Abstract
Background
To study survival and long-term morbidities of children with nasopharyngeal carcinoma (NPC).
Patients and Methods
Retrospective review of children with NPC treated at St. Jude Children’s Research Hospital between 1961 and 2004. Prognostic factors and long term effects of therapy were analyzed.
Results
Fifty-nine patients (median age 14.1 years) were identified. Most were male (66.1%) and Black (54.2%) and had lymphoepithelioma (93.2%). Thirty-five (59.3%), 20 (33.9%) and 4 (6.8%) patients were staged IV, III and II respectively. All patients received radiotherapy (RT) to primary tumor, and most received cervical RT (98.3%) and chemotherapy (88.1%). The 15-year survival and event-free survival (EFS) were 67.2%±7.5% and 63.5%±7.8%, respectively. Five patients (8.5%) developed subsequent malignancies 8.6–27 years after NPC diagnosis. EFS was improved in patients diagnosed after 1980 (74.8%±10.0% vs. 45.5%±10.1%, p=0.031), patients with stage III compared to stage IV (79.3%±9.6% vs. 56.2%±11.8%, p=0.049), patients that received cisplatin (81.0%±10.7% vs. 45.8%±9.7%, p=0.013), and patients treated with RT≥50Gy (71.4%±9.3% vs. 43.8%±11.6%, p=0.048). Whites had higher distant failure than Blacks (41.7%±10.4% vs. 15.6±6.5%, p=0.045). The 15-year cumulative incidence (CI) of any morbidity, sensorineural hearing loss (SNHL), primary hypothyroidism, and growth hormone deficiency (GHD) were 83.7%±5.4%, 52.9%±6.7%, 42.7%±6.6%, and 14.1%±4.7%, respectively. There were dose-response relationships between RT dose and primary hypothyroidism and GHD.
Conclusion
Outcome of children with NPC improved over the past 4 decades with cisplatin-based chemotherapy and higher RT doses. However, many survivors had long-term treatment-related morbidities.
Keywords: Nasopharyngeal carcinoma, children, long-term effects
INTRODUCTION
Nasopharyngeal carcinoma (NPC) is an uncommon malignancy in children, with an incidence of 0.1–1.5 per million per year in the US. 1 It is classified pathologically into 3 subtypes; type III, or undifferentiated carcinoma (also known as lymphoepithelioma), is the most common subtype in children.2 Clinically, the most common presenting symptom of childhood NPC is cervical lymphadenopathy.2, 3
Treatment recommendations for childhood NPC follow guidelines established for adults. Standard of care for patients with loco-regional disease includes radiotherapy (RT) to the nasopharynx and cervical lymph nodes.1 Because of high incidence of local and systemic failure in locally advanced disease, chemotherapy has been incorporated into the treatment of those patients. In recent years, randomized and non-randomized studies have documented the advantage of concomitant administration of cisplatin-based chemotherapy and RT. The use of adjuvant and neoadjuvant chemotherapy is a matter of much debate; however, available data would suggest an advantage to the use of neoadjuvant therapy.4
Several studies have analyzed the clinical characteristics and treatment outcomes of NPC in children and young adults.1, 5–29 With combined chemotherapy and radiotherapy, survival rates in excess of 60–70%, and up to 91%, have been reported.12, 17 However, morbidities such as endocrinopathies, hearing loss, bone demineralization, and second neoplasms are not uncommon.5, 6, 12–18, 20, 27–29 Since most published series are small and have short follow-up, the long-term outcome of children with NPC has not been well characterized. Therefore, we performed a retrospective review of all children with NPC treated at our institution during the past 43 years to investigate the long-term survival and morbidity in different eras, as well as factors associated with those outcomes.
PATIENTS and METHODS
Clinical review
With institutional review board approval, we reviewed the medical records of all NPC patients younger than 20 years treated at St. Jude Children’s Research Hospital between 1961 and 2004. The subjects were retrospectively identified in our institutional database. The survivorship program at St. Jude Children’s Research Hospital performs active, on site follow-up for 10 years from diagnosis. Afterwards, patients are followed using annual comprehensive questionnaires and phone calls. We extracted data on the presenting features, histopathology, imaging findings, treatment, outcome, and late morbidities. Since staging of NPC has evolved over the past decades, we attempted to re-stage all patients according to the most recent version of the American Joint Committee on Cancer (AJCC) NPC staging classification,30 using the available clinical information and the original radiology reports.
Treatment protocols
Four major treatment protocols were used for NPC in the past 43 years. Patients diagnosed in 1966–1980, 1985–1990 and 1991–2000 were mostly treated using the institutional NPC-77, 85N2, and NPC1 protocols. In some instances, patients were treated outside of those studies but following the same regimens. For purpose of simplification, those patients were analyzed with the corresponding protocol. Patients diagnosed after 2000 were treated with a non-protocol treatment plan using a chemoradiotherapy (NPTP-CRT-PF) regimen that included neoadjuvant and concomitant chemotherapy and RT. Patients diagnosed before 1966, in 1981–1984 and those not eligible for the above treatment protocols were treated with other non-protocol treatment plans involving radiotherapy and different chemotherapy regimens. The treatment regimens are summarized in Table 2. The NPC-77 protocol consisted of radiotherapy to the nasopharynx and cervical lymph nodes, with cyclophosphamide for 1 year. The 85N2 and NPC1 protocols included RT alone for T1N0 and T2N0 disease, and 4 cycles of neoadjuvant chemotherapy followed by radiotherapy for T3-4 and/or N1-3 disease. The NPTP-CRT-PF regimen consisted of neoadjuvant chemotherapy followed by concurrent chemo-radiotherapy. Fifty-two patients received 2D-irradiation, one patient was treated using 3D-conformal irradiation, and six patients were treated using intensity modulated radiation therapy. Six patients received amifostine given prior to cisplatin administration and prior to daily irradiation.
Table 2.
