Abstract
Objective
The aim of the current study is to determine whether pharmacotherapy normalizes cognitive circuitry function supporting voluntary behavioral inhibition in adolescent bipolar disorder (ABD).
Method
Healthy controls (HC) and unmedicated patients with ABD in manic, mixed or hypomanic episodes are matched on demographics and IQ (n=13 per group; Mean age=14.4 ±2.4 years). fMRI studies are performed at baseline and after 14 weeks, during which time patients with ABD are treated initially with second generation antipsychotics (SGAs) followed by lamotrigine monotherapy. A Response Inhibition Task is administered in which a planned motor response, already on the way’ to execution, has to be voluntarily inhibited on the trials where a stop signal is presented at varying delays between the cue to respond and response initiation. There are six blocks, each with a predominant rate of “Go” or “Stop” trials and separated by a 10 sec rest/fixation period.
Results
All patients showed significant improvement in both the manic and depressive symptoms from baseline (p<.001). Behavioral data showed that accuracy improved over 14 weeks in patients and HC. Significant time by group interaction effects [F(1, 24)=5.34, p<.03] for the difference between Stop vs. Go blocks showed greater increases of activation in prefrontal (left inferior and middle frontal gyri and medial frontal gyrus bilaterally) and temporal (left superior temporal gyrus and right middle temporal gyrus) regions, and greater decreases in activation in right putamen and bilateral thalamus at follow-up in the ABD group. Increased ventrolateral prefrontal cortex function was related to clinical treatment response.
Conclusions
Treatment with SGAs followed by lamotrigine monotherapy enhances prefrontal and temporal lobe activity during a Response Inhibition Task demonstrating the reversal of disorder-relevant neural circuitry dysfunction in patients with ABD. Patients are not slowed down in performance with this treatment regime.
Keywords: frontal, striatum, bipolar, child, fMRI, cognition
In adolescent bipolar disorder (ABD), inattention, impulsivity and behavioral dis-inhibition are prominent and persist even after achieving mood stability.4 The frontostriatal circuitry that supports motor response inhibition has been shown to be affected in ABD.5 Appropriate goals of treatment, therefore, are to aim for mood stabilization, reducing motor response inhibition problems, and reversing the related frontostriatal deficits in ABD. Lamotrigine is one such medication that is used to stabilize mood in ABD6 and adult bipolar disorder (BD)7;8 due to its glutamatergic attenuating function that is believed to have the potential to improve cognitive function related to motor response inhibition problems.9–11
There is preliminary evidence from two fMRI studies indicating that lamotrigine may enhance brain circuitry function in bipolar disorder.6;12 In a study of patients with ABD performing an affective task requiring the rating of emotions in pictures, reduction in depressive symptoms was correlated with decreased right amygdala activation over an 8-week period.6 In an adult study of euthymic bipolar patients performing an “N back” working memory task, greater activation in the left medial prefrontal cortex (MPFC) and bilateral pregenual anterior cingulate cortex (ACC) was observed after 6 weeks of treatment with lamotrigine.12 Methodological limitations in these studies include a short treatment period and lack of demonstration of greater improvement in brain function after treatment in patients relative to matched healthy controls (HC) retested over a similar time period. This control group is essential for interpreting change over time as representing a drug treatment effect rather than a benefit from practice effects with the task or the scanning experience. Further, meta-analysis of studies of response inhibition in HC has demonstrated increased activity in the right prefrontal cortex.13 The task of response inhibition is complex and multifactorial with attention, perceptual discrimination, and motor executive control. Response suppression involves a situation that is highly stereotyped, requiring repetitive responses with little deliberation, except when an unusual event occurs in the midst of this routine task with clear stimulus-response requirements. This effort is shown to deploy dorsal, ventral, and medial PFC regions in HC.13–15 It was also shown that in ABD poor performance on voluntary motor response inhibition tasks is related to the high degree of impulsivity and inattention5 with underlying frontostriatal disturbances.5;16;17 Patients with adult BD, relative to HC, have also shown decreased activation in the orbitofrontal cortex during response suppression.18 Therefore, the primary aim of the current study is to evaluate the differences between ABD and HC in the domain of neurobehavioral deficits in frontostriatal circuitry that support the ability to voluntarily suppress behavioral responses. Our aim is to see if pharmacotherapy can reverse the frontostriatal circuitry dysfunction in ABD. Our pharmacotherapy consists of second generation antipsychotics (SGAs) for acute mania followed by lamotrigine for continued mood stabilization. We did not use SGAs for maintenance treatment due to high risk for metabolic side effects if prescribed for long-term treatment,19 in addition to our interest in determining the beneficiary effects of lamotrigine for optimal symptomatic recovery and cognitive function. We designed a Response Inhibition Task to probe the neural circuitry supporting “stopping process and motor inhibition.” We administered this task at baseline and after 14 weeks to HC and to ABD patients, who were acutely ill and unmedicated at baseline and at 14 weeks after mood stabilization with pharmacotherapy. The concept underlying this task is to compare the process of motor response inhibition to motor execution in ABD patients compared to HC, rather than examining response inhibition in the context of a pre-potent tendency to respond.20 This is an important first step in clarifying the basic neural circuits for motor inhibition versus execution in these patients. To this end, we contrasted blocks of trials that mainly required motor inhibition (Stop trials) to blocks of trials that mainly required motor action (Go trials). We predicted that there would be differences in prefrontal activation between the ABD subjects and HC at baseline. We hypothesized that lamotrigine, by virtue of pharmacological effects on prefrontal and striatal systems, would reverse the dysfunction in these regions that are believed to contribute to behavioral control deficits in ABD.
