Abstract
Although errant saccadic eye movements may mark genetic factors in schizophrenia, little is known about abnormal brain activity that precedes saccades in individuals with genetic liability for schizophrenia. We investigated electrophysiological activity preceding prosaccades and antisaccades in schizophrenia patients, first-degree biological relatives of schizophrenia patients, and control subjects. Prior to antisaccades patients had reduced potentials over lateral prefrontal cortex. Smaller potentials were associated with worse antisaccade performance. Relatives also exhibited reduced pre-saccadic potentials over lateral frontal cortex but additionally had reduced potentials over parietal cortex. Both patients and relatives tended toward increased activity over orbital frontal cortex prior to saccades. Results are consistent with lateral prefrontal dysfunction marking genetic liability for schizophrenia and underlying deficient saccadic control.
Introduction
Individuals with schizophrenia have difficulty inhibiting the reflexive movement of the eye toward a stimulus in the periphery when instructed to direct their gaze in the opposite direction (Hutton & Ettinger, 2006). Because excessive saccadic errors during an “antisaccade” task have been observed in first-degree biological relatives of schizophrenia patients (see Calkins, Curtis, Iacono, & Grove, 2004 for a recent review), it has been suggested that deficits in eye-movement control are indicators of genetic liability (i.e., endophenotypes) for schizophrenia and therefore mark brain abnormalities that reflect central nervous system effects of genes predisposing schizophrenia (Gottesman & Gould, 2003; Schulze et al., 2006). Recently, large multi-site studies have used antisaccade performance as an endophenotype for studying the genetics of the disorder (Calkins et al., 2007). Nonetheless, since the first identification of elevated error rates in biological relatives of schizophrenia patients on antisaccade tasks (Clementz, McDowell, & Zisook, 1994), there has been debate about which biological relatives under what experimental conditions exhibit impaired antisaccade performance (Levy et al., 2004).
Because errors in ocular motion may serve as a behavioral marker of genetically-related pathophysiology in schizophrenia, it is informative to investigate neural processes that underlie the control of saccades. Two fMRI studies that investigated brain function of biological relatives of schizophrenia patients during a volitional saccade tasks reported diminished brain activations in relatives. Relatives exhibited limited activity in the caudate nucleus during antisaccades as compared to prosaccades (Raemaekers, Ramsey, Vink, van den Heuvel, & Kahn, 2006) as well as decreased activations in dorsolateral prefrontal cortex, anterior cingulate cortex, cuneus, insula, and middle occipital gyrus during volitional saccade tasks (e.g., antisaccade and ocular motor delayed response) (Camchong, Dyckman, Austin, Clementz, & McDowell, 2008). Although these findings are suggestive of frontal-striatal and prefrontal abnormalities marking risk for schizophrenia, it is unclear whether the functional brain abnormalities precede eye movements leaving open the possibility that the activations were a consequence rather than a cause of errant saccades. To date, no published studies of individuals who carry genetic liability for schizophrenia have specifically isolated neural anomalies that precede the onset of saccadic eye movements, therefore it remains to be determined which neural events are the cause of impairments on the antisaccade task. To characterize neural events contributing to poor antisaccade performance in schizophrenia we used electrophysiological recordings to capture brain activity prior to the execution of saccades. Specifically, we examined the neural responses of schizophrenia patients, unaffected first-degree biological relatives of schizophrenia patients, and control subjects during antisaccade and prosaccade tasks. The study employed two fixation conditions to facilitate delineation of relevant pre-saccadic neural events. Inclusion of unaffected biological relatives provided a test of whether anomalies were independent of schizophrenia spectrum psychopathology in a sample likely to carry genetic liability for the disorder.
In schizophrenia deficient ocular-motor control, as well as deficits in working memory and sustained attention, are associated with dysfunction of both prefrontal (PFC) and parietal cortices (Carter et al., 1998; McDowell et al., 2002; Ojeda et al., 2002). Scientists have proposed that these functional abnormalities result from a failure in a dopaminergic PFC mechanism (Servan-Schreiber, Cohen, & Steingard, 1996), a dysfunction in a frontoparietal attentional network (Maruff, Danckert, Pantelis, & Currie, 1998), or a functional disconnection between PFC and parietal cortex (Kim et al., 2003). Functional magnetic resonance imaging (fMRI) studies indicate that schizophrenia patients fail to normally activate PFC during antisaccade execution (Fukumoto-Motoshita et al., 2009; McDowell et al., 2002; Tu, Yang, Kuo, Hsieh, & Su, 2006). These studies suggest that antisaccade deficits in schizophrenia are primarily due to dysfunction in the PFC and are consistent with the cortical region being central to saccadic control (Guitton, Buchtel, & Douglas, 1985; Pierrot-Deseilligny, Rivaud, Gaymard, Muri, & Vermersch, 1995; Rivaud, Muri, Gaymard, Vermersch, & Pierrot-Deseilligny, 1994). Yet, slowed saccadic responses of schizophrenia patients have also been related to reduced white matter organization in anterior cingulate cortex, parietal cortex, and the frontal eye field (Manoach et al., 2007). Studies that have specifically examined neural activity preceding eye movements showed impaired modulation of brain activity (i.e., the contingent negative variation) in schizophrenia patients prior to antisaccades suggesting poor eye movement preparation, selection, and execution (Franke, Reuter, Schulz, & Kathmann, 2007; Reuter, Herzog, Endrass, & Kathmann, 2006; Reuter, Jager, Bottlender, & Kathmann, 2007) which has been interpreted as reflective of prefrontal dysfunction (Klein, Heinks, Andresen, Berg, & Moritz, 2000). In addition, previous investigations of eye movement control in schizophrenia have solely examined pre-saccadic neural activity locked to stimulus events and not the saccade itself and therefore may not fully reveal brain abnormalities related to saccade preparation and generation in schizophrenia. Finally, given evidence of parietal cortex involvement in goal-directed eye-movement through spatial computation and sensorimotor transformations (Moon et al., 2007; Rafal, 2006; Zhang & Barash, 2000) and parietal cortex activity during inhibitory periods prior to saccade generation (Ettinger et al., 2007), it is possible that dysfunction of parietal brain regions contributes to abnormal antisaccadic control in schizophrenia patients and their biological relatives.
Researchers have noted that characteristics of the fixation stimulus before saccades have effects on saccade latencies. Onset latencies of correct antisaccades are reduced for a “step” condition (where the fixation stimulus extinguishes simultaneously with the appearance of the peripheral stimulus) compared to an “overlap” condition (where fixation and peripheral stimuli briefly overlap in time) (Braun & Breitmeyer, 1988; Fischer & Weber, 1993, 1997). McDowell and Clementz (1997) found that overlap fixation was superior for increasing group separation between schizophrenia patients and nonpsychiatric control subjects. Yet it is unclear what mechanism for antisaccade generation is affected by overlap fixation and how such a mechanism is altered in schizophrenia. Overlap effects can be understood in terms of Posner’s theory of visual attention which posits three different elements of attention – disengage, move, engage (Fischer & Weber, 1993; Posner, 1980; Posner, Walker, Friedrich, & Rafal, 1984). Accordingly, a benefit in latency is obtained when attention is engaged at the position where the stimulus is going to be presented and a cost is exacted when the stimulus appears somewhere else. Studies of patients with brain lesions suggest disengagement of attention is unique to the parietal lobe (Posner et al., 1984). The overlap fixation condition requires subjects to disengage visual attention from the fixation location and thus likely elicits additional parietal activation before the eyes are directed to the target location. The increased demand to disengage attention by parietal cortex does not exist in the step fixation. Therefore, an antisaccade task with overlap fixation may effectively elicit abnormal activity not only in prefrontal regions but also in parietal cortex in schizophrenia patients.
Cue disengagement for successful saccade performance has been proposed as mediated by parietal cortex (Richards, 2003). Analyzing electrophysiological activity of normal subjects from pre-stimulus, post-stimulus, and pre-saccadic periods, Richards (2003) identified pre-saccadic components that peaked over fronto-central, parietal, fronto-polar, and lateral frontal brain regions (the maximum of principal components were found at scalp sites FCz/Cz, PZ, Fp1/Fp2, and Af2/Af4/F6 in the 10–20 electrode system, respectively). The component with the parietal maximum was reduced when cues were provided prior to target onset that informed subjects of the upcoming target location. In the absence of such a cue, the parietal component was increased possibly reflecting demands on spatial attention to efficiently locate the upcoming target stimulus. The fronto-central negative component observed by Richards (2003) was maximal in the pre-stimulus period and interpreted as the contingent negative variation (CNV) that had been previously identified prior to saccades (Everling et al., 1997 Klein et al., 2000). Richards (2003) identified fronto-polar and lateral frontal electrophysiological components perhaps due to the use of a high density of electrodes across the scalp and various target cueing conditions where pro- and antisaccade trials were mixed in a block. The fronto-polar and lateral frontal components steadily increased from about 300 to 200 ms before saccade onset and the magnitude was greater in antisaccades than prosaccades. Richards (2003) localized the components to orbital PFC and anterior/lateral PFC, respectively. Although the role of the orbital PFC neural activity is not fully understood, the lateral PFC neural activity was found to be increased in antisaccades compared to prosaccades as well as when a cued condition compared to the no-cue condition. These saccade type and cue effects suggest that the underlying neural activity may reflect planning of the targeted eye movement. In other words, the lateral frontal component activity might reflect contextual processing by dorsolateral PFC to guide targeted eye-movements. Given previously identified PFC and posterior parietal cortex dysfunction in schizophrenia, pre-saccadic electrophysiological components identified by Richards (2003) may well be altered in the disorder.
