room temperature for 30 min. For intracellular staining, cells were permeabilized with saponin permeabilization buffer (SPB), (0.1% saponin, 0.1% BSA in PBS) for 30 min at room temperature, and then stained with an Oct-4 PE conjugated (BD) antibody, or nanog PE conjugated (BD) antibody overnight. Cells were washed 2 times with SPB before analysis. For Sox-2 staining, cells were permeabilized with 90% ice-cold methanol, washed with SPB, incubated with Sox-2 Alexa Fluor 488 or 647 conjugated antibody (BD) for 1 hour at 4 °C, and then washed 2 times with SPB. Data were obtained using a FACSCalibur or a BD LSR II and analyzed using FlowJo software. The percentage of positive cells was established by comparing experimental cells to differentiated hES cells. Gating for positive and negative populations was established by analyzing the bimodal peaks of partially differentiated hES cells.
Real-time Quantitative PCR Analysis
Total RNA was isolated from samples using the RNeasy Plus Kit (Qiagen). 200–600ng RNA was reverse transcribed via AffinityScript QPCR cDNA Synthesis Kit (Stratagene). QPCR was performed on a 7500 Fast Real-Time PCR System (Applied Biosciences) using SYBR Green JumpStart Taq ReadyMix (Sigma) and lineage-specific gene primers. The cycling parameters were 95°C for 2 minutes for initial denaturation followed by 40 cycles of 95°C for 15 seconds for denaturation, then 56°C for 1 minute for primer annealing and extension. Melting curve analysis ensured primer specificity, and no reverse transcripase samples controlled for genomic DNA contamination. Relative gene expression levels (fold induction) were determined using the ⊗⊗Ct method, and the error bars represent the propagated error determined from the standard deviation of triplicate reactions. Briefly, gene of interest threshold cycle (Ct) values were normalized to Ct values for the housekeeping gene SRP72 and compared to the Ct value determined for undifferentiated cells cultured long-term on Matrigel. In the event that the Ct value for the gene of interest was not detected in the undifferentiated sample, the earliest time point with detectable expression was substituted. The primer sets are listed in Supplementary Table 2. The human embryonic stem cell RT2 Profiler PCR Array (SABiosciences) was performed according the manufacturer’s protocol.
Harvest and G-Banding of Human ES Cells for Cytogenetic Analysis
The harvest procedure for human ES cells was adapted from standard cytogenetics protocols. Detailed protocols can be obtained from the WiCell research institute at www.wicell.org.
Adhesion Assay for GAG Involvement
Human ES cells (H9) cultured on Matrigel were dissociated using an enzyme-free, Hanks’-based cell dissociation buffer (Sigma) for 10–15 minutes. Cells were resuspended in DMEM/F12 (Gibco), or DMEM/F12 supplemented with 2 units/mL chondroitinase ABC (Sigma), or 500 ⎧g/mL heparin (Sigma). Cells treated with the GAG-degrading enzymes were incubated for 1 hour in suspension at 37 °C. Cell suspensions were seeded on to Matrigel-coated surfaces, recombinant vitronectin-coated surfaces (10 ⎧g/mL, R&D Systems), or SAMs presenting the peptide GKKQRFRHRNRKG at a 5% surface density. After 1 hour, surfaces were washed 3 times with PBS and the cells were lysed with M-PER buffer (Pierce). The cell lysate was mixed with CellTiter-Glo (Promega), which is a homogenous and sensitive method to determine the number of viable cells in culture based on the presence of ATP. The luminescence was measured on 20/20n luminometer from Turner Biosystems.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank R. Derda, B. P. Orner, and J. A. Thomson for helpful discussions. They also acknowledge J. R. Torrealba for analysis and interpretation of the teratoma data and G. L. Case at the University of Wisconsin Biotechnology Center for help with the automated peptide synthesis. This research was supported by the National Institutes of Health (R01 grants AI055258 and GM49975), and the University of Wisconsin Materials Research Science and Engineering Center (DMR-0520527). We thank the W. M. Keck Foundation for supporting the Center for Chemical Genomics, and the WiCell Research Institute and the University of Wisconsin Paul P. Carbone Comprehensive Cancer Center Flow Cytometry Facility (5P30 CA014520-3S) for technical assistance.
Footnotes
Note: Supplementary information available on the Nature Methods website.
AUTHOR CONTRIBUTIONS
JRK, LL, PJW, and LLK conceived the experiments and interpreted the results. JRK performed the in vitro experiments. LL synthesized and purified the molecules used to fabricate the surfaces. JRK and MSP conducted the teratoma assay, and PJW conducted the directed differentiation assays. JRK and LLK wrote the manuscript.
COMPETING FINANCIAL INTRESTS
The authors declare competing financial interests: details accompany the full-text HTML version of the paper on the Nature Methods website. LLK is an author on a patent on defined surfaces of self-assembled and stem cells (US patent 2007/0207543 A1). LLK, JRK, and LL are authors on a patent pending for peptide presenting surfaces for the long-term culture of pluripotent cells (US patent application 20100087004).