Chemotherapy regimens
| Protocol | Years | N | Radiotherapy* | Chemotherapy |
|---|---|---|---|---|
| NPC-77 | 1966–1980 | 18 | 45–70 Gy | CYC 200 mg/m2/week x 6 weeks CYC 300 mg/m2 every other week x 12 months |
| 85N2 | 1985–1990 | 5 | 45–70 Gy |
Neoadjuvant (4 cycles) MTX 120 mg/m2 d 1 CDDP 100 mg/m2 d 2 5-FU 1000 mg/m2/d d 1–5 LV 25 mg/m2 q6h x 6 d 2–4 |
| NPC-1 | 1991–2000 | 12 | 45–70 Gy |
Neoadjuvant (4 cycles) MTX 120 mg/m2 d 1 CDDP 100 mg/m2 d 2 5-FU 1000 mg/m2/d d 1–5 LV 25 mg/m2 q6h x 6 d 2–4 |
| NPTP-CRT-PF | 2000–2004 | 6 | 45–70 Gy |
Neoadjuvant (3 cycles) CDDP 80 mg/m2 d 1 5-FU 1000 mg/m2 d 1–4 Concurrent chemoradiation (3 cycles) CDDP 100 mg/m2 d 1 |
| Other regimens | 12 | 45–70 Gy | VCR/BLE/CDDP VCR/DNR/CDDP BLE/MTX/CDDP CYC/MTX CYC/MTX/5FU VCR/CYC VCR/CYC/MTX |
|
| No chemotherapy | 6 | 45–70 Gy | - |
Abbreviations: CYC: Cyclophosphamide; MTX: Methotrexate; CDDP: Cisplatin; 5-FU: 5-Fluorouracyl; LV: Leucovorin; VCR: Vincristine; BLE: Bleomycin; DNR: Daunorubicin
Radiotherapy to primary tumor and nodal areas was individualized.
Statistical Methods
Overall survival (OS) was defined as the time interval from date of diagnosis to date of death from any cause or to date of last contact. Event-free survival (EFS) was defined as the time interval from date of diagnosis to date of first event (relapsed or progressive disease, second malignancy or death from any cause) or to date of last contact for patients without events. OS and EFS were estimated using the Kaplan-Meier method. Standard errors (SE) were calculated using the method of Peto and Pike.31 Differences in OS and EFS were examined using the exact log rank test.
Local failure and distant failure were defined as the time intervals from date of diagnosis to date of local disease recurrence/progression (involving either the nasopharynx or cervical lymphatics) and distant disease recurrence/progression, respectively. The cumulative incidences (CI) of local and distant failure were estimated.32 Competing events for local failure included distant failure, second neoplasm or death before local failure. Competing events for distant failure included local failure, second neoplasm or death before distant failure. Patients with simultaneous local and distant failures were considered as having local or distant failure in the respective analysis. Differences in CI were examined using Gray’s test.33
RESULTS
Patient and treatment characteristics (Table 1)
Table 1.
Patient Demographics and Clinical Features (n=59)
| N (%1) | |
|---|---|
| Gender | |
| Male | 39 (66.1) |
| Female | 20 (33.9) |
| Race | |
| Black | 32 (54.2) |
| White | 24 (40.7) |
| Hispanic | 2 (3.4) |
| American Indian | 1 (1.7) |
| Histology | |
| Lymphoepithelioma | 55 (93.2) |
| Non-keratinizing squamous cell carcinoma | 4 (6.8) |
| T stage | |
| T1 | 2 (3.4) |
| T2 | 6 (10.2) |
| T2a | 3 (5.1) |
| T2b | 6 (10.2) |
| T3 | 20 (33.9) |
| T4 | 22 (37.3) |
| N stage | |
| N0 | 5 (8.5) |
| N1 | 7 (11.9) |
| N2 | 27 (45.8) |
| N3 | 2 (3.4) |
| N3a | 12 (20.3) |
| N3b | 6 (10.2) |
| M stage | |
| M0 | 57 (96.6) |
| M1 | 2 (3.4) |
| AJCC combined stage | |
| IIA | 2 (3.4) |
| IIB | 2 (3.4) |
| III | 20 (33.9) |
| IVA | 15 (25.4) |
| IVB | 18 (30.5) |
| IVC | 2 (3.4) |
Percentages may not sum to 100% due to rounding.
Fifty-nine patients were diagnosed with NPC from December 1961 to March 2004. The median age at diagnosis was 14.1 years (range, 6.1–19.7 years). Most patients were male (66.1%). Blacks were significantly over-represented in NPC compared to other malignancies in the same period of time, as Blacks constituted 54.2% of NPC diagnoses, compared to 20.4% for other malignancies (p<0.001).
Lymphoepithelioma (type III NPC) was the most common histology (93.2%), with the remaining patients having the diagnosis of non-keratinizing squamous cell carcinoma. The majority of patients had advanced T stage, regional lymph node metastases and high AJCC stage (Table 1). All patients received RT to the primary tumor at doses of 30–70Gy (median, 55.1Gy). All but 1 patient received RT to nodal sites at doses 30–66.6Gy (median, 53.9Gy). Seven patients did not receive chemotherapy.
Survival and second neoplasms
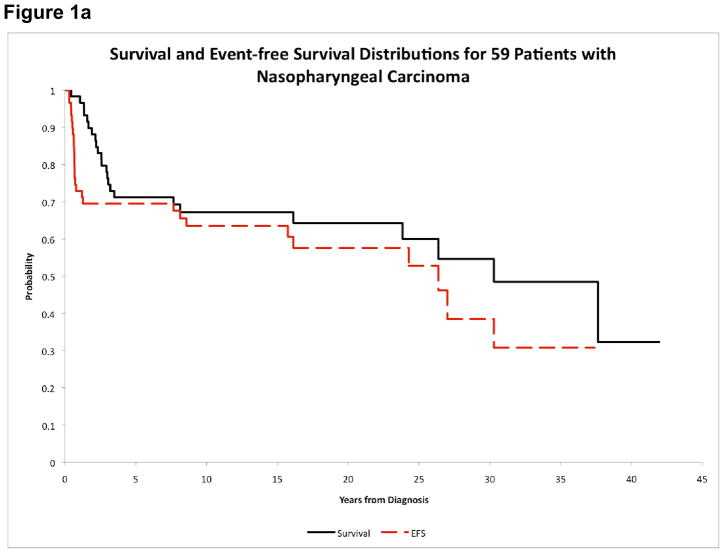
Thirty-five patients (59.3%) were alive with a median follow-up of 16.5 years (range, 4.9–41.9 years). First events included relapsed or progressive disease in 18 patients (3 local, 13 distant, 2 simultaneous local/distant), second neoplasm in 4 patients, and death in 5 patients. Of the 5 patients with death as their first event, 2 died in car accidents and 1 died due to aspiration pneumonia. Causes of death were not available for the other 2 patients. Median time to disease progression and death as first events were 7.6 months (range 3.7–15.3 months) and 16.1 years (range 7.6–30.3 years), respectively. OS estimates at 5 and 15 years were 71.2%±5.9% and 67.2%±7.5%, respectively. The corresponding EFS estimates were 69.5%±6.0% and 63.5%±7.8%, respectively (Figure 1a). Both patients with distant metastatic disease died; one patient with metastases to bone and mediastinum died 19 months from diagnosis, and one patient with metastases to bone and liver died 16 months from diagnosis of NPC. The 15-year CIs of local and distant failure were 8.5%±3.7% and 25.4%±5.7%, respectively. Only 1 of the 18 patients who had relapsed or progressive disease was alive. The 15-year post-relapse survival was 5.6%±3.8%.