To investigate neural activity preceding saccades and examine possible abnormal PFC and parietal function associated with antisaccade performance in schizophrenia we studied electrophysiological responses of schizophrenia patients and first-degree biological relatives of schizophrenia patients using antisaccade and prosaccade paradigms that included step and overlap fixation conditions. We hypothesized that schizophrenia patients and their relatives would show abnormal electrophysiological activity over PFC and parietal cortex, especially in the overlap fixation condition. We focused on pre-saccadic positive potentials that have been observed prior to visually triggered saccades and modulated by whether the eye movement is a prosaccade or antisaccade (Everling et al., 1997), as well as signals possibly related to the three electrophysiological components of greatest magnitude at lateral frontal, parietal, and fronto-polar electrodes prior to saccades (Richards, 2003). Through contrasts of the electrophysiological activity preceding antisaccades and prosaccades we were able to demonstrate abnormal neural responses that appear to reflect genetic liability for schizophrenia.
Methods
Participants
Twenty participants diagnosed with DSM-IV schizophrenia, 21 of their first-degree biological relatives, and 20 control subjects were studied. Five schizophrenia patients and two relatives were excluded in the later analysis due to having too few trials without blink and saccadic errors for computing event-related potentials (ERPs). Table 1 presents the characteristics of participants. Schizophrenia patients were recruited from the Minneapolis VA Medical Center and regional mental health centers, and research staff identified first-degree biological relatives of patients by completing a pedigree from the patient’s report. Interested relatives completed a telephone interview to determine their demographic and medical characteristics and were excluded if they had a physical problem that would render study measures impossible to obtain, or were younger than age 18 or older than age 68. Control subjects were recruited through flier advertisement throughout the community.
Table 1.
Participant characteristics.
| Variable | Patients |
Relatives |
Controls |
Test value (df) | p value |
|---|---|---|---|---|---|
| N=15 |
N=19 |
N=20 |
|||
| Mean (S.D.) | Mean (S.D.) | Mean (S.D.) | |||
| Age (year) | 38.20 (9.77) | 49.16 (9.52) | 40.60 (10.25) | F(2,51)=6.07 | .004 |
| Percent female | 26.67 | 68.42 | 45.00 | χ2(2)=5.98 | .050 |
| Education (years) | 12.80 (3.10) | 14.26 (2.47) | 15.60 (2.11) | F(2,51)=5.23 | .009 |
| Estimated IQ | 96.80 (15.19) | 100.11 (14.91) | 111.90 (10.35) | F(2,51)=6.34 | .003 |
| BPRS total score | 40.87(12.00)a | NA | NA | NA | NA |
| SPQ total score b | NA | 13.35 (10.27) | 7.94 (4.70) | F(1,32)=3.90 | .057 |
S.D.=standard deviation. n.s.=not significant. IQ=intelligence quotient. Estimated IQ was derived from Vocabulary and Block Design subtests (Brooker & Cyr, 1986) of the Wechsler Adult Intelligence Scale- Revised. BPRS=Brief Psychiatric Rating Scale 24-item version (Ventura et al., 2000). NA=not applicable. SPQ=Schizotypal Personality Questionnaire (Raine, 1991).
Mean BPRS score was equivalent to an average item rating of 1.8 which is between absent (1) and very mild (2) for prominence.
SPQ score failed to be associated with saccade accuracy and latency indices.
To obtain diagnostic information a trained doctoral-level clinical psychologist completed the Diagnostic Interview for Genetic Studies(Nurnberger et al., 1994) (DIGS) with each patient. Using all available clinical information for a patient the interviewer completed the Operational Criteria for Psychotic Illness (McGuffin, Farmer, & Harvey, 1991) (OPCRIT) to derive a DSM-IV diagnosis. A second doctoral-level clinical psychologist functioned as a consensus reviewer and also completed the OPCRIT for the participant. Any diagnostic disagreement between the interviewer and consensus reviewer was resolved by reviewing OPCRIT items on which ratings differed. From the clinical interview the psychologist rated current symptomatology using the 24-item version of the Brief Psychiatric Rating Scale (Ventura, Nuechterlein, Subotnik, Gutkind, & Gilbert, 2000) (BPRS). Of the patients in the study 14 were being prescribed antipsychotic medication (exclusively novel antipsychotics). The average chlorpromazine equivalent for antipsychotic dosages was 565 mg (448 SD). Nine patients were being prescribed antidepressant medication.
To assess psychopathology in first-degree relatives and control subjects a doctoral-level psychologist or a trained and supervised research assistant completed the Structured Clinical Interview for DSM-IV Axis I Disorders (First, Spitzer, Gibbon, & Williams, 1996) (SCID-I). Relatives and controls also completed the Structured Clinical Interview for DSM-IV Axis II Personality Disorders Personality Questionnaire (Ekselius, Lindstrom, von Knorring, Bodlund, & Kullgren, 1994) (SCID-II-PQ), the Structured Interview for Schizotypy (Kendler, Lieberman, & Walsh, 1989) (SIS) and completed ratings of schizotypal, schizoid, and paranoid personality disorders. To assess self-reported schizotypal characteristics we administered the Schizotypal Personality Questionnaire (Raine, 1991) (SPQ) to first-degree biological relatives of patients and control subjects. IQ was estimated using the Block Design and Vocabulary subtests of the Wechsler Adult Intelligence Scale, Third Edition (Brooker & Cyr, 1986).
No relatives had a schizophrenia spectrum disorder. Although four relatives had past major depressive episodes, they were in full remission when they participated in the study. Gender had no effect on behavioral performance and age was only associated with prosaccade latency (see Table 1). All participants were free of serious physical health problems, and absent of known neurological hard signs. Exclusion criteria included English as a second language, charted IQ less than 70 or a diagnosis of mental retardation, current alcohol or drug abuse, past drug dependence, a current or past central nervous system disease or condition, a medical condition or disease with likely significant central nervous system effects, history of head injury with skull fracture or loss of consciousness of greater than 20 min, significant tardive dyskinesia as indicated by a Dyskinesia Identification System: Condensed User Scale (Sprague, Kalachnik, & Slaw, 1989) (DISCUS).
Procedure
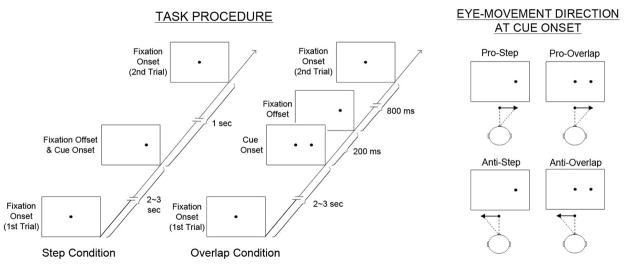
Each participant was seated in a dimly lit room with their head stabilized by a chin and forehead rest. A computer display faced subjects at 51 cm from the eyes. Before the task subjects were presented with calibration targets at 5°, 10°, and 15° on either side of the central fixation. Experimental trials started with the 0.5° illuminated target dot at fixation. Following a 2 to 3 second pseudorandom interval, the dot was extinguished and then appeared at 15° to the right or left of central fixation for 1 second. The trial ended with the dot returning to central fixation. In the prosaccade task, subjects were instructed to move their eyes as quickly and accurately as possible to the position of the dot. In the antisaccade task, subjects were instructed to look as quickly as possible to the position opposite the dot with respect to the central fixation point. The subjects were instructed not to blink during the saccades. Two different, pseudorandomly intermixed central fixation conditions were used. In the step condition, the central fixation disappeared contemporaneous with the illumination of the cue. In the overlap condition, the central fixation dot remained illuminated for 200 ms after illumination of the target dot on the periphery (See Figure 1 for task procedures). Eight blocks of 24 trials were presented (pro, anti, anti, pro, pro, anti, anti, pro). There were 48 total trials per fixation condition (step, overlap) per saccade type (pro, anti) with equal numbers of trials requiring saccades to the left and right of central fixation.
Figure 1.
Antisaccade and prosaccade task procedure showing the order of stimulus presentation for step and overlap conditions (left panel) and intended eye-movement directions at cue onset in each task condition (right panel).
Electrophysiological Data Collection and Analyses
Electroencephalograms (EEG) were recorded using a 16-bit analog-to-digital amplifier and 29 tin electrodes embedded in an elastic cap (Electrode Cap International [ECI]). Electrodes were placed on the head to conform to 10–10 nomenclature and referenced to the left earlobe. Electrodes were filled with ECI gel and sites were abraded to yield impedances below 5kΩ. EEG signals were digitized at a rate of 500Hz with 0.5 low frequency and 100Hz high frequency filters and a 60 Hz notch filter. Vertical electro-oculograms (VEOG) recorded from above and below the right eye were used to reject epochs containing ocular artifact, while horizontal electro-oculograms (HEOG) recorded from outer ocular canthi were used to measure horizontal eye-movements. Following data collection, recordings from scalp sites were rereferenced to linked earlobes.
