Abstract
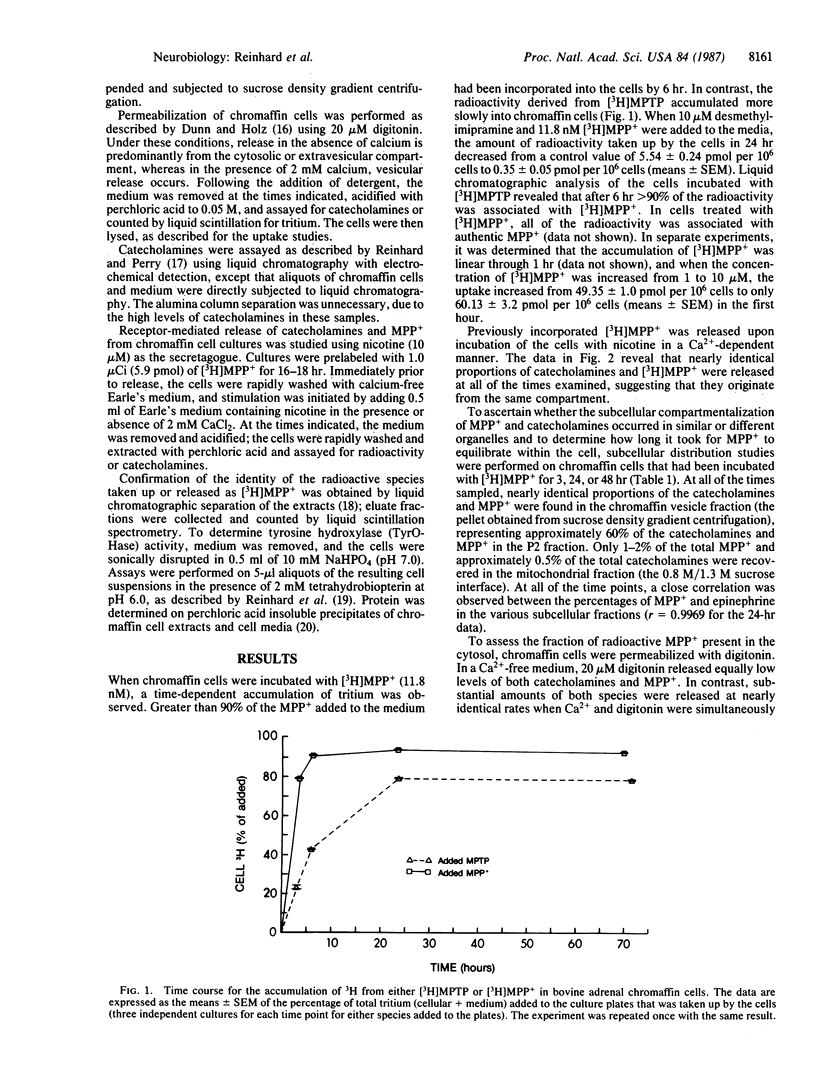
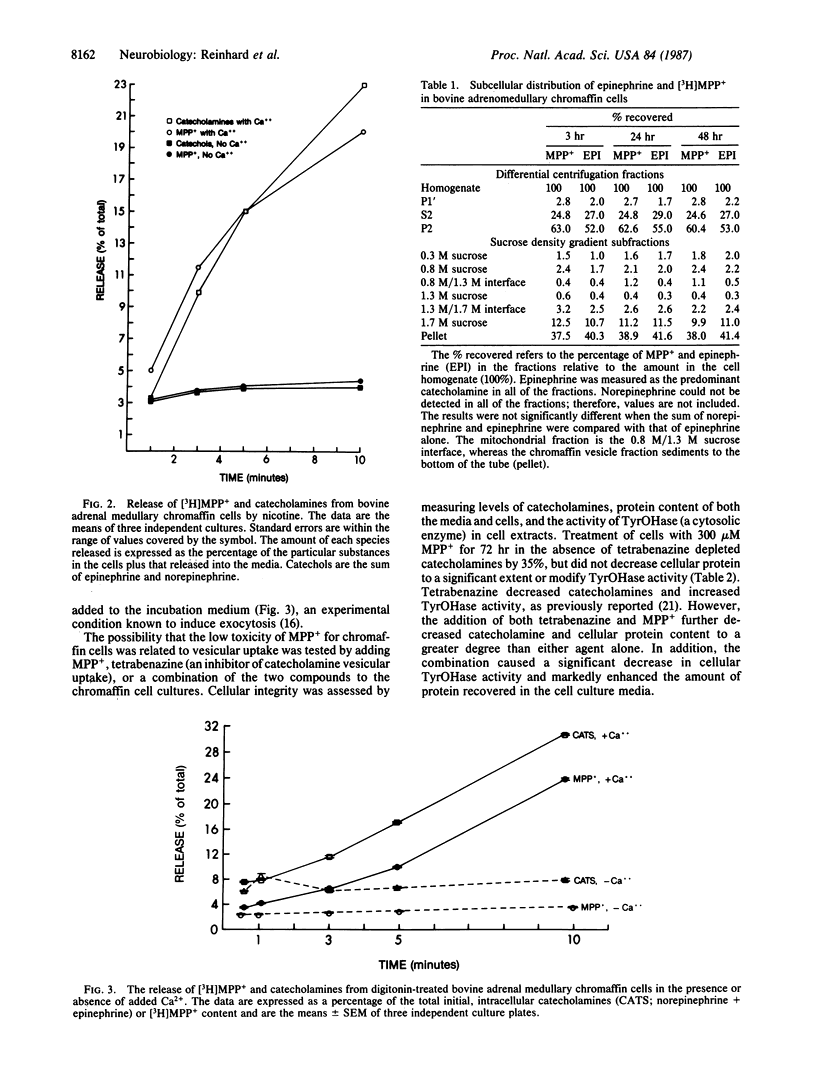
Cultures of bovine adrenomedullary chromaffin cells accumulated 1-methyl-4-phenylpyridinium (MPP+) in a time- and concentration-dependent manner by a process that was prevented by desmethylimipramine. The subcellular localization of the incorporated [methyl-3H]MPP+ was examined by differential centrifugation and sucrose density gradient fractionation and was found to be predominantly colocalized with catecholamines in chromaffin vesicles, and negligible amounts were detected within the mitochondrial fraction. When chromaffin cell membranes were made permeable with the detergent digitonin in the absence of calcium, there was no increase in the release of [3H]MPP+, indicating that there is negligible accumulation of the neurotoxin in the cytosol. Simultaneous exposure to digitonin and calcium induced cosecretion of MPP+ and catecholamines. Stimulation of the cells with nicotine released both catecholamines and MPP+ at identical rates and percentages of cellular content in a calcium-dependent manner. Last, when cells were incubated with MPP+ in the presence of tetrabenazine (an inhibitor of vesicular uptake), the chromaffin cell toxicity of MPP+ was potentiated. We submit that the ability of the chromaffin cells to take up and store MPP+ in the chromaffin vesicle prevents the toxin's interaction with other structures and, thus, prevents cell damage. As an extension of this hypothesis, the relative resistance of some brain monoaminergic neurons to the toxic actions of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine may result from the subcellular sequestration of MPP+ in the storage vesicle.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burns R. S., Chiueh C. C., Markey S. P., Ebert M. H., Jacobowitz D. M., Kopin I. J. A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4546–4550. doi: 10.1073/pnas.80.14.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calne D. B., Langston J. W. Aetiology of Parkinson's disease. Lancet. 1983 Dec 24;2(8365-66):1457–1459. doi: 10.1016/s0140-6736(83)90802-4. [DOI] [PubMed] [Google Scholar]
- Chiba K., Trevor A., Castagnoli N., Jr Metabolism of the neurotoxic tertiary amine, MPTP, by brain monoamine oxidase. Biochem Biophys Res Commun. 1984 Apr 30;120(2):574–578. doi: 10.1016/0006-291x(84)91293-2. [DOI] [PubMed] [Google Scholar]
- Daniels A. J., Williams R. J., Wright P. E. The character of the stored molecules in chromaffin granules of the adrenal medulla: a nuclear magnetic resonance study. Neuroscience. 1978;3(6):573–585. doi: 10.1016/0306-4522(78)90022-2. [DOI] [PubMed] [Google Scholar]