Figure 1.
Figure 1a. Overall survival and event-free survival of NPC patients
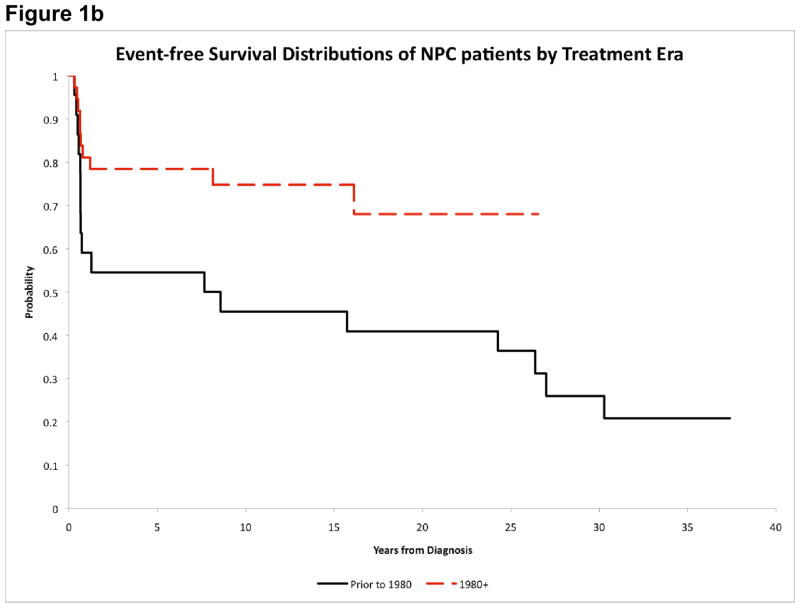
Figure 1b. Event-free survival of NPC patients by treatment era
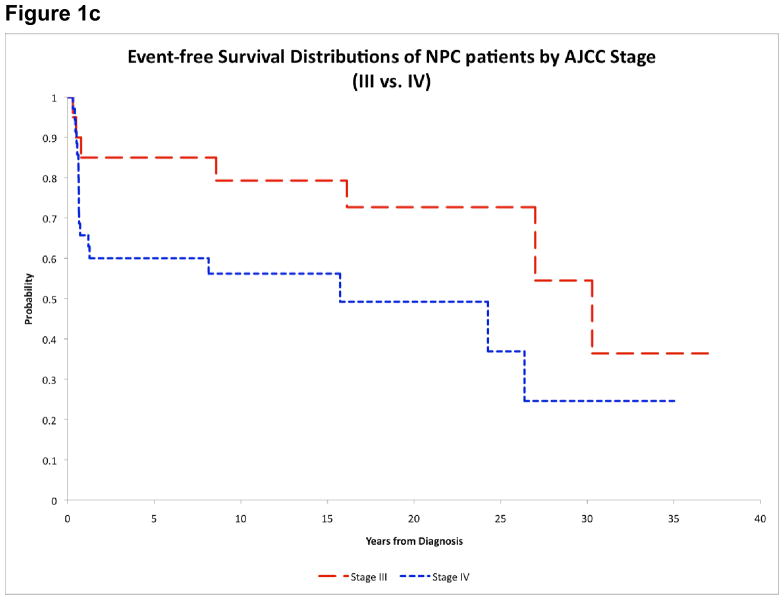
Figure 1c. Event-free survival of NPC patients by AJCC stage
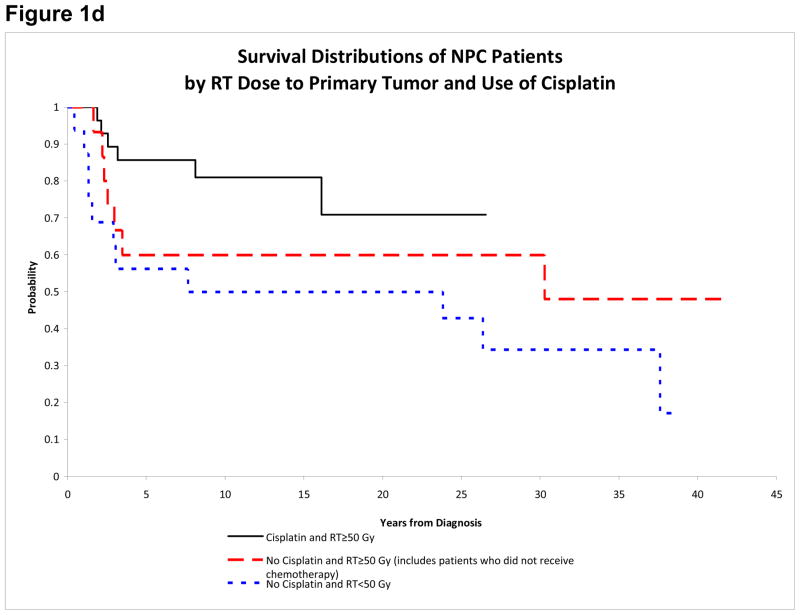
Figure 1d. Survival of NPC patients by RT dose to primary tumor and cisplatin
Five patients (8.5%) (3 Whites, 2 Blacks) developed subsequent neoplasms. One patient had a basal cell carcinoma 27 years after his diagnosis of NPC. Twenty-two months later he developed mucoepidermoid carcinoma in the parotid gland and died 8.8 years later. Another patient with a germline TP53 mutation developed colorectal adenocarcinoma with liver metastasis 8.6 years after his diagnosis of NPC. He developed subsequent malignancies (right maxillary squamous cell carcinoma, esophageal adenocarcinoma, adenocarcinoma of minor salivary gland of lip) and died 15.2 years after the diagnosis of colorectal adenocarcinoma. Three patients were alive 17.7, 22.7, and 8.8 years after diagnosis of a brainstem tumor, thyroid adenoma, and basal cell carcinoma of the neck, respectively. The latency of these second neoplasms was quite long (24.3, 15.7, and 23.4 years).
Predictors of outcome (Table 3)
Table 3.