Using HEOG channel potentials, we quantified saccade performance in terms of saccade latency and direction (i.e., not amplitude gain). The onset of a saccade was identified in the HEOG as the beginning of an abrupt deviation from baseline. For saccadic eye-movement identification four criteria were used: 1) velocity of eye-movement (>30°/sec), 2) degree of eye-movement (>25% of target movement), 3) timing of saccade (from 100 to 800 ms after cue onset), and 4) absence of blink (from 250 ms before cue onset to onset of saccade). The target saccade movement for each subject was determined by the mean of eye-movements of calibration trials. Inspection of saccade latency distributions showed that 800 ms was an appropriate limit for saccade response. Saccades that occurred −250 to 100 ms relative to stimulus onset were regarded as anticipatory saccades (Fischer & Weber, 1993). During the saccade scoring, trials whose saccade onsets could not be clearly determined were rejected. To verify the accuracy of saccade scoring using HEOG signals, we also scored saccade performances with infra-red eye-movement tracking data which were simultaneously collected with HEOG and EEG recordings for 10 subjects (1 schizophrenia patient, 5 control subjects, 4 relatives). Saccade latencies measured through HEOG and infra-red signals correlated .985 and saccade direction for the two signals perfectly agreed (i.e., tetrachoric correlation was 1.00). Thus, HEOG provided a very accurate indication of both saccade latency and direction.
To examine potentials that were response-related the 0 ms point for each EEG epoch was set at the saccade onset. EEG recordings were epoched to extend from 500 ms pre-saccade to 250 ms post-saccade in order to include saccade-related brain activity from the onset of a cue to the onset of a saccade and beyond. EEG epochs were visually inspected and some epochs whose amplitudes exceeded ±100 μV before saccade onsets were rejected to ensure only epochs free of eye- and body-movement artifacts were included for averaging. Only trials with correct saccadic responses were averaged. The minimum number of trials averaged for a subject in any condition was 25. Average response-related potentials were computed for each task and fixation condition and the baseline for averages was defined as the median voltage from 500 to 400 before saccade onset (i.e., first 100 ms of the epoch). The 500 to 400 baseline period was selected to use a time-period prior to any stimulus movement but that was near the onset of the saccade stimulus (most saccade latencies ranged between −250 and −200 ms for pro-saccades and −350 and −300 ms for anti-saccades). Saccade accuracy reflected the percentage of trials where the subject emitted a saccade in the correct direction and was defined as the number of correct trials divided by number of trials without artifacts. Thus, the accuracy measure was computed excluding both incorrect and anticipatory saccades. We subtracted scalp-recorded potentials of prosaccades from antisaccade potentials to generate anti – pro difference potentials that revealed activity specific to antisaccade generation.
Statistical Analyses
For analyses of saccade performance (i.e., accuracy and latency), we conducted repeated measures ANOVAs with one between subjects factor (group: schizophrenia, relatives, controls) and two within subjects factors (task: pro-, antisaccade; fixation: overlap, step). Demographic variables (see Table 1) were included as covariates in ANOVAs when groups differed on the variable and it was significantly associated with the behavioral or ERP indices1. Consistent with the findings in normal controls (Everling et al., 1997; Richards, 2003) temporally and spatially broad saccade-related activity was observed at midline frontal (Fp1, Fp2, Af3, Af4, F3, FZ, F4) and centro-parietal (C3, CZ, C4, P3, PZ, P4) electrodes beginning about 210 ms before saccade onset (see Supplementary Materials for depiction of waveforms from all these electrode sites). Because of an absence of clear peaks and valleys in the saccade-locked ERP activity, we computed mean amplitudes for every 30 ms period from −210 to 0 ms at the thirteen frontal and the centro-parietal electrodes to quantify changes in the magnitude of preparatory neural responses across time. The analysis strategy involved three steps intended to characterize group effects on the broad pre-saccadic potentials and determine at which points the pre-saccadic potentials were most sensitive to the fixation, saccade task, and group effects. First, to test the hypothesis that schizophrenia patients and their relatives had neural abnormalities that were more evident prior to antisaccades than prosaccades we conducted a repeated measure ANOVA with one between subjects factor (group; schizophrenia, relatives, controls) and two within subjects factors (task: pro- and antisaccades; electrodes: Fp1, Fp2, Af3, Af4, F3, FZ, F4, C3, CZ, C4, P3, PZ, P4), with the dependent variable being the mean amplitude for all 30 ms time bins from −210 to 0 ms. The analysis allowed examination of whether groups differed on the broad temporal pre-saccadic potentials noted in other studies to be modulated by saccade type (e.g., Everling et al. 1997). Second, follow-up analyses were used to determine whether the two saccade tasks (pro and anti) and two fixation conditions (step and overlap) were associated with modulation of the pre-saccadic potentials during specific time periods and over select brain regions. We carried out two repeated measures ANOVAs – one with task, electrode, and time as within subjects factors and the other with fixation, electrode, and time as within subjects factors. Third, to fully investigate group differences in pre-saccadic potentials previously identified in studies of normative samples (Everling et al., 1997, Richards 2003) we conducted one-way ANOVAs with an independent variable of group and a dependent variable of mean amplitudes of difference (anti – prosaccades) potentials recorded at select frontal and parietal electrodes. To investigate fixation-specific group differences in the pre-saccadic potentials, these ANOVAs were separately run for each fixation condition. Electrode selection was based on previously observed scalp locations of pre-saccadic potentials (Richards, 2003) and the hypothesized location of relevant neural activity. Electrodes F4 and F3 represented the lateral frontal component, Cz and Pz represented the parietal component, and Fp1 and Fp2 were chosen for the fronto-polar component. Given the amplitude change of the predominant pre-saccadic ERP components across time (Richards, 2003), we defined the largest time windows for the group comparisons based on exploratory analyses that compared mean amplitudes of the difference potentials in seven 30 ms time windows at the select electrode across groups. Magnitude of group difference in mean amplitude was estimated as effect size (Cohen’s d). All reported within-subjects effects were Huynh-Feldt corrected. To examine the relationship of electrophysiological activity with saccade performance and medication status, Pearson correlation coefficients were computed between mean amplitude indices and measures of accuracy, latency, and chlorpromazine equivalents.
Results
Saccade Accuracy and Latency
Prosaccade vs. Antisaccade Task
Schizophrenia patients, relatives, and control subjects had lower accuracy on the antisaccade task than the prosaccade task (F1,51=167.10, p<.001). There was an interaction between group and task (F2,51=4.11, p=.02). Follow-up analyses indicated that schizophrenia patients had lower saccade accuracy than controls and relatives, and that they exhibited much larger accuracy deficit in the antisaccade task than the prosaccade task (see Table 2). Saccade latency was strongly influenced by task type (F1,49=26.13, p<.001) with longer latencies evident in the antisaccade task compared to the prosaccade task. Analyses of the percentage of anticipatory saccades revealed a group difference only on the antisaccade task (F2,51=13.15, p<.001). Follow-up analyses indicated that in the antisaccade task schizophrenia patients showed anticipatory saccades on more trials than controls (8.3% of trials vs. 3.3%, respectively), while relatives (3.8% of trials) failed to differ from controls.
Table 2.
Behavioral performances in saccade tasks
| Patients | Relatives | Controls | |
|---|---|---|---|
| (N=15) | (N=19) | (N=20) | |
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Accuracy | |||
| Pro-Overlap | .83 (.11)a | .89 (.11) | .92 (.07) |
| Pro-Step | .87 (.10) | .88 (.09) | .90 (.07) |
| Anti-Overlap | .59 (.17)b | .75 (.11)c | .73 (.11) |
| Anti-Step | .58 (.15)b | .71 (.12)c | .72 (.11) |
| Latencyd | |||
| Pro-Overlap | 252.10 (50.94) | 255.55 (41.92) | 235.34 (46.70) |
| Pro-Step | 209.77 (33.96) | 198.43 (35.59) | 188.73 (38.87) |
| Anti-Overlap | 344.75 (52.97) | 336.77 (64.60) | 326.91 (62.16) |
| Anti-Step | 306.11 (60.67) | 302.39 (58.44) | 286.49 (64.85) |
Patients had significantly lower accuracy in prosaccades for overlap fixations than controls (t33=2.64, p=.013).
Patients had significantly lower accuracy in antisaccades than controls (t33=3.79, p=.001) and relatives (t32=3.45, p=.001), regardless of fixation condition.
Relatives had significantly higher antisaccade accuracy for overlap fixations compared to step fixations (t18=2.56, p=.020).
All groups had significantly longer saccade latency for overlap fixations compared to step fixations, regardless of saccade tasks. There was no significant group difference in saccade latency.
Note. Pro = prosaccade, Anti = antisaccade, Step = step fixation condition, Overlap = overlap fixation conditions.
Step vs. Overlap Fixation
There was an interaction of group and fixation type for saccade accuracy (F2,51=3.21, p=.049). Analyses revealed that schizophrenia patients had lower prosaccade accuracy for overlap fixation trials compared to step fixation trials, while relatives had higher antisaccade accuracy for overlap fixation compared to step fixation, and the control group’s saccade accuracy did not vary by fixation condition. In addition, schizophrenia patients had a lower proportion of correct prosaccades for overlap fixation trials than controls (.83 vs. .92). The overlap condition produced much longer saccade latencies than the step condition in all groups. There was a main effect of fixation (F1,49=4.89, p=.03), but no group main effect (F2,49=1.10, p=.34) on saccade latency.