METHODS
This was a prospective outpatient open-label trial of SGAs for acute mania/hypomania followed by lamotrigine monotherapy for 13 adolescents with bipolar disorder type I (n=8) and II (n=5). The fMRI Response Inhibition Task was administered at baseline and at the end of 14 weeks, and the BOLD signal activation based on this paradigm was used as the primary outcome. The total duration of the trial was 14 weeks. All patients received an initial 4 weeks of prospective treatment with SGAs and they were discontinued in weeks 4–6. During the 14 weeks of the trial, lamotrigine was prospectively titrated up alongside SGAs over the first 8 weeks, followed by 6 weeks of full dose treatment with lamotrigine alone. The SGAs served the role of rescue medication for acute symptoms of mania while lamotrigine was still being titrated up to full dose. IQ and demographically matched HC and patients with ABD (n=13 per group; Mean age=14.4 ±2.4 years) had fMRI studies performed at baseline and again at 14 weeks. HC did not receive treatment, but were retested to control for potential changes at retest due to familiarity with the behavioral paradigm or MRI scanning procedure. This study was approved by the University of Illinois at Chicago (UIC) Institutional Review Board. Parents gave written consent and children gave assent to participate in this trial.
Inclusion criteria for patients with ABD were: a DSM- IV21 diagnosis of Bipolar Disorder Type, mixed or manic episode or hypomanic episode, 10 to 18 years of age, and a baseline score of >12 on the Young Mania Rating Scale (YMRS).22 Patients were already medication free, not requiring a wash-out at study entry, or were sufficiently unstable on prior medications to justify discontinuation of an ineffective treatment prior to beginning treatment with lamotrigine with the consent of parents and assent of patients. The washout period consisted of tapering previous medications over one week prior to study entry except for those who received aripiprazole who required a 4-week washout period. All patients were medication free for at least 4–7 days prior to scanning. None of the patients were on fluoxetine, which would have required a longer washout period. Inclusion criteria for HC were: not meeting DSM-IV criteria for an Axis I disorder, not having a family history of affective illness, 10 to 18 years of age, and a baseline score of <12 on the YMRS. The ABD group and the HC group did not differ significantly in age (14.4 ±2.4 years), gender, race, parental socio-economic status, IQ, or word reading ability (Table 1). Exclusion criteria for all subjects included: active substance abuse; comorbid psychiatric diagnosis requiring pharmacotherapy including ADHD; serious medical problems; previous exposure to lamotrigine; IQ<80; and contraindications to MRI studies including metallic implants, retractors or braces and claustrophobia. IQ was estimated using the Wechsler Abbreviated Scale of Intelligence (WASI).23
Table 1.
Demographic Variables and Clinical Characteristics
| HC | ABD | Analysis | |
|---|---|---|---|
| Mean (SD/%) | Mean (SD/%) | p value | |
| Variables | |||
| Age (years) | 14.4 (2.8) | 14.4 (2.2) | P=.97 |
| Gender | P=.99 | ||
| Male | 4 (30%) | 10 (77%) | |
| Female | 9 (70%) | 3 (23%) | |
| Race | P=.71 | ||
| Caucasian | 7 (54%) | 8 (62%) | |
| Other | 6 (66%) | 5 (38%) | |
| WASI- IQ * | 109.7 (4.8) | 106.2 (6.8) | P=.09 |
| WRAT-3, Reading Subtest † | 45 (3.7) | 42.6 (5.5) | P=.10 |
| Socioeconomic Status** | 2.5 (.52) | 2 (1) | P=.27 |
| Pre YMRS | 1.2 (1.6) | 17.1 (6.4) | p=.00002 |
| Post YMRS | 1.5 (2.1) | 4.77 (6.9) | p=.11 |
| Pre CDRS-R | 19.5 (2.4) | 52.12(11.5) | p=.00001 |
| Post CDRS-R | 19.08 (1.6) | 25.4 (6.6) | P=.003 |
WASI IQ = Wechsler Abbreviated Scale of Intelligence, Intelligence Quotient (Matrix Reasoning and Vocabulary Subtests);
Wide Range Achievement Test – Third Edition (WRAT-3);
Mean revised Hollingshead socio-economic status; ABD= Adolescent Bipolar Disorder; HC = Healthy Control; YMRS = Young Mania Rating Scale; CDRS-R=Child Depression Rating Scale-Revised; Ns: Not significant.
Assessment Procedures
A board certified child psychiatrist (MNP) completed the Washington University Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS)24 with all subjects. Subsequently, all available clinical information was reviewed to make a consensus clinical diagnosis. Live diagnostic interviews of ten cases were independently coded by two researchers to establish inter-rater diagnostic reliability, which by Cohen’s Kappa was 0.94.25 The primary clinical treatment efficacy measures were the YMRS and the Child Depression Rating Scale-Revised(CDRS-R).26
SGAs During the First Four Weeks
Manic and hypomanic symptoms were treated in the acute phase of illness by using SGAs given that we recruited acutely ill unmedicated patients to this study and it takes at least 8 weeks to titrate up the dose of lamotrigine. The order of preference for SGAs given for the first 4 weeks of acute illness was risperidone, aripiprazole, quetiapine, and ziprasidone. The order was modified according to reported previous ill effects of any SGA. For example, if a patient did not respond to risperidone and was agitated on aripiprazole they received quetiapine. The SGA was slowly withdrawn over 2–4 weeks as tolerated (i.e., between the 4th to 8th week period). An overall guideline for withdrawal of SGAs was followed with reduction at .25 mg of risperidone; 2.5–5 mg of aripiprazole; 25–50 mg of seroquel; or 20–40 mg of ziprasidone—every other day until they were off of the SGA. Benzotropine was allowed on as-needed basis for extrapyramidal symptoms if on SGAs, but only during