Potential prognostic factors for overall survival (OS) and event-free survival (EFS) at 15 years for NPC Patients
| Factor | n | OS ± 1 SE (%) | P1 | EFS ± 1 SE (%) | P1 |
|---|---|---|---|---|---|
| Age at Diagnosis | |||||
| ≤14 years | 28 | 71.4 ± 10.2 | 0.525 | 67.9 ± 10.7 | 0.737 |
| >14 years | 31 | 63.7 ± 10.6 | 59.9 ± 11.0 | ||
| Gender | |||||
| Female | 20 | 79.3 ± 10.9 | 0.088 | 79.3 ± 10.9 | 0.101 |
| Male | 39 | 61.2 ± 9.5 | 55.4 ± 9.9 | ||
| Race | |||||
| White | 24 | 58.3 ± 10.9 | 0.130 | 50.0 ± 11.2 | 0.141 |
| Black | 32 | 70.3 ± 9.9 | 70.3 ± 9.9 | ||
| Treatment Era | |||||
| Before 1980 | 22 | 54.5 ± 10.2 | 0.145 | 45.5 ± 10.1 | 0.031 |
| After 1980 | 37 | 74.8 ± 10.0 | 74.8 ± 10.0 | ||
| T Stage | |||||
| T1/T2 | 17 | 76.5 ± 11.2 | 0.772 | 70.1 ± 12.1 | 0.409 |
| T3 | 20 | 70.0 ± 11.1 | 65.0 ± 11.6 | ||
| T4 | 22 | 57.3 ± 16.7 | 57.3 ± 16.7 | ||
| N Stage | |||||
| N0/N1/N2 | 39 | 76.4 ± 8.3 | 0.112 | 70.6 ± 9.0 | 0.103 |
| N3 | 20 | 49.5 ± 13.3 | 49.5 ± 13.3 | ||
| AJCC Stage | |||||
| IIA/IIB | 4 | 50.0 ± 25.0 | 0.1022 | 50.0 ± 25.0 | 0.0492 |
| III | 20 | 90.0 ± 7.1 | 79.3 ± 9.6 | ||
| IVA/IVB/IVC | 35 | 56.2 ± 11.8 | 56.2 ± 11.8 | ||
| Use of Cisplatin | |||||
| Yes3 | 28 | 81.0 ± 10.7 | 0.070 | 81.0 ± 10.7 | 0.013 |
| No4 | 24 | 54.2 ± 9.8 | 45.8 ± 9.7 | ||
| Protocol/Treatment | |||||
| NPC-77 | 18 | 61.1 ± 11.0 | 0.427 | 55.6 ± 11.2 | 0.238 |
| NPC1-like6 | 17 | 94.1 ± 8.1 | 94.1 ± 8.1 | ||
| NPTP-CRT-PF | 6 | 83.3 ± 15.25 | 83.3 ± 15.25 | ||
| NPTP-VBP | 5 | 60.0 ± 19.0 | 60.0 ± 19.0 | ||
| No Protocol | 6 | 50.0 ± 20.4 | 50.0 ± 20.4 | ||
| RT to Primary Tumor | |||||
| RT to primary <50 Gy | 16 | 50.0 ± 11.8 | 0.044 | 43.8 ± 11.6 | 0.048 |
| RT to primary ≥50 Gy | 43 | 73.8 ± 8.9 | 71.4 ± 9.3 | ||
| RT to Nodes | |||||
| RT to nodes <50 Gy | 21 | 47.6 ± 11.5 | 0.018 | 38.1 ± 11.3 | 0.054 |
| RT to nodes ≥50 Gy | 38 | 78.2 ± 8.6 | 78.2 ± 8.6 | ||
| Cisplatin & Primary RT Dose | |||||
| Cisplatin & primary RT ≥50 Gy | 28 | 81.0 ± 10.7 | 0.088 | 81.0 ± 10.7 | 0.042 |
| No cisplatin & primary RT ≥50 Gy7 | 15 | 60.0 ± 13.4 | 53.3 ± 13.8 | ||
| No cisplatin & primary RT <50 Gy | 16 | 50.0 ± 11.8 | 43.8 ± 11.6 |
P-values were obtained using exact log rank tests.
Comparison of AJCC stages III vs. IV.
Includes the following protocols/treatments: 85N2, NPC1 (excluding the 1 patient who received RT only without chemotherapy on NPC1), NPTP-CRT-PF, NPTP-BPM and NPTP-VBP.
Includes the following protocols/treatments: NPC-77, NPTP-CM, NPTP-CMF, NPTP-VC, NPTP-VCM and NPTP-VDC
5-year estimate; data not mature to 15 years.
Includes NPC1 and 85N2 protocols.
Includes 7 patients who had no chemotherapy (but received >50 Gy RT to primary)
Males and White patients appeared to have worse outcome, but the differences were not statistically significant (Table 3). Patients diagnosed after 1980 had improved EFS (15-year estimates: 74.8%±10.0% vs. 45.5%±10.1%, p=0.031) (Figure 1b). There was also a significant difference in EFS between AJCC stage III and IV patients (79.3%±9.6% vs. 56.2%±11.8%, p=0.049) (Figure 1c). Outcome distributions among the 5 major treatment groups were not significantly different (Table 3). However, patients who received cisplatin had improved EFS (81.0%±10.7% vs. 45.8%±9.7%, p=0.013). Nevertheless, the use of cisplatin was highly correlated with treatment era (p<0.001); all patients who received cisplatin were diagnosed after 1980, while 22 of 24 patients who did not receive cisplatin were diagnosed before 1980. Patients who received RT≥50Gy to the primary tumor had improved OS (73.8%±8.9% vs. 50.0%±11.8%, p=0.044) and EFS (71.4%±9.3% vs. 43.8%±11.6%, p=0.048). Similarly, patients who received RT≥50Gy to the nodal sites had better OS (78.2%±8.6% vs. 47.6%±11.5%, p=0.018) and EFS (78.2%±8.6% vs. 38.1%±11.3%, p=0.054). We further investigated the use of cisplatin in conjunction with primary tumor RT dose. Patients who received cisplatin and RT≥50Gy had the highest 15-year OS and EFS (81.0%±10.7%). Patients who did not receive cisplatin but received RT≥50Gy had intermediate OS (60.0%±13.4%) and EFS (53.3%±13.8%). Patients who did not receive cisplatin and received RT<50Gy had the lowest OS (50.0%±11.8%) and EFS (43.8%±11.6%) (Figure 1d).
We did not examine predictors of local failure due to the small number of patients with this event. We did examine potential predictors of distant failure. White patients had significantly higher CI of distant failure compared to Black patients (15-year estimates: 41.7%±10.4% vs. 15.6%±6.5%, p=0.045). Tumor and nodal staging did not correlate with distant failure. However, treatment-related parameters strongly correlated with distant relapse. The CI of distant failure was lower in patients who received cisplatin (14.3%±6.7% vs. 37.5%±10.2%, p=0.047) and patients who received RT≥50Gy to the primary tumor (18.6%±6.0% vs. 43.8%±13.0%, p=0.041) or nodal sites (13.2%±5.6% vs. 47.6%±11.3%, p=0.003). The 15-year CI of distant failure was lowest in patients who received cisplatin and RT≥50Gy (14.3%±6.77%), intermediate in patients who had no cisplatin but received RT≥50Gy (26.7%±11.9%), and highest in patients who had no cisplatin and received RT<50Gy (43.8%±13.0%).