Pre-saccadic Neural Activity
All groups showed increasingly positive scalp-recorded potentials over time that peaked immediately before saccade onset2. A repeated measures ANOVA with the three factors (group, task, and electrode) and dependent variable of the mean amplitudes computed for all time-ranges (−210~0 ms) revealed interactions of group, task, and electrode (F12,612=2.82, p=.01) and task and electrode (F12,612=8.45, p<.001) indicating that group differences in pre-saccade potentials were evident during specific task conditions (i.e., antisaccade) at particular electrodes. There were no main effects of group (F2,51=.37, p=.69) and task (F2,51=.56, p=.46), and trends of a main effect of electrode (F12,612=2.53, p=.09) and a group by electrode interaction effect (F24,612=2.09, p=.10). Potentials prior to prosaccades were characterized by the largest amplitude increases over the centro-parietal area, while those preceding antisaccades were characterized by amplitudes increases over frontal brain regions. Follow-up ANOVAs revealed that there was a significant 3-way interaction of task, electrode, and time (F72,3816=9.09, p<.001), suggesting that spatial and temporal variation of pre-saccadic potentials were dependent on saccade type. Pre-saccadic potentials also showed a main effect of fixation (F1,51=10.46, p=.002), an interaction of fixation and time (F6,306=2.55, p=.034), and a trend for an interaction of fixation, time, and electrode (F72,3672=1.72, p=.067), thereby indicating that the effect of different fixations prior to stimulus onset varied across time and scalp location. Hence, omnibus analyses of pre-saccadic potentials indicated that the scalp distribution and time course of the responses were sensitive to diagnostic group, task, and fixation conditions, suggesting that group differences in the neural activity prior to saccades was likely isolated to select electrodes for specific time periods. Therefore, to fully describe group differences leading up to saccade generation we carried out one-way ANOVAs for difference potentials (anti – prosaccades) at electrodes selected to be at scalp regions where pre-saccadic potentials were previously observed (Everling et al., 1997, Richards 2003).
Potentials over Lateral Frontal Cortex
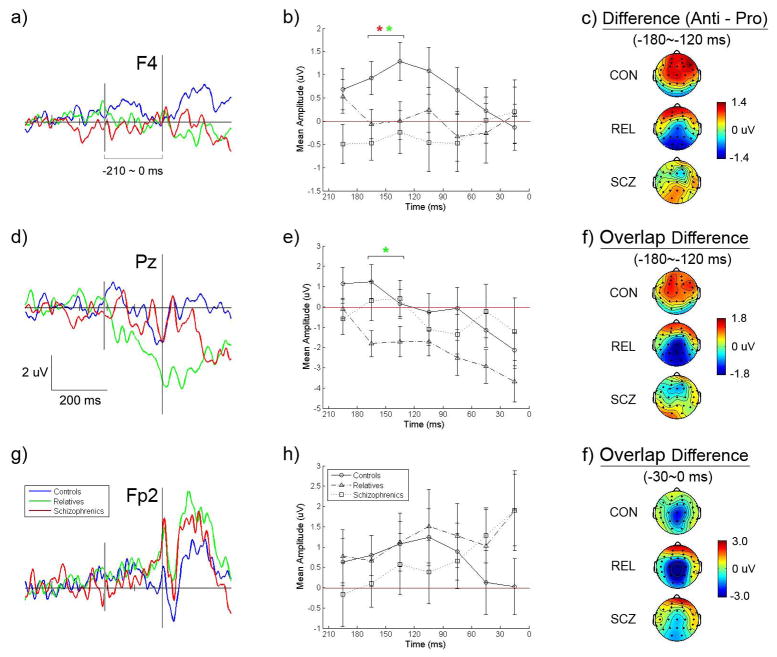
Because lateral frontal cortex has been shown to be involved with the control of saccadic eye movements we examined electrodes over this region for group differences. To isolate activity that varied depending on the saccade task we submitted the difference in mean amplitudes between antisaccade and prosaccade potentials to analyses. Figure 2 depicts difference waveforms for selected electrodes and the scalp topography of difference potentials. Groups varied in anti – pro difference potentials over right lateral frontal cortex (site F4) from 180 to 120 ms before saccade onset regardless of fixation type (F2,51=4.68, p=.01). Post hoc analyses indicated that both the schizophrenia subjects (t33=−2.73, p=.01, Cohen’s d =−.94) and the relatives (t37=−2.33, p=.02, d=−.75) had smaller difference potentials than controls from −180 to −120 ms. A trend for a group difference was also found in difference potentials for overlap fixations over left lateral frontal cortex (site F3) from 180 to 120 ms (F2,51=2.99, p=.059). In post hoc analyses, it was found that schizophrenia patients had smaller difference potentials than controls from −180 to −120 ms (t33=−2.15, p=.04, d=−.75), while in the same time window relatives had somewhat decreased difference potentials which was not statistically significant (t37=−1.674, p=.10, d=−.55). Thus, prior to antisaccades schizophrenia patients and their relatives exhibited diminished amplitudes over lateral frontal cortex. Figure 3 depicts separate prosaccade and antisaccade potentials for schizophrenia, relative, and control groups at site F4. Difference potentials over lateral frontal cortex for which group effects were evident showed associations with saccade performance. Schizophrenia patients who had larger lateral frontal difference potentials between 180 and 120 ms before saccade onset had better antisaccade accuracy for step fixation trials (F4: r=.57, p=.03; F3: r=.54, p=.04), but not for overlap fixation trials. Also, relatives who had larger difference potentials at F3 between 150 and 120 ms before saccade onset for overlap fixations had shorter antisaccade latencies for overlap fixations (r=−.53, p=.02), but an association was absent for overlap difference potentials at other lateral frontal sites as well as for step antisaccade trials.
Figure 2.
Grand averages of difference potentials (antisaccade – prosaccade) for schizophrenia patients, biological relatives of schizophrenia patients, and control subjects preceding saccadic eye movements. Potentials are displayed for three electrodes (left panel) and mean difference amplitudes are depicted across 7 time ranges in 30 ms intervals (middle panel) to show voltage change across time (See text for further descriptions. Note: In contrasts of the patients to the controls, * = p<.05; in contrasts of the relatives to the control, * = p<.05).
Figure 3.
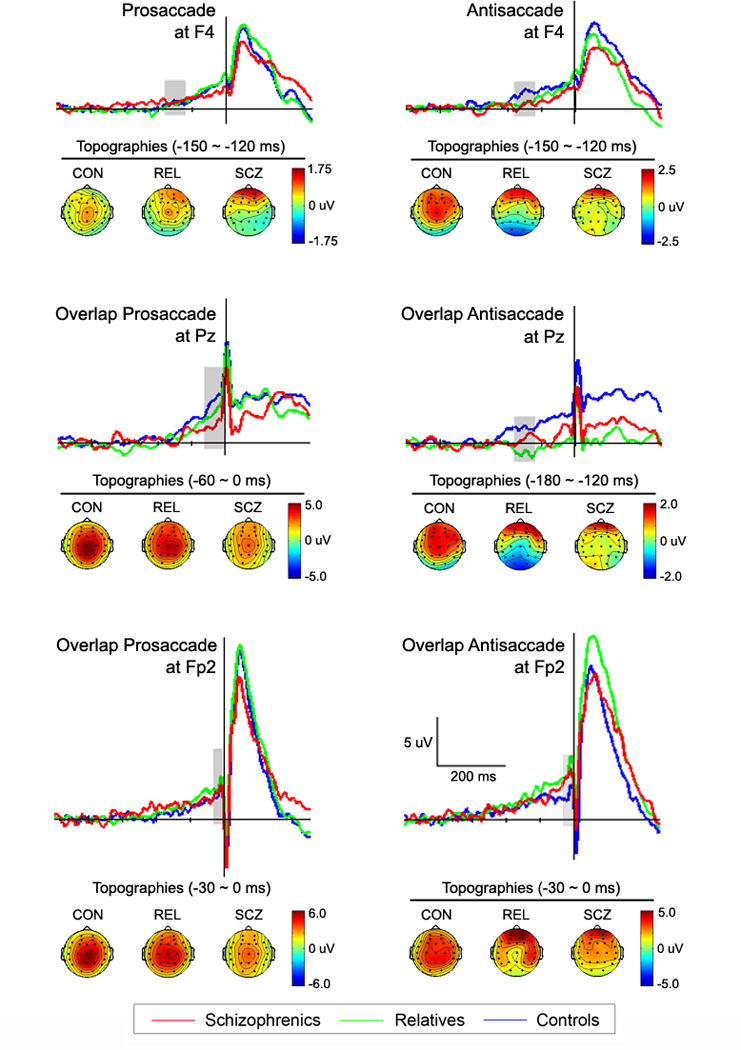
Pro- and antisaccade potentials over lateral frontal (site F4), parietal (site Pz), and orbital frontal (site Fp2) cortices from which difference potentials were derived. Scalp topographies for controls, relatives and schizophrenia patients for time bins (shaded regions on waveforms) showing group differences in pre-saccadic difference potentials.
Potentials over Parietal Cortex
Because of the suspected role of parietal cortex in spatial attention and previously observed augmentation of potentials over the region prior to saccades, we examined activity recorded at central and parietal electrodes. Group main effects were evident for anti – pro difference potentials for overlap fixation trials from −180 to −120 ms at Cz (F2,57=4.61, p=.01), and from −180 to −120 ms at Pz (F2,57=3.55, p=.04). Post hoc tests indicated that relatives had more negative amplitudes than controls (Cz: t37=−3.02, p=.005, d=−.99; Pz: t37=−2.64, p=.01, d=−.87), while schizophrenia patients failed to differ from controls (Cz: t33=−1.23, p=.227, d=−.43; Pz: t33=−.32, p=.75, d=−.11) and relatives (Cz: t32=−1.69, p=.101, d=−.60; Pz: t32=−1.98, p=.06, d=−.70). Figure 2 panel d depicts anti – pro difference potentials at site Pz and the increasingly negative difference potential from −200 ms to saccade onset for overlap fixation trials. Inspection of separate prosaccade and antisaccade potentials at site Pz displayed in Figure 3 revealed relatives to have higher amplitudes for prosaccade trials than antisaccade trials during −210 to 0 ms period. Statistical tests of antisaccade overlap fixation potentials confirmed effects observed in difference potentials by revealing group differences over central and parietal sites from 180 to 120 ms before saccades (Cz: F2,51=3.91, p=.03; Pz: F2,51=4.21, p=.02; P3: F2,51=3.79, p=.03). Post hoc tests indicated that amplitudes during this time window at Cz and Pz were smaller for relatives than controls (Cz: t37=−2.70, p=.01, d=−.89; Pz: t37=−3.05, p=.004, d=−1.00), while those of the schizophrenia subjects were intermediate and failed to differ from controls (Cz: t33=−1.51, p=.14, d=−.53; Pz: t33=−.99, p=.33, d=−.34) and relatives (Cz: t33=1.13, p=.27, d=.40; Pz: t33=1.63, p=.11, d=.58). This group difference was not found in the overlap prosaccade potentials. Thus, in contrast with control subjects, relatives had diminished amplitudes over parietal cortex for antisaccades but normal amplitudes for prosaccades on overlap trials. Control subjects and schizophrenia patients did not show substantial parietal voltage differences between prosaccades and antisaccades for overlap fixations over the same pre-saccadic period. Correlation analyses also revealed that relatives who had lower overlap antisaccade potentials from −180 to −120 ms at the central and parietal sites had longer latencies for antisaccades on overlap fixation trials (Cz: r=−.58, p=.009; Pz: : r=−.49, p=.03).