References
- 1.Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 4.Ludwig TE, et al. Derivation of human embryonic stem cells in defined conditions. Nat. Biotechnol. 2006;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- 5.Yao S, et al. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc. Natl. Acad. Sci. USA. 2006;103:6907–6912. doi: 10.1073/pnas.0602280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L, et al. Self-renewal of human embryonic stem cells requires insulin-like growth factor-1 receptor and ERBB2 receptor signaling. Blood. 2007;110:4111–4119. doi: 10.1182/blood-2007-03-082586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu C, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nature Biotechnology. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 8.Martin MJ, Muotri A, Gage F, Varki A. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med. 2005;11:228–232. doi: 10.1038/nm1181. [DOI] [PubMed] [Google Scholar]
- 9.Amit M, Shariki C, Margulets V, Itskovitz-Eldor J. Feeder layer- and serum-free culture of human embryonic stem cells. Biol. Reprod. 2004;70:837–845. doi: 10.1095/biolreprod.103.021147. [DOI] [PubMed] [Google Scholar]
- 10.Braam SR, et al. Recombinant Vitronectin is a Functionally Defined Substrate that Supports Human Embryonic Stem Cell Self Renewal via {alpha}V{beta}5 Integrin. Stem Cells. 2008;26:2257–2265. doi: 10.1634/stemcells.2008-0291. [DOI] [PubMed] [Google Scholar]
- 11.Vuoristo S, et al. Laminin isoforms in human embryonic stem cells: synthesis, receptor usage and growth support. J. Cell. Mol. Med. 2009;13:2622–2633. doi: 10.1111/j.1582-4934.2008.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derda R, et al. Defined substrates for human embryonic stem cell growth identified from surface arrays. ACS Chem. Biol. 2007;2:347–355. doi: 10.1021/cb700032u. [DOI] [PubMed] [Google Scholar]
- 13.Gerecht S, et al. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2007;104:11298–11303. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li YJ, Chung EH, Rodriguez RT, Firpo MT, Healy KE. Hydrogels as artificial matrices for human embryonic stem cell self-renewal. J. Biomed. Mater. Res. A. 2006;79:1–5. doi: 10.1002/jbm.a.30732. [DOI] [PubMed] [Google Scholar]
- 15.Harb N, Archer TK, Sato N. The Rho-Rock-Myosin signaling axis determines cell-cell integrity of self-renewing pluripotent stem cells. PLoS ONE. 2008;3:e3001. doi: 10.1371/journal.pone.0003001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melkoumian Z, et al. Synthetic peptide-acrylate surfaces for long-term self-renewal and cardiomyocyte differentiation of human embryonic stem cells. Nature Biotechnology. 2010;28:606–610. doi: 10.1038/nbt.1629. [DOI] [PubMed] [Google Scholar]
- 17.Villa-Diaz LG, et al. Synthetic polymer coatings for long-term growth of human embryonic stem cells. Nature Biotechnology. 2010;28:581–583. doi: 10.1038/nbt.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derda R, et al. High-Throughput Discovery of Synthetic Surfaces That Support Proliferation of Pluripotent Cells. J. Am. Chem. Soc. 2010 doi: 10.1021/ja906089g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meng Y, et al. Characterization of integrin engagement during defined human embryonic stem cell culture. FASEB J. 2010;24:1056–1065. doi: 10.1096/fj.08-126821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolhar P, Kotamraju VR, Hikita ST, Clegg DO, Ruoslahti E. Synthetic surfaces for human embryonic stem cell culture. J. Biotechnol. 2010 doi: 10.1016/j.jbiotec.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Orner BP, Derda R, Lewis RL, Thomson JA, Kiessling LL. Arrays for the combinatorial exploration of cell adhesion. J. Am. Chem. Soc. 2004;126:10808–10809. doi: 10.1021/ja0474291. [DOI] [PubMed] [Google Scholar]
- 22.Whitesides GM, Ostuni E, Takayama S, Jiang X, Ingber DE. Soft lithography in biology and biochemistry. Annu.Rev. Biomed Eng. 2001;3:335–373. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- 23.Mrksich M, Whitesides GM. Using self-assembled monolayers to understand the interactions of man-made surfaces with proteins and cells. Annu. Rev. Bioph. Biom. 1996;25:55–78. doi: 10.1146/annurev.bb.25.060196.000415. [DOI] [PubMed] [Google Scholar]
- 24.Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238:491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 25.Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature. 2009;462:433–441. doi: 10.1038/nature08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watanabe K, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 27.Vogel BE, et al. A novel integrin specificity exemplified by binding of the alpha v beta 5 integrin to the basic domain of the HIV Tat protein and vitronectin. J. Cell. Biol. 1993;121:461–468. doi: 10.1083/jcb.121.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446:1030–1037. doi: 10.1038/nature05817. [DOI] [PubMed] [Google Scholar]
- 29.Ying Q, et al. The ground state of embryonic stem cell self-renewal. Nature. 2008;453 doi: 10.1038/nature06968. 519-U515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 31.Ludwig TE, et al. Feeder-independent culture of human embryonic stem cells. Nat. Methods. 2006;3:637–646. doi: 10.1038/nmeth902. [DOI] [PubMed] [Google Scholar]
- 32.Prokhorova T, et al. Teratoma Formation by Human Embryonic Stem Cells is site-dependent and enhanced by the presence of Matrigel. Stem Cells Dev. 2008;18:47–54. doi: 10.1089/scd.2007.0266. [DOI] [PubMed] [Google Scholar]
- 33.Laflamme MA, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat. Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 34.D'Amour KA, et al. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 35.Chambers SM, et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luk YY, Kato M, Mrksich M. Self-assembled monolayers of alkanethiolates presenting mannitol groups are inert to protein adsorption and cell attachment. Langmuir. 2000;16:9604–9608. [Google Scholar]
- 37.Klein EA, Yung Y, Castagnino P, Kothapalli D, Assoian RK. Cell adhesion, cellular tension, and cell cycle control. Meth. Enzymol. 2007;426:155–175. doi: 10.1016/S0076-6879(07)26008-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.