Morbidities
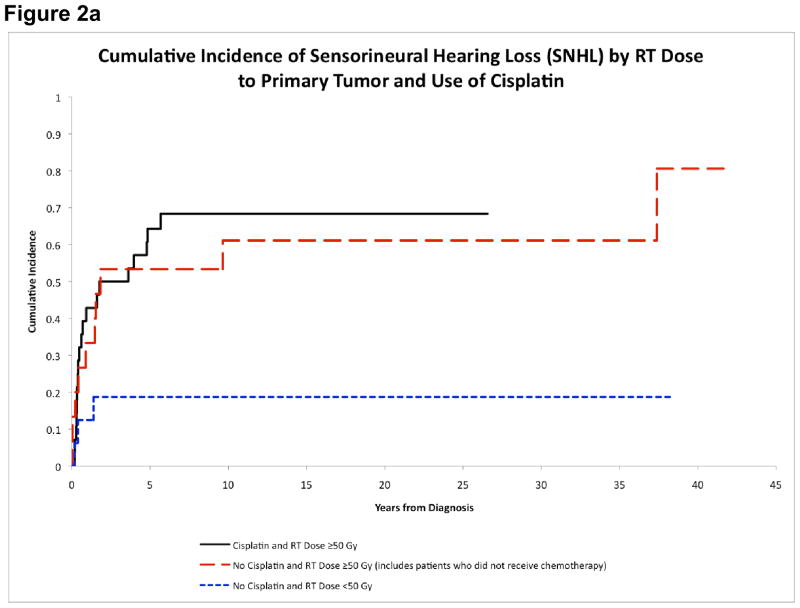
The evaluation of long-term morbidities must be considered in the context of the limitations imposed by the retrospective nature of the study. The CI of various screened morbidities is shown in Table 4. The CI of primary hypothyroidism was much higher in the modern treatment era (after 1980) (15-year estimates: 65.6%±8.3% vs. 4.6%±4.6%, p<0.001), and a similar phenomenon was observed for growth hormone deficiency (GHD) (19.5%±6.8% vs. 4.6%±4.6%, p=0.041), and osteopenia (17.1%±6.6% vs. 0.0%±0.0%, p=0.044). The CI of any degree of sensorineural hearing loss (SNHL) appeared higher in patients who received cisplatin (68.4%±9.3% vs. 37.5%±10.2%, p=0.052). There was also a dose-response relationship between RT dose and development of SNHL or severe SNHL (grade 3 or higher) (Table 5). Patients who received RT≥50Gy had significantly higher CI of SNHL (65.9%±7.6% vs. 18.8%±10.1%, p=0.002); and patients who received RT>65Gy had significantly higher CI of severe SNHL (25.0%±10.0% vs. 2.7%±2.7%, p=0.012). The 15-year CI of SNHL was higher in patients who received cisplatin and RT≥50Gy (68.4%±9.3%), and in patients who did not received cisplatin but received RT≥50Gy (61.1%±13.7%), than in patients who neither received cisplatin nor RT≥50Gy (18.8%±10.1%) (Figure 2a).
Table 4.
Estimates of the 15-Year cumulative incidence (CI) of various morbidities
| No. of patients with morbidity | CI at 15-years ± 1 Standard Error (%) | |
|---|---|---|
| Any morbidity | 49 | 83.7 ± 5.4 |
| Sensorineural hearing Loss | 32 | 52.9 ± 6.7 |
| Primary hypothyroidism | 27 | 42.7 ± 6.6 |
| Trismus | 20 | 32.3 ± 6.2 |
| Chronic/recurrent sinusitis | 12 | 18.9 ± 5.2 |
| Chronic/recurrent otitis media | 10 | 17.6 ± 5.2 |
| Growth hormone deficiency | 10 | 14.1 ± 4.7 |
| LH/FSH deficiency | 10 | 14.4 ± 4.8 |
| Visual impairment | 7 | 10.9 ± 4.3 |
| Osteopenia | 7 | 10.5 ± 4.1 |
| TSH deficiency | 6 | 8.5 ± 3.7 |
| Cranial nerve palsy | 5 | 8.5 ± 3.7 |
| Renal dysfunction | 5 | 8.5 ± 3.7 |
| Pulmonary fibrosis | 5 | 6.8 ± 3.3 |
| ACTH deficiency | 5 | 8.6 ± 3.7 |
| Hyperprolactinemia | 4 | 6.8 ± 3.3 |
| Esophageal stricture | 2 | 3.4 ± 2.4 |
| Osteonecrosis | 1 | 1.7 ± 1.7 |
Table 5.
Proportions of patients with sensorineural hearing loss (SNHL) at different radiotherapy dose to primary tumor
| RT dose (Gy) | N | SNHL (grade 1/2/3) | Severe SNHL (≥ grade 3) |
|---|---|---|---|
| >65 | 20 | 14 (70.0%) | 5 (25.0%) |
| 50–65 | 23 | 15 (65.2%) | 1 (4.3%) |
| <50 | 16 | 3 (18.8%) | 0 (0.0%) |
Figure 2.
Figure 2a. Cumulative incidence of sensorineural hearing loss by RT dose to primary tumor and cisplatin
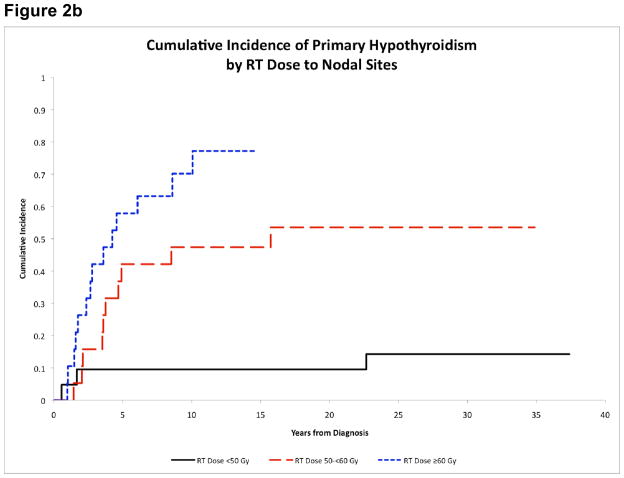
Figure 2b. Cumulative incidence of primary hypothyroidism by RT dose to nodal sites
There was also a dose-response relationship between cervical RT dose and CI of primary hypothyroidism (p<0.001). The highest 15-year CI of hypothyroidism occurred in patients who received RT≥60Gy (77.2%±11.7%), followed by 50–60Gy (47.4%±12.0%) and <50Gy (9.5%±6.6%) (Figure 2b). Patients who received RT≥50Gy to the primary tumor also had higher CI of GHD (19.6%±6.4% vs. 0.0%, p=0.015).