There was some indication of parietal dysfunction in schizophrenia patients suggested by a trend toward group differences in amplitudes at Pz (F2,58=2.60, p=.08) from 60 to 0 ms prior to prosaccade onset for overlap fixation trials (see Figure 3). Post hoc tests revealed schizophrenia subjects had lower amplitudes than controls (t33=−2.38, p=.02, d=−.83), while relatives failed to differ from controls (t37=−1.01, p=.32, d=−.33) and patients (t33=−1.29, p=.21, d=.46).
Potentials over Frontal Polar Area
Given findings of frontal polar brain responses being associated antisaccades (Richards, 2003; Sweeney et al., 1996) and the role of orbital or inferior frontal cortex in response inhibition (Aron, Robbins, & Poldrack, 2004), we examined activity at Fp1 and Fp2 electrode sites where positive pre-saccadic potentials were observed. There was a trend toward an interaction of group and time for anti – pro difference potentials over right frontal polar area (site Fp2) for overlap fixation trials (−210 to 0 ms, F12,306=1.88, p=.06) suggesting that schizophrenia patients, relatives, and controls had different trajectories in pre-saccadic potentials over the front of the brain. Figure 2 depicts changes in pre-saccadic amplitudes at site Fp2, where controls exhibited increased activity for antisaccade trials from about 120 to 90 ms before saccade onset followed by an amplitude reduction (see panel h in Figure 2). In contrast, the schizophrenia subjects had continuously increasing potentials for antisaccades that achieved maximum amplitude just before saccade onset (i.e., −30 to 0 ms). The relatives showed a pattern of difference potentials similar to that of schizophrenia subjects reaching a maximum just prior to saccade onset. Relatives who had larger Fp2 potentials from 210 to 60 ms before step antisaccades had lower saccade accuracies (r=−.49, p=.03), suggesting that frontal polar functions might be relied upon by relatives to inhibit reflexive prosaccades in a failed attempt to compensate for lateral frontal dysfunction. Follow-up analyses revealed that frontal polar potentials preceding eye movements (−210 to 0 ms) were larger before antisaccades than prosaccades (Fp1: F1,51=10.28, p=.002; Fp2: F1,51=13.19, p=.001) which is consistent with frontal polar activity reflecting inhibition of reflexive prosaccades. Nevertheless, paired-comparisons of difference potentials failed to reveal group differences at frontal polar electrodes. When potentials for the antisaccades and prosaccades were separately considered (see Figure 3), antisaccade trials showed a trend toward a group effect at FP2 from −30 to 0 ms for step fixations (F2,51=2.61, p=.08). Post-hoc tests for the −30 to 0 ms period revealed that relatives had larger amplitudes than controls prior to antisaccades, especially for step fixation trials (t37=2.72, p=.01, d=.89), while schizophrenia patients failed to differ from controls (t33= .93, p=.36, d=.33) and relatives (t32=−.96, p=.34, d=−.34). There was also a significant group difference in frontopolar potentials at FP2 from −120 to 0 ms for step prosaccades (F2,51=3.35, p=.04). Follow-up analyses revealed that schizophrenia patients had larger FP2 potentials than controls (t33=2.70, p=.01, d=.94), while relatives showed a trend toward larger FP2 potentials than controls (t37=2.02, p=.051, d=.64), and there was no group difference between patients and relatives (t37=.32, p=.75, d=.11).
Medications
Associations of chlorpromazine equivalents with behavioral and electrophysiological indices indicated that a higher antipsychotic dosage was related to lower accuracy for overlap antisaccades (r=−.73, p=.002), and lower anti – pro difference potentials during −210 to −180 ms period at frontal electrodes (Af3: r=−.55, p=.03; Af4: r=−.58, p=.02; F3: r=−.62, p=.02; F4: r=−.58, p=.02). Compared to schizophrenia patients who were not on antidepressants, patients who were on this class of medication had higher accuracies for step prosaccades (F1,13=9.52, p=.009) and antisaccades (F1,13=4.78, p=.05), greater frontal anti – pro difference potentials during the −180 to −120 ms period (Af3: F1,13=7.97, p=.01) and smaller frontal anti – pro difference potentials during the −90 to −60 ms period (Af4: F1,13=5.56, p=.03; F3: F1,13=4.71, p=.05; F4: F1,13=8.80, p=.01). Although medication status of patients was statistically related to some behavioral and electrophysiological indices, relatives who were generally not taking psychiatric medications exhibited electrophysiological abnormalities similar to patients. Therefore, the electrophysiological abnormalities during saccade generation are unlikely to be the product of medications.
Discussion
Results provide evidence that in the 200 milliseconds before executing a volitional saccade both individuals with schizophrenia and first-degree biological relatives of schizophrenia patients exhibit neural abnormalities over prefrontal cortex. Because unaffected relatives who are likely carriers of genes for schizophrenia manifested similar electrophysiological activity to patients, the neural abnormalities appear to represent deviant cortical functions associated with genetic liability for the disorder. Specifically, schizophrenia patients and unaffected relatives of schizophrenia patients showed diminished anti – pro saccade difference potentials over lateral frontal cortex from 180 to 120 ms before the onset of saccades. Importantly, smaller pre-saccadic difference potentials over lateral frontal cortex were associated with lower antisaccade accuracies in schizophrenia patients and longer antisaccade latencies in relatives. Diminished potentials were also evident in relatives over parietal cortex prior to antisaccades for overlap fixations, while schizophrenia patients had diminished pre-saccadic potentials over parietal cortex for prosaccades for overlap fixations. Reduction of pre-saccadic potentials over parietal cortex in relatives was associated with longer latencies on the overlap antisaccade task. Electrophysiological anomalies over parietal cortex may reflect difficulties in attentional disengagement from the fixation stimulus during saccade preparation (Evdokimidis, Smyrnis, Constantinidis, Gourtzelidis, & Papageorgiou, 2001; Richards, 2003). We also observed relatives to have a tendency toward augmented potentials at the right frontal polar site prior to antisaccades, particularly during step fixation trials. Relatives who had larger frontal polar potentials had lower antisaccade accuracy, suggesting that orbital frontal activity may represent ineffective compensatory inhibition of reflexive saccades in response to compromised control of volitional eye movements by lateral frontal cortex (Everling & Fischer, 1998).
Positive potentials over lateral prefrontal areas have been observed prior to antisaccades in a study that analyzed principal components of scalp recorded voltages during short pre-saccadic time periods (Figure 8 in Richards, 2003). In the study, the potential was localized to lateral middle and anterior middle frontal gyrus and interpreted to reflect planning of targeted eye movements. In the present study source estimations localized scalp-recorded voltages prior to antisaccades to lateral PFC (see Supplementary Materials). Given the demand to control and plan voluntary eye-movements in the antisaccade task, the primary source of the lateral frontal potentials may be lateral PFC, especially the DLPFC (Everling & Fischer, 1998). Given additional evidence for dorsolateral PFC activation during antisaccade generation (DeSouza, Menon, & Everling, 2003; Pierrot-Deseilligny, Milea, & Muri, 2004; Pierrot-Deseilligny et al., 2003), the role of the PFC in providing contextual information through biasing signals to achieve cognitive control (Miller & Cohen, 2001; Pierrot-Deseilligny et al., 2004; Pierrot-Deseilligny et al., 2003), and the well-known dorsolateral PFC deficit in schizophrenia (Dracheva et al., 2001; MacDonald & Carter, 2003; Weinberger, Berman, & Zec, 1986), diminished activity at lateral frontal recording sites in schizophrenia may indeed reflect impairment of dorsolateral PFC during saccade generation. Neuroimaging in humans has revealed bilateral activation of dorsolateral PFC during antisaccade tasks (Sweeney et al., 1996), and lesions of the dorsolateral PFC result in increases of erroneous reflexive saccades (Fukushima, Fukushima, Miyasaka, & Yamashita, 1994; Pierrot-Deseilligny, Rivaud, Gaymard, & Agid, 1991).