- Di Monte D., Jewell S. A., Ekström G., Sandy M. S., Smith M. T. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and 1-methyl-4-phenylpyridine (MPP+) cause rapid ATP depletion in isolated hepatocytes. Biochem Biophys Res Commun. 1986 May 29;137(1):310–315. doi: 10.1016/0006-291x(86)91211-8. [DOI] [PubMed] [Google Scholar]
- Diliberto E. J., Jr, Heckman G. D., Daniels A. J. Characterization of ascorbic acid transport by adrenomedullary chromaffin cells. Evidence for Na+-dependent co-transport. J Biol Chem. 1983 Nov 10;258(21):12886–12894. [PubMed] [Google Scholar]
- Dunn L. A., Holz R. W. Catecholamine secretion from digitonin-treated adrenal medullary chromaffin cells. J Biol Chem. 1983 Apr 25;258(8):4989–4993. [PubMed] [Google Scholar]
- Fuller R. W., Robertson D. W., Hemrick-Luecke S. K. Persistent depletion of striatal dopamine in mice by 1-methyl-4-(2-thienyl)-1,2,3,6-tetrahydropyridine (MTTP). Biochem Pharmacol. 1986 Jan 15;35(2):143–144. doi: 10.1016/0006-2952(86)90506-x. [DOI] [PubMed] [Google Scholar]
- Heikkila R. E., Hess A., Duvoisin R. C. Dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine in mice. Science. 1984 Jun 29;224(4656):1451–1453. doi: 10.1126/science.6610213. [DOI] [PubMed] [Google Scholar]
- Iversen L. L. Catecholamine uptake processes. Br Med Bull. 1973 May;29(2):130–135. doi: 10.1093/oxfordjournals.bmb.a070982. [DOI] [PubMed] [Google Scholar]
- Javitch J. A., D'Amato R. J., Strittmatter S. M., Snyder S. H. Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6 -tetrahydropyridine: uptake of the metabolite N-methyl-4-phenylpyridine by dopamine neurons explains selective toxicity. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2173–2177. doi: 10.1073/pnas.82.7.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopell W. N., Westhead E. W. Osmotic pressures of solutions of ATP and catecholamines relating to storage in chromaffin granules. J Biol Chem. 1982 May 25;257(10):5707–5710. [PubMed] [Google Scholar]
- Langston J. W., Ballard P., Tetrud J. W., Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983 Feb 25;219(4587):979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Langston J. W., Irwin I., Langston E. B., Forno L. S. Pargyline prevents MPTP-induced parkinsonism in primates. Science. 1984 Sep 28;225(4669):1480–1482. doi: 10.1126/science.6332378. [DOI] [PubMed] [Google Scholar]
- Markey S. P., Johannessen J. N., Chiueh C. C., Burns R. S., Herkenham M. A. Intraneuronal generation of a pyridinium metabolite may cause drug-induced parkinsonism. Nature. 1984 Oct 4;311(5985):464–467. doi: 10.1038/311464a0. [DOI] [PubMed] [Google Scholar]