DISCUSSION
Epidemiology
We observed that Blacks were over-represented in our cohort of NPC patients compared to other malignancies. The higher incidence of NPC in Blacks has been described but the underlying reason for this racial disparity is uncertain.34 This is in contrast with other EBV-related malignancies where the incidence of Blacks is the same or lower than Whites.35 The racial disparity in NPC appears to be more pronounced in children compared to adults.34 Taken together, these data suggest that different pathways of EBV tumorigenesis according to ethnicity and age may be involved.
Survival and Event-free Survival
The 5-year OS (71.2±5.9%) and EFS (69.5±6.0%) of our cohort are comparable to previous reports. The more relevant prognostic factors for outcome were related to locoregional stage and treatment administered. Patients with AJCC stage IV had significantly lower EFS. Likewise, the presence of progressive or recurrent disease resulted in dismal outcomes despite salvage treatments. As observed previously, most recurrences were distant rather than loco-regional. 5, 15, 18, 21, 27, 36 An important finding of our study was the association of race with outcome; Black patients tended to have better EFS with significantly lower incidence of distant failure compared to White patients. The reason for the difference was not apparent and requires further investigation.
Long-term outcome of childhood NPC has been seldom reported; the 10-year survival has been reported to be 36–58%.1, 14, 20, 21 Uzel reported a 15-year survival of 62.4% in 32 patients with a median follow-up of 8.9 years.16 We estimated the OS and EFS to be 67.2±7.5% and 63.5±7.8%, and 64.3±8.8% and 57.6±9.4%, respectively, at 15 and 20 years. A longer follow-up will be required to document whether the disease-related outcome estimates continue to decrease. EFS estimates increased from 46% to 75% after 1980. Improvements in radiation planning and delivery, chemotherapy, and supportive care likely contributed to this trend. Higher RT doses were used in the recent era, and RT≥50Gy to primary tumor or nodal sites was associated with lower distant failure and better EFS. Similar association between RT dose and survival has been previously reported.5, 13, 14, 21, 23 The switch from cyclophosphamide-based to cisplatin-based chemotherapy in the most recent era might also contribute to improved outcome; patients who received cisplatin had lower incidence of distant failure and better EFS, consistent with the recent report by Kupeli.20 We further demonstrated that cisplatin and higher RT doses might have additive or synergistic beneficial effect; patients treated with cisplatin and RT≥50Gy had superior EFS compared to patients treated without cisplatin and those treated without cisplatin and RT<50Gy had the worst outcome. Data from adult NPC studies suggest that concurrent cisplatin and RT is superior to RT alone.4, 37 Likewise, there appears to be an added benefit to the incorporation of neoadjuvant chemotherapy.4 Whether the same benefit of concurrent chemo-radiotherapy applies to children is currently uncertain; this issue is currently being studied by the ongoing COG ARAR331 trial.
Morbidities
The incidence of long-term morbidities in survivors of childhood NPC has not been well reported. With a median follow-up of 16.5 years and up to almost 42 years, we attempted to estimate the CI of long-term morbidities. Most morbidities started early, within 5 years from diagnosis. Some complications tended to cluster within the first 2 years, including trismus, chronic or recurrent sinusitis or otitis media, and pulmonary fibrosis. In contrast, the frequency of some endocrinopathies, such as growth hormone deficiency, gonadotropin deficiency and primary hypothyroidism, increased with time and some cases were diagnosed after 30 years.
Xerostomia and dental problems, well-known unavoidable adverse effects of RT to the salivary glands, occurred in most patients, consistent with previous reports.6, 15–17, 19 Intensity modulated RT (IMRT) can potentially ameliorate mucosal damage and xerostomia in NPC patients.38, 39 The administration of amifostine may also be beneficial in patients receiving radiotherapy to the head and neck area. In a report by Brizel,40 adult patients with head and neck cancer receiving radiotherapy were randomized to receive amifostine, given daily before radiotherapy. Moderate to severe chronic xerostomia was significantly less prevalent with the use of amifostine. Importantly, the long-term outcome was similar for both groups of patients, demonstrating that the use of amifostine does not influence the antitumor efficacy of the treatment.40 Nevertheless, there is significant controversy on the role of this radioprotectant in patients receiving treatment for NPC.41 The currently open COG ARAR0331 is exploring this question.
SNHL is a debilitating complication of cisplatin and radiotherapy to the head and neck region. However, there is no data documenting the CI of SNHL in childhood NPC. SNHL affected over half of the patients by 15 years. Most patients had grade 2 SNHL, but in nearly one-fifth of patients SNHL was ≥ grade 3 requiring hearing aids. We noted a higher incidence of SNHL in patients treated with cisplatin and RT≥50Gy, and a dose-response relationship with RT doses. Whether concurrent chemo-radiotherapy increases the risk of SNHL remains to be known.
We observed a relatively high incidence of primary hypothyroidism. Hypopituitarism was also common, especially growth hormone and gonadotropin deficiencies. The endocrinopathies were probably secondary to RT, which was supported by dose-response relationships between RT dose and primary hypothyroidism and GHD. However, the observation might represent detection bias as we performed screening of endocrinopathies more stringently in the more recent era. It has been suggested that reduction of RT dose based on the response to neoadjuvant chemotherapy may not compromise survival.42 Therefore, response-adapted RT is worth further evaluation to minimize endocrine morbidities. Of note, four of the six patients treated with IMRT developed neuroendocrine deficits.
Pulmonary fibrosis occurred in 5 patients in our cohort, probably secondary to bleomycin. Two patients died subsequently and the pulmonary fibrosis was a contributory cause of death. One patient had symptomatic restrictive lung disease and two patients were asymptomatic. Visual impairment occurred infrequently, was usually mild, and mostly secondary to radiation-induced cataracts, retinopathy or optic atrophy. Although not major health issues, these problems may compromise the quality of life of the affected patients. Osteopenia was observed in some patients as we started screening for bone density with DEXA scan and quantitative computed tomography recently. Therefore, the incidence of osteopenia is likely underestimated.
The current study is one of the largest studies in childhood NPC and adds important information regarding treatment and outcomes. A limitation of our study is its retrospective nature. Monitoring for long-term morbidities was heterogeneous and the incidence of various morbidities was likely underestimated. Also, since patients were treated on different regimens over a 40-year period, it is difficult to determine whether differences in outcome were truly related to treatment or to changes over time.