Abnormalities of the parietal cortex may also contribute to saccade control deficits in schizophrenia. In the overlap fixation condition where disengagement of spatial attention from the overlapping fixation is required, schizophrenia patients tended to have diminished activity over parietal cortex and lower prosaccade accuracies than control subjects. The results of the present study are consistent with evidence of centro-parietal pre-saccadic positive potentials observed during prosaccades and voluntary saccades (Everling, Krappmann, & Flohr, 1997; Kurtzberg & Vaughan, 1982; Moster & Goldberg, 1990; Richards, 2003). Increases in centro-parietal potentials have been related to cellular activity in parietal area 7 that is associated with the deployment of visual attention during saccadic eye-movements (Lynch, Mountcastle, Talbot, & Yin, 1977). Reduced pre-saccadic centro-parietal potentials prior to antisaccades as compared to prosaccades that were observed in the present study are consistent with pre-saccadic activations identified in other studies (Everling et al., 1997; Kurtzberg & Vaughan, 1982). The abnormal potentials over parietal cortex may reflect schizophrenia patients’ poor disengagement of spatial attention from the fixation stimulus before execution of the saccade. Failure to disengage from fixation might underlie prosaccade accuracy deficits in patients, which were found in overlap fixations and not step fixations. This is consistent with a previous finding of deficits in attentional disengagement and inhibitory control in schizophrenia patients as indicated by prolonged latency and decreased accuracy on a saccade task with overlap fixations and distracters (Schwartz & Evans, 1999). Through transcranial magnetic stimulation (TMS) investigators have demonstrated that prolonged saccadic latency can result from applying electromagnetic fields to parietal cortex (Kapoula, Isotalo, Muri, Bucci, & Rivaud-Pechoux, 2001; Yang & Kapoula, 2004).
Pre-saccadic negative potentials were not observed in the present study although they have been noted in many studies of volitional saccades. Pre-saccadic negativity has been interpreted to reflect preparatory activity of the frontal eye field or supplementary eye field (Everling et al., 1997; Kurtzberg & Vaughan, 1982; Moster & Goldberg, 1990). The discrepancy appears to stem from differences in the task design and analysis of pre-saccadic potentials. Compared to previous studies that included block-designs with a single fixation condition where subjects responded to stimuli in a fixed manner (Everling et al., 1997; Kurtzberg & Vaughan, 1982; Thickbroom & Mastaglia, 1990), we used a task in which subjects had to respond to pseudo-randomly mixed fixation conditions. The predictable nature of the fixation in the previous studies likely allows the preparatory neural activity to be reliably elicited. Pre-saccadic negative potentials have not been observed by other investigators who have employed unpredictable cues for saccade generation (Evdokimidis, Liakopoulos, Constantinidis, & Papageorgiou, 1996). Additionally, studies that have observed pre-saccadic negative potentials include longer pre-saccadic periods than in the present study (Everling et al., 1997; Kurtzberg & Vaughan, 1982; Moster & Goldberg, 1990; Thickbroom & Mastaglia, 1990), and the pre-saccadic negativity was related to stimulus onsets rather than saccade onsets (Everling, Spantekow, Krappmann, & Flohr, 1998; Richards, 2003). Richards (2003) also noted that the pre-saccadic negativity is likely the contingent negativity variation (CNV) related to preparatory neural activity which can occur without overt motor movements. Hence, there is limited possibility of the pre-saccadic activity observed in the present study is affected by a CNV given that we employed unpredictable fixation conditions prior to movements and relatively short time period between fixation onset on saccade generation.
Given that relatives had essentially normal accuracy on the antisaccade task despite what may be PFC deficits, and their performance improved on overlap fixation for the antisaccade task (see Table 2), their diminished potentials at parietal sites preceding overlap antisaccades may reflect something other than dysfunction. One possibility is that relatives with lateral frontal cortex dysfunction rely on compensatory suppression of parietal activity to inhibit disengagement of spatial attention and prevent reflexive prosaccades during the antisaccade task. Reduction of activity in parietal cortex may inhibit reflexive saccades by persistently engaging spatial attention on the fixation stimulus during overlap trials. Estimated sources (see Supplemental Materials) of potentials observed in the overlap fixation condition were consistent with unaffected first-degree biological relatives having little parietal activity prior to antisaccades but greater activity prior to prosaccades. Finally, relatives exhibited reduced centro-parietal potentials for correct antisaccades compared to incorrect antisaccades, especially in the overlap fixation condition (Cz during −90 to −60 ms: t18=−3.14, p=.006; Pz during −90 to −60 ms: t18=−3.19, p=.005). These findings support the interpretation that the reduced potentials over parietal cortex in relatives reflect inhibition of disengagement from the fixation stimulus (i.e., improved fixation), thus preventing errant reflexive saccades. In other words, the diminished parietal site amplitudes in biological relatives could reflect a compensatory mechanism for executing correct antisaccades during overlap fixation trials.
A tendency for relatives and schizophrenia patients to exhibit larger frontal polar potentials may also represent a dynamic response to failure in lateral prefrontal cortex. The frontal polar pre-saccadic potentials may reflect orbital frontal cortex or ventromedial PFC activity given a report of pre-saccadic activity of similar topography that was localized to the orbital frontal gyrus and was larger prior to antisaccades than prosaccades (Richards, 2003). Source analysis suggested that the orbital frontal cortex (Brodman area 11) may be a main contributor to the frontal polar activity prior to antisaccades observed in the current study (see Supplementary Materials). Recruitment of additional prefrontal regions in schizophrenia is also suggested by recent work that revealed schizophrenia patients to activate ventrolateral prefrontal cortex in performing an N-back working memory task while control subjects largely augmented dorsolateral PFC (Tan, Callicott, & Weinberger, 2007; Tan et al., 2006). Neuroimaging studies of cognitive tasks requiring inhibitory control, such as go-nogo and antisaccade tasks, suggest that the orbital frontal cortex assists with inhibiting unwanted behavioral responses (Aron et al., 2004; Chikazoe, Konishi, Asari, Jimura, & Miyashita, 2007). As a result of impaired dorsolateral PFC activity prior to antisaccades, relatives may rely more heavily upon orbital PFC than control subjects in an attempt to inhibit prepotent reflexive saccades. Recent work has provided evidence that a failure of schizophrenia patients to inhibit reflexive saccades (Manoach et al., 2002) and not deficient switching between tasks (Greenzang, Manoach, Goff, & Barton, 2007) leads to poor antisaccade performance. We found schizophrenia patients had larger frontal polar potentials than controls from 120 to 0 ms prior to step prosaccade potentials. Because prosaccades do not require inhibition of saccades and the topographies of potentials prior to prosaccades were different from those of antisaccades, future work is required to clarify the source and function of increased frontal polar potentials in schizophrenia patients.
Although analyses focused on potentials locked to saccade onset (i.e., response-locked), one cannot rule out the possibility that neural activity reflecting processing of the fixation stimulus may contribute to the pre-saccadic potentials. Inspection of stimulus-locked potentials from 0 to 250 ms after stimulus onset (the time period largely before saccadic eye movements) revealed essentially no notable components at frontal sites (Fp1, Fp2, F3, F4) shortly after onset of the fixation stimulus; however, a broad shallow positive potential at central and parietal sites was observed from 50 to 150 ms after fixation onset. A recent report of a stimulus-locked P2 response recorded in two monkeys described a decrement in the potential at a parietal recording site for fixations prior to antisaccades (Sander, Soper, & Everling, 2010). This is consistent with the possibility that pre-saccadic potentials over parietal cortex reflect modulation spatial attention in preparation for saccade generation. Additionally, the potentials prior to visually-triggered saccades in the present study are similar to a pre-saccadic positivity over the parietal region previously noted as diminished prior to antisaccades (Everling et al., 1997). Finally, a spike potential noted at or near saccade onset by other studies (Everling et al., 1997, Richards, 2003) was evident with the expected frontal-negative-positive-elsewhere distribution in data from the current investigation.
In summary, electrophysiological abnormalities over lateral frontal cortex prior to saccades in an antisaccade task provide evidence suggestive of prefrontal cortical dysfunction preceding saccadic errors in schizophrenia patients and biological relatives of schizophrenia patients. Because anatomical locations of neural activity are not definitively identified and instead are estimated from scalp recordings, work remains to precisely isolate the location of pre-saccadic abnormalities associated with schizophrenia. Nevertheless, the present study provides evidence consistent with lateral frontal cortical dysfunction during volitional saccade generation being a marker of genetic liability for schizophrenia. Relatives also exhibited reduced potentials compared to control subjects over parietal cortex prior to saccade execution. Despite these electrophysiological abnormalities the relatives successfully performed the antisaccade task. Reduced activity over parietal cortex may reflect suppression of attentional disengagement, leading to enhanced inhibition of reflexive saccades and normative performance in relatives. To conclude, the present study suggests that PFC and parietal cortex are elements of a neural substrate associated poor saccadic control in schizophrenia and reflective of genetic liability for the disorder.
Supplementary Material
Acknowledgments
We thank Monica Calkins Ph.D., Clayton Curtis Ph.D., and Kevin Haroian for assistance in task design and implementation. Kathryn A. McGuire Ph.D., John. J. Stanwyck, Sarah M. Sass, Robb Hunter assisted with task implementation and data acquisition, and Carly Smitkowski assisted in scoring the eye movement data. We are also grateful to Jennifer McDowell Ph.D. for consultation and guidance during the processing and quantification of eye movement data.
This work was supported by grants from the Department of Veterans Affairs Medical Research Service, the National Institutes of Mental Health (5R24MH069675) to Dr. Scott Sponheim, as well as by the Mental Illness and Neuroscience Discovery (MIND) Institute and the Mental Health Patient Service Line at the Veterans Affairs Medical Center, Minneapolis Minnesota.