- Melamed E., Rosenthal J., Cohen O., Uzzan A., Globus M. Amphetamine, but not reserpine, protects mice against dopaminergic neurotoxicity of MPTP. Neuropharmacology. 1985 Sep;24(9):923–925. doi: 10.1016/0028-3908(85)90047-4. [DOI] [PubMed] [Google Scholar]
- Nicklas W. J., Vyas I., Heikkila R. E. Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci. 1985 Jul 1;36(26):2503–2508. doi: 10.1016/0024-3205(85)90146-8. [DOI] [PubMed] [Google Scholar]
- Ramsay R. R., Singer T. P. Energy-dependent uptake of N-methyl-4-phenylpyridinium, the neurotoxic metabolite of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, by mitochondria. J Biol Chem. 1986 Jun 15;261(17):7585–7587. [PubMed] [Google Scholar]
- Reinhard J. F., Jr, Smith G. K., Nichol C. A. A rapid and sensitive assay for tyrosine-3-monooxygenase based upon the release of 3H2O and adsorption of [3H]-tyrosine by charcoal. Life Sci. 1986 Dec 8;39(23):2185–2189. doi: 10.1016/0024-3205(86)90395-4. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sundström E., Jonsson G. Pharmacological interference with the neurotoxic action of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on central catecholamine neurons in the mouse. Eur J Pharmacol. 1985 Apr 16;110(3):293–299. doi: 10.1016/0014-2999(85)90555-2. [DOI] [PubMed] [Google Scholar]
- Wilson S. P., Abou-Donia M. M., Chang K. J., Viveros O. H. Reserpine increases opiate-like peptide content and tyrosine hydroxylase activity in adrenal medullary chromaffin cells in culture. Neuroscience. 1981;6(1):71–79. doi: 10.1016/0306-4522(81)90244-x. [DOI] [PubMed] [Google Scholar]
- Wilson S. P., Viveros O. H. Primary culture of adrenal medullary chromaffin cells in a chemically defined medium. Exp Cell Res. 1981 May;133(1):159–169. doi: 10.1016/0014-4827(81)90366-9. [DOI] [PubMed] [Google Scholar]
- Youngster S. K., Duvoisin R. C., Hess A., Sonsalla P. K., Kindt M. V., Heikkila R. E. 1-Methyl-4-(2'-methylphenyl)-1,2,3,6-tetrahydropyridine (2'-CH3-MPTP) is a more potent dopaminergic neurotoxin than MPTP in mice. Eur J Pharmacol. 1986 Mar 18;122(2):283–287. doi: 10.1016/0014-2999(86)90115-9. [DOI] [PubMed] [Google Scholar]
- Zimmerman D. M., Cantrell B. E., Reel J. K., Hemrick-Luecke S. K., Fuller R. W. Characterization of the neurotoxic potential of m-methoxy-MPTP and the use of its N-ethyl analogue as a means of avoiding exposure to a possible Parkinsonism-causing agent. J Med Chem. 1986 Aug;29(8):1517–1520. doi: 10.1021/jm00158a033. [DOI] [PubMed] [Google Scholar]