In conclusion, survival of children with NPC has improved over the past 4 decades with improved radiotherapy and chemotherapy. However, life expectancy is reduced by second neoplasms and quality of life may be impaired by long-term morbidities. Better treatments are needed to improve the cure of advanced or recurrent disease and to reduce long-term morbidities. New chemotherapeutic agents, such as taxanes, along with improved radiation techniques, as well as the use of EBV-directed cytotoxic T lymphocytes will likely provide further advance in the management of these patients, while more efforts will have to be placed in minimizing acute and late effects, with the incorporation of chemo- and radiation-protectants and imaging-guided and risk-adapted radiation.
Acknowledgments
Supported by Grants P30-CA23099 and CA21765, and the American Lebanese Associated Charities (ALSAC).
References
- 1.Ayan I, Altun M. Nasopharyngeal carcinoma in children: retrospective review of 50 patients. Int J Radiat Oncol Biol Phys. 1996;35(3):485–92. doi: 10.1016/s0360-3016(96)80010-1. [DOI] [PubMed] [Google Scholar]
- 2.Ayan I, Kaytan E, Ayan N. Childhood nasopharyngeal carcinoma: from biology to treatment. Lancet Oncol. 2003;4(1):13–21. doi: 10.1016/s1470-2045(03)00956-2. [DOI] [PubMed] [Google Scholar]
- 3.Vokes EE, Liebowitz DN, Weichselbaum RR. Nasopharyngeal carcinoma. Lancet. 1997;350(9084):1087–91. doi: 10.1016/S0140-6736(97)07269-3. [DOI] [PubMed] [Google Scholar]
- 4.Langendijk JA, Leemans CR, Buter J, Berkhof J, Slotman BJ. The additional value of chemotherapy to radiotherapy in locally advanced nasopharyngeal carcinoma: a meta-analysis of the published literature. J Clin Oncol. 2004;22(22):4604–12. doi: 10.1200/JCO.2004.10.074. [DOI] [PubMed] [Google Scholar]
- 5.Pao WJ, Hustu HO, Douglass EC, Beckford NS, Kun LE. Pediatric nasopharyngeal carcinoma: long term follow-up of 29 patients. Int J Radiat Oncol Biol Phys. 1989;17(2):299–305. doi: 10.1016/0360-3016(89)90443-4. [DOI] [PubMed] [Google Scholar]
- 6.Ghim TT, Briones M, Mason P, Crocker I, Davis P, Bell B, et al. Effective adjuvant chemotherapy for advanced nasopharyngeal carcinoma in children: a final update of a long-term prospective study in a single institution. J Pediatr Hematol Oncol. 1998;20(2):131–5. doi: 10.1097/00043426-199803000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Lobo-Sanahuja F, Garcia I, Carranza A, Camacho A. Treatment and outcome of undifferentiated carcinoma of the nasopharynx in childhood: a 13-year experience. Med Pediatr Oncol. 1986;14(1):6–11. doi: 10.1002/mpo.2950140103. [DOI] [PubMed] [Google Scholar]
- 8.Roper HP, Essex-Cater A, Marsden HB, Dixon PF, Campbell RH. Nasopharyngeal carcinoma in children. Pediatr Hematol Oncol. 1986;3(2):143–52. doi: 10.3109/08880018609031210. [DOI] [PubMed] [Google Scholar]
- 9.Gasparini M, Lombardi F, Rottoli L, Ballerini E, Morandi F. Combined radiotherapy and chemotherapy in stage T3 and T4 nasopharyngeal carcinoma in children. J Clin Oncol. 1988;6(3):491–4. doi: 10.1200/JCO.1988.6.3.491. [DOI] [PubMed] [Google Scholar]
- 10.Lombardi F, Gasparini M, Gianni C, De Marie M, Molinari R, Pilotti S. Nasopharyngeal carcinoma in childhood. Med Pediatr Oncol. 1982;10(3):243–50. doi: 10.1002/mpo.2950100304. [DOI] [PubMed] [Google Scholar]
- 11.Ayan I, Meral R, Kebudi R, Ayan N, Gorgun O, Darendeliler E, et al. Efficacy of cisplatinum based chemotherapy in childhood nasopharyngeal carcinoma. Med Pediatr Oncol. 2000;35:277. [Google Scholar]
- 12.Mertens R, Granzen B, Lassay L, Gademann G, Hess CF, Heimann G. Nasopharyngeal carcinoma in childhood and adolescence: concept and preliminary results of the cooperative GPOH study NPC-91. Gesellschaft fur Padiatrische Onkologie und Hamatologie. Cancer. 1997;80(5):951–9. [PubMed] [Google Scholar]
- 13.Serin M, Erkal HS, Elhan AH, Cakmak A. Nasopharyngeal carcinoma in childhood and adolescence. Med Pediatr Oncol. 1998;31(6):498–505. doi: 10.1002/(sici)1096-911x(199812)31:6<498::aid-mpo6>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 14.Wolden SL, Steinherz PG, Kraus DH, Zelefsky MJ, Pfister DG, Wollner N. Improved long-term survival with combined modality therapy for pediatric nasopharynx cancer. Int J Radiat Oncol Biol Phys. 2000;46(4):859–64. doi: 10.1016/s0360-3016(99)00493-9. [DOI] [PubMed] [Google Scholar]
- 15.Sahraoui S, Acharki A, Benider A, Bouras N, Kahlain A. Nasopharyngeal carcinoma in children under 15 years of age: a retrospective review of 65 patients. Ann Oncol. 1999;10(12):1499–502. doi: 10.1023/a:1008325925164. [DOI] [PubMed] [Google Scholar]
- 16.Uzel O, Yoruk SO, Sahinler I, Turkan S, Okkan S. Nasopharyngeal carcinoma in childhood: long-term results of 32 patients. Radiother Oncol. 2001;58(2):137–41. doi: 10.1016/s0167-8140(00)00310-8. [DOI] [PubMed] [Google Scholar]
- 17.Zubizarreta PA, D’Antonio G, Raslawski E, Gallo G, Preciado MV, Casak SJ, et al. Nasopharyngeal carcinoma in childhood and adolescence: a single-institution experience with combined therapy. Cancer. 2000;89(3):690–5. [PubMed] [Google Scholar]
- 18.Berberoglu S, Ilhan I, Cetindag F, Sunter O. Nasopharyngeal carcinoma in Turkish children: review of 33 cases. Pediatr Hematol Oncol. 2001;18(5):309–15. doi: 10.1080/088800101300312573. [DOI] [PubMed] [Google Scholar]
- 19.Selek U, Ozyar E, Ozyigit G, Varan A, Buyukpamukcu M, Atahan IL. Treatment results of 59 young patients with nasopharyngeal carcinoma. Int J Pediatr Otorhinolaryngol. 2005;69(2):201–7. doi: 10.1016/j.ijporl.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Kupeli S, Varan A, Ozyar E, Atahan IL, Yalcin B, Kutluk T, et al. Treatment results of 84 patients with nasopharyngeal carcinoma in childhood. Pediatr Blood Cancer. 2006;46(4):454–8. doi: 10.1002/pbc.20433. [DOI] [PubMed] [Google Scholar]
- 21.Ingersoll L, Woo SY, Donaldson S, Giesler J, Maor MH, Goffinet D, et al. Nasopharyngeal carcinoma in the young: a combined M.D. Anderson and Stanford experience. Int J Radiat Oncol Biol Phys. 1990;19(4):881–7. doi: 10.1016/0360-3016(90)90008-8. [DOI] [PubMed] [Google Scholar]
- 22.Cao KJ, Li Y, Xie GF, Hong MH. Prognostic factors in nasopharyngeal carcinoma in childhood and adolescence. Zhonghua Zhong Liu Za Zhi. 2006;28(2):134–7. [PubMed] [Google Scholar]
- 23.Cao KJ, Li Y, Huang PY, Xie GF, Huang TB, Hong MH. Long-term efficacy of radiotherapy on children with nasopharyngeal carcinoma. Ai Zheng. 2004;23(11):1322–4. [PubMed] [Google Scholar]
- 24.Bakkal BH, Kaya B, Berberoglu S, Aksu G, Sayin MY, Altundag MB, et al. The efficiency of different chemoradiotherapy regimens in patients with paediatric nasopharynx cancer: review of 46 cases. Int J Clin Pract. 2007;61(1):52–61. doi: 10.1111/j.1742-1241.2006.00872.x. [DOI] [PubMed] [Google Scholar]
- 25.Sheen JM, Yu HR, Huang EY, Wu KS, Wei SH, Hsiao CC. Nasopharyngeal carcinoma in children: a single institution’s experience. Acta Paediatr Taiwan. 2005;46(5):268–71. [PubMed] [Google Scholar]
- 26.Sham JS, Poon YF, Wei WI, Choy D. Nasopharyngeal carcinoma in young patients. Cancer. 1990;65(11):2606–10. doi: 10.1002/1097-0142(19900601)65:11<2606::aid-cncr2820651135>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 27.Daoud J, Toumi N, Bouaziz M, Ghorbel A, Jlidi R, Drira MM, et al. Nasopharyngeal carcinoma in childhood and adolescence: analysis of a series of 32 patients treated with combined chemotherapy and radiotherapy. Eur J Cancer. 2003;39(16):2349–54. doi: 10.1016/s0959-8049(03)00512-4. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura RA, Novaes PE, Antoneli CB, Fogaroli RC, Pellizzon AC, Ferrigno R, et al. High-dose-rate brachytherapy as part of a multidisciplinary treatment of nasopharyngeal lymphoepithelioma in childhood. Cancer. 2005;104(3):525–31. doi: 10.1002/cncr.21207. [DOI] [PubMed] [Google Scholar]
- 29.Haimi M, Arush MW, Bar-Sela G, Gez E, Bernstein Z, Postovsky S, et al. Nasopharyngeal carcinoma in the pediatric age group: the northern Israel (Rambam) medical center experience, 1989–2004. J Pediatr Hematol Oncol. 2005;27(10):510–6. doi: 10.1097/01.mph.0000183271.22947.64. [DOI] [PubMed] [Google Scholar]
- 30.Green FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, et al. AJCC Cancer Staging Manual. 6. Philadelphia: Lippincott-Raven; 2002. [Google Scholar]
- 31.Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. New York: Wiley; 1980. p. 169. [Google Scholar]
- 33.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141–54. [Google Scholar]
- 34.Richey LM, Olshan AF, George J, Shores CG, Zanation AM, Cannon T, et al. Incidence and survival rates for young blacks with nasopharyngeal carcinoma in the United States. Arch Otolaryngol Head Neck Surg. 2006;132(10):1035–40. doi: 10.1001/archotol.132.10.1035. [DOI] [PubMed] [Google Scholar]
- 35.Herrinton LJ. Epidemiology of the Revised European-American Lymphoma Classification subtypes. Epidemiol Rev. 1998;20(2):187–203. doi: 10.1093/oxfordjournals.epirev.a017980. [DOI] [PubMed] [Google Scholar]
- 36.Lim LH, Goh CH, Loong SL, Khin LW, Balakrishnan A, Wee J. Nasopharyngeal carcinoma in young patients. Int J Clin Pract. 2003;57(10):871–4. [PubMed] [Google Scholar]
- 37.Al-Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, Vuong T, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16(4):1310–7. doi: 10.1200/JCO.1998.16.4.1310. [DOI] [PubMed] [Google Scholar]
- 38.Kwong DL, Pow EH, Sham JS, McMillan AS, Leung LH, Leung WK, et al. Intensity-modulated radiotherapy for early-stage nasopharyngeal carcinoma: a prospective study on disease control and preservation of salivary function. Cancer. 2004;101(7):1584–93. doi: 10.1002/cncr.20552. [DOI] [PubMed] [Google Scholar]
- 39.Laskar S, Bahl G, Muckaden M, Pai SK, Gupta T, Banavali S, et al. Nasopharyngeal carcinoma in children: comparison of conventional and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72(3):728–36. doi: 10.1016/j.ijrobp.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 40.Brizel DM, Wasserman TH, Henke M, Strnad V, Rudat V, Monnier A, et al. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol. 2000;18(19):3339–45. doi: 10.1200/JCO.2000.18.19.3339. [DOI] [PubMed] [Google Scholar]
- 41.Brizel DM, Overgaard J. Does amifostine have a role in chemoradiation treatment? Lancet Oncol. 2003;4(6):378–81. doi: 10.1016/s1470-2045(03)01132-x. [DOI] [PubMed] [Google Scholar]
- 42.Orbach D, Brisse H, Helfre S, Klijanienko J, Bours D, Mosseri V, et al. Radiation and chemotherapy combination for nasopharyngeal carcinoma in children: Radiotherapy dose adaptation after chemotherapy response to minimize late effects. Pediatr Blood Cancer. 2008;50(4):849–53. doi: 10.1002/pbc.21372. [DOI] [PubMed] [Google Scholar]
- 43.Hui EP, Ma BB, Leung SF, King AD, Mo F, Karn MK, et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol. 2009;27(2):242–9. doi: 10.1200/JCO.2008.18.1545. [DOI] [PubMed] [Google Scholar]
- 44.Straathof KCM, Bollard CM, Popat U, Huls H, Lopez T, Morriss MC, et al. Treatment of nasopharyngeal carcinoma with Epstein-Barr virus-specific T lymphocytes. Blood. 2005;105(5):1898–1904. doi: 10.1182/blood-2004-07-2975. [DOI] [PubMed] [Google Scholar]