Footnotes
Gender failed to be associated with any behavioral measure or indices of pre-saccadic potentials. Age and IQ were correlated with saccade latencies (age: r=.38, p=.005; IQ: r=−.34, p=.011), but not saccade accuracy or any index of pre-saccadic potentials. Inclusion of IQ and age as covariates failed to alter the pattern of significant effects and resulted in main effects of task (without covariates: F1,51=276.68, p<.001; with covariates: F1,49=26.13, p<.001) and fixation (without covariates: F1,51=217.72, p<.001; with covariates: F1,49=4.89, p=.032) effects but no group effect (without covariates: F2,51=.80, p=.450; with covariates: F2,49=1.10, p=.341) on saccade latencies.
To test for an effect of saccade direction on neural data, pro- and anti- presaccadic average potentials were also computed for leftward and rightward saccades, collapsing across fixation conditions. A repeated measure ANOVA was used to test for the effect of saccade direction on pre-saccadic potentials. The analysis included within subjects factors of laterality, time bin, and electrode, with a between subjects factor of group, and dependent variables being difference potentials between prosaccades and antisaccades at the various time bins. The analysis revealed no effects of laterality (F1,51=2.17, p=.15) or laterality by group (F1,51=.20, p=.82).
References
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8(4):170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Braun D, Breitmeyer BG. Relationship between directed visual attention and saccadic reaction times. Exp Brain Res. 1988;73(3):546–552. doi: 10.1007/BF00406613. [DOI] [PubMed] [Google Scholar]
- Brooker B, Cyr J. Tables for clinicians to use to convert WAIS-R short forms. J Clin Psychol. 1986;42(6):983–986. [Google Scholar]
- Calkins ME, Curtis CE, Iacono WG, Grove WM. Antisaccade performance is impaired in medically and psychiatrically healthy biological relatives of schizophrenia patients. Schizophr Res. 2004;71(1):167–178. doi: 10.1016/j.schres.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Calkins ME, Dobie DJ, Cadenhead KS, Olincy A, Freedman R, Green MF, et al. The Consortium on the Genetics of Endophenotypes in Schizophrenia: model recruitment, assessment, and endophenotyping methods for a multisite collaboration. Schizophr Bull. 2007;33(1):33–48. doi: 10.1093/schbul/sbl044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, Dyckman KA, Austin BP, Clementz BA, McDowell JE. Common neural circuitry supporting volitional saccades and its disruption in schizophrenia patients and relatives. Biol Psychiatry. 2008;64(12):1042–1050. doi: 10.1016/j.biopsych.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Perlstein W, Ganguli R, Brar J, Mintun M, Cohen JD. Functional hypofrontality and working memory dysfunction in schizophrenia. Am J Psychiatry. 1998;155(9):1285–1287. doi: 10.1176/ajp.155.9.1285. [DOI] [PubMed] [Google Scholar]
- Chikazoe J, Konishi S, Asari T, Jimura K, Miyashita Y. Activation of right inferior frontal gyrus during response inhibition across response modalities. J Cogn Neurosci. 2007;19(1):69–80. doi: 10.1162/jocn.2007.19.1.69. [DOI] [PubMed] [Google Scholar]
- Clementz BA, McDowell JE, Zisook S. Saccadic system functioning among schizophrenia patients and their first-degree biological relatives. J Abnorm Psychol. 1994;103(2):277–287. [PubMed] [Google Scholar]
- DeSouza JF, Menon RS, Everling S. Preparatory set associated with pro-saccades and anti-saccades in humans investigated with event-related FMRI. J Neurophysiol. 2003;89(2):1016–1023. doi: 10.1152/jn.00562.2002. [DOI] [PubMed] [Google Scholar]
- Dracheva S, Marras SA, Elhakem SL, Kramer FR, Davis KL, Haroutunian V. N-methyl-D-aspartic acid receptor expression in the dorsolateral prefrontal cortex of elderly patients with schizophrenia. Am J Psychiatry. 2001;158(9):1400–1410. doi: 10.1176/appi.ajp.158.9.1400. [DOI] [PubMed] [Google Scholar]
- Ekselius L, Lindstrom E, von Knorring L, Bodlund O, Kullgren G. SCID II interviews and the SCID Screen questionnaire as diagnostic tools for personality disorders in DSM-III-R. Acta Psychiatr Scand. 1994;90(2):120–123. doi: 10.1111/j.1600-0447.1994.tb01566.x. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Ffytche DH, Kumari V, Kathmann N, Reuter B, Zelaya F, et al. Decomposing the Neural Correlates of Antisaccade Eye Movements Using Event-Related fMRI. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm147. [DOI] [PubMed] [Google Scholar]
- Evdokimidis I, Liakopoulos D, Constantinidis TS, Papageorgiou C. Cortical potentials with antisaccades. Electroencephalogr Clin Neurophysiol. 1996;98(5):377–384. doi: 10.1016/0013-4694(96)94699-4. [DOI] [PubMed] [Google Scholar]
- Evdokimidis I, Smyrnis N, Constantinidis TS, Gourtzelidis P, Papageorgiou C. Frontal-parietal activation differences observed before the execution of remembered saccades: an event-related potentials study. Brain Res Cogn Brain Res. 2001;12(1):89–99. doi: 10.1016/s0926-6410(01)00037-4. [DOI] [PubMed] [Google Scholar]
- Everling S, Fischer B. The antisaccade: a review of basic research and clinical studies. Neuropsychologia. 1998;36(9):885–899. doi: 10.1016/s0028-3932(98)00020-7. [DOI] [PubMed] [Google Scholar]
- Everling S, Krappmann P, Flohr H. Cortical potentials preceding pro- and antisaccades in man. Electroencephalogr Clin Neurophysiol. 1997;102(4):356–362. doi: 10.1016/s0013-4694(96)96569-4. [DOI] [PubMed] [Google Scholar]
- Everling S, Spantekow A, Krappmann P, Flohr H. Event-related potentials associated with correct and incorrect responses in a cued antisaccade task. Exp Brain Res. 1998;118(1):27–34. doi: 10.1007/s002210050252. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorder (SCID-I, Research Version) New York: Biometric Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- Fischer B, Weber H. Express saccades and visual attention. Behavioral and Brain Sciences. 1993;16:553–610. [Google Scholar]
- Fischer B, Weber H. Effects of stimulus conditions on the performance of antisaccades in man. Exp Brain Res. 1997;116(2):191–200. doi: 10.1007/pl00005749. [DOI] [PubMed] [Google Scholar]
- Franke C, Reuter B, Schulz L, Kathmann N. Schizophrenia patients show impaired response switching in saccade tasks. Biol Psychol. 2007;76(1–2):91–99. doi: 10.1016/j.biopsycho.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Fukumoto-Motoshita M, Matsuura M, Ohkubo T, Ohkubo H, Kanaka N, Matsushima E, et al. Hyperfrontality in patients with schizophrenia during saccade and antisaccade tasks: a study with fMRI. Psychiatry Clin Neurosci. 2009;63(2):209–217. doi: 10.1111/j.1440-1819.2009.01941.x. [DOI] [PubMed] [Google Scholar]
- Fukushima J, Fukushima K, Miyasaka K, Yamashita I. Voluntary control of saccadic eye movement in patients with frontal cortical lesions and parkinsonian patients in comparison with that in schizophrenics. Biol Psychiatry. 1994;36(1):21–30. doi: 10.1016/0006-3223(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Greenzang C, Manoach DS, Goff DC, Barton JJ. Task-switching in schizophrenia: active switching costs and passive carry-over effects in an antisaccade paradigm. Exp Brain Res. 2007;181(3):493–502. doi: 10.1007/s00221-007-0946-8. [DOI] [PubMed] [Google Scholar]
- Guitton D, Buchtel HA, Douglas RM. Frontal lobe lesions in man cause difficulties in suppressing reflexive glances and in generating goal-directed saccades. Exp Brain Res. 1985;58(3):455–472. doi: 10.1007/BF00235863. [DOI] [PubMed] [Google Scholar]
- Hutton SB, Ettinger U. The antisaccade task as a research tool in psychopathology: a critical review. Psychophysiology. 2006;43(3):302–313. doi: 10.1111/j.1469-8986.2006.00403.x. [DOI] [PubMed] [Google Scholar]
- Kapoula Z, Isotalo E, Muri RM, Bucci MP, Rivaud-Pechoux S. Effects of transcranial magnetic stimulation of the posterior parietal cortex on saccades and vergence. Neuroreport. 2001;12(18):4041–4046. doi: 10.1097/00001756-200112210-00037. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Lieberman JA, Walsh D. The Structured Interview for Schizotypy (SIS): a preliminary report. Schizophr Bull. 1989;15(4):559–571. doi: 10.1093/schbul/15.4.559. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Kwon JS, Park HJ, Youn T, Kang DH, Kim MS, et al. Functional disconnection between the prefrontal and parietal cortices during working memory processing in schizophrenia: a[15(O)]H2O PET study. Am J Psychiatry. 2003;160(5):919–923. doi: 10.1176/appi.ajp.160.5.919. [DOI] [PubMed] [Google Scholar]
- Klein C, Heinks T, Andresen B, Berg P, Moritz S. Impaired modulation of the saccadic contingent negative variation preceding antisaccades in schizophrenia. Biol Psychiatry. 2000;47(11):978–990. doi: 10.1016/s0006-3223(00)00234-1. [DOI] [PubMed] [Google Scholar]
- Kurtzberg D, Vaughan HG., Jr Topographic analysis of human cortical potentials preceding self-initiated and visually triggered saccades. Brain Res. 1982;243(1):1–9. doi: 10.1016/0006-8993(82)91115-5. [DOI] [PubMed] [Google Scholar]
- Levy DL, O’Driscoll G, Matthysse S, Cook SR, Holzman PS, Mendell NR. Antisaccade performance in biological relatives of schizophrenia patients: a meta-analysis. Schizophr Res. 2004;71(1):113–125. doi: 10.1016/j.schres.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Lynch JC, Mountcastle VB, Talbot WH, Yin TC. Parietal lobe mechanisms for directed visual attention. J Neurophysiol. 1977;40(2):362–389. doi: 10.1152/jn.1977.40.2.362. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Carter CS. Event-related FMRI study of context processing in dorsolateral prefrontal cortex of patients with schizophrenia. J Abnorm Psychol. 2003;112(4):689–697. doi: 10.1037/0021-843X.112.4.689. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Ketwaroo GA, Polli FE, Thakkar KN, Barton JJ, Goff DC, et al. Reduced microstructural integrity of the white matter underlying anterior cingulate cortex is associated with increased saccadic latency in schizophrenia. Neuroimage. 2007;37(2):599–610. doi: 10.1016/j.neuroimage.2007.04.062. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Lindgren KA, Cherkasova MV, Goff DC, Halpern EF, Intriligator J, et al. Schizophrenic subjects show deficient inhibition but intact task switching on saccadic tasks. Biol Psychiatry. 2002;51(10):816–826. doi: 10.1016/s0006-3223(01)01356-7. [DOI] [PubMed] [Google Scholar]
- Maruff P, Danckert J, Pantelis C, Currie J. Saccadic and attentional abnormalities in patients with schizophrenia. Psychol Med. 1998;28(5):1091–1100. doi: 10.1017/s0033291798007132. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Brown GG, Paulus M, Martinez A, Stewart SE, Dubowitz DJ, et al. Neural correlates of refixation saccades and antisaccades in normal and schizophrenia subjects. Biol Psychiatry. 2002;51(3):216–223. doi: 10.1016/s0006-3223(01)01204-5. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Clementz BA. The effect of fixation condition manipulations on antisaccade performance in schizophrenia: studies of diagnostic specificity. Exp Brain Res. 1997;115(2):333–344. doi: 10.1007/pl00005702. [DOI] [PubMed] [Google Scholar]
- McGuffin P, Farmer A, Harvey I. A polydiagnostic application of operational criteria in studies of psychotic illness. Development and reliability of the OPCRIT system. Arch Gen Psychiatry. 1991;48(8):764–770. doi: 10.1001/archpsyc.1991.01810320088015. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Moon SY, Barton JJ, Mikulski S, Polli FE, Cain MS, Vangel M, et al. Where left becomes right: a magnetoencephalographic study of sensorimotor transformation for antisaccades. Neuroimage. 2007;36(4):1313–1323. doi: 10.1016/j.neuroimage.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moster ML, Goldberg G. Topography of scalp potentials preceding self-initiated saccades. Neurology. 1990;40(4):644–648. doi: 10.1212/wnl.40.4.644. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51(11):849–859. doi: 10.1001/archpsyc.1994.03950110009002. discussion 863–844. [DOI] [PubMed] [Google Scholar]
- Ojeda N, Ortuno F, Arbizu J, Lopez P, Marti-Climent JM, Penuelas I, et al. Functional neuroanatomy of sustained attention in schizophrenia: contribution of parietal cortices. Hum Brain Mapp. 2002;17(2):116–130. doi: 10.1002/hbm.10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Milea D, Muri RM. Eye movement control by the cerebral cortex. Curr Opin Neurol. 2004;17(1):17–25. doi: 10.1097/00019052-200402000-00005. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Muri RM, Ploner CJ, Gaymard B, Demeret S, Rivaud-Pechoux S. Decisional role of the dorsolateral prefrontal cortex in ocular motor behaviour. Brain. 2003;126(Pt 6):1460–1473. doi: 10.1093/brain/awg148. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Rivaud S, Gaymard B, Agid Y. Cortical control of reflexive visually-guided saccades. Brain. 1991;114(Pt 3):1473–1485. doi: 10.1093/brain/114.3.1473. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Rivaud S, Gaymard B, Muri R, Vermersch AI. Cortical control of saccades. Ann Neurol. 1995;37(5):557–567. doi: 10.1002/ana.410370504. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32(1):3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Walker JA, Friedrich FJ, Rafal RD. Effects of parietal injury on covert orienting of attention. J Neurosci. 1984;4(7):1863–1874. doi: 10.1523/JNEUROSCI.04-07-01863.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raemaekers M, Ramsey NF, Vink M, van den Heuvel MP, Kahn RS. Brain activation during antisaccades in unaffected relatives of schizophrenic patients. Biol Psychiatry. 2006;59(6):530–535. doi: 10.1016/j.biopsych.2005.07.030. [DOI] [PubMed] [Google Scholar]
- Rafal RD. Oculomotor functions of the parietal lobe: Effects of chronic lesions in humans. Cortex. 2006;42(5):730–739. doi: 10.1016/s0010-9452(08)70411-8. [DOI] [PubMed] [Google Scholar]
- Raine A. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr Bull. 1991;17(4):555–564. doi: 10.1093/schbul/17.4.555. [DOI] [PubMed] [Google Scholar]
- Reuter B, Herzog E, Endrass T, Kathmann N. Brain potentials indicate poor preparation for action in schizophrenia. Psychophysiology. 2006;43(6):604–611. doi: 10.1111/j.1469-8986.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- Reuter B, Jager M, Bottlender R, Kathmann N. Impaired action control in schizophrenia: the role of volitional saccade initiation. Neuropsychologia. 2007;45(8):1840–1848. doi: 10.1016/j.neuropsychologia.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Richards JE. Cortical sources of event-related potentials in the prosaccade and antisaccade task. Psychophysiology. 2003;40(6):878–894. doi: 10.1111/1469-8986.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivaud S, Muri RM, Gaymard B, Vermersch AI, Pierrot-Deseilligny C. Eye movement disorders after frontal eye field lesions in humans. Exp Brain Res. 1994;102(1):110–120. doi: 10.1007/BF00232443. [DOI] [PubMed] [Google Scholar]
- Sander V, Soper B, Everling S. Nonhuman primate event-related potentials associated with pro- and anti-saccades. Neuroimage. 2010;49(2):1650–1658. doi: 10.1016/j.neuroimage.2009.09.038. [DOI] [PubMed] [Google Scholar]
- Schulze K, MacCabe JH, Rabe-Hesketh S, Crawford T, Marshall N, Zanelli J, et al. The relationship between eye movement and brain structural abnormalities in patients with schizophrenia and their unaffected relatives. J Psychiatr Res. 2006;40(7):589–598. doi: 10.1016/j.jpsychires.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Schwartz BD, Evans WJ. Neurophysiologic mechanisms of attention deficits in schizophrenia. Neuropsychiatry Neuropsychol Behav Neurol. 1999;12(4):207–220. [PubMed] [Google Scholar]
- Servan-Schreiber D, Cohen JD, Steingard S. Schizophrenic deficits in the processing of context. A test of a theoretical model. Arch Gen Psychiatry. 1996;53(12):1105–1112. doi: 10.1001/archpsyc.1996.01830120037008. [DOI] [PubMed] [Google Scholar]
- Sprague RL, Kalachnik JE, Slaw KM. Psychometric properties of the Dyskinesia Identification System: Condensed User Scale (DISCUS) Ment Retard. 1989;27(3):141–148. [PubMed] [Google Scholar]
- Sweeney JA, Mintun MA, Kwee S, Wiseman MB, Brown DL, Rosenberg DR, et al. Positron emission tomography study of voluntary saccadic eye movements and spatial working memory. J Neurophysiol. 1996;75(1):454–468. doi: 10.1152/jn.1996.75.1.454. [DOI] [PubMed] [Google Scholar]
- Tan HY, Callicott JH, Weinberger DR. Dysfunctional and compensatory prefrontal cortical systems, genes and the pathogenesis of schizophrenia. Cereb Cortex. 2007;17(Suppl 1):i171–181. doi: 10.1093/cercor/bhm069. [DOI] [PubMed] [Google Scholar]
- Tan HY, Sust S, Buckholtz JW, Mattay VS, Meyer-Lindenberg A, Egan MF, et al. Dysfunctional prefrontal regional specialization and compensation in schizophrenia. Am J Psychiatry. 2006;163(11):1969–1977. doi: 10.1176/ajp.2006.163.11.1969. [DOI] [PubMed] [Google Scholar]
- Thickbroom GW, Mastaglia FL. Premotor negativity associated with saccadic eye movement and finger movement: a comparative study. Brain Res. 1990;506:223–226. doi: 10.1016/0006-8993(90)91254-e. [DOI] [PubMed] [Google Scholar]
- Tu PC, Yang TH, Kuo WJ, Hsieh JC, Su TP. Neural correlates of antisaccade deficits in schizophrenia, an fMRI study. J Psychiatr Res. 2006;40(7):606–612. doi: 10.1016/j.jpsychires.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Ventura J, Nuechterlein KH, Subotnik KL, Gutkind D, Gilbert EA. Symptom dimensions in recent-onset schizophrenia and mania: a principal components analysis of the 24-item Brief Psychiatric Rating Scale. Psychiatry Res. 2000;97(2–3):129–135. doi: 10.1016/s0165-1781(00)00228-6. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43(2):114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Yang Q, Kapoula Z. TMS over the left posterior parietal cortex prolongs latency of contralateral saccades and convergence. Invest Ophthalmol Vis Sci. 2004;45(7):2231–2239. doi: 10.1167/iovs.03-1291. [DOI] [PubMed] [Google Scholar]
- Zhang M, Barash S. Neuronal switching of sensorimotor transformations for antisaccades. Nature. 2000;408(6815):971–975. doi: 10.1038/35050097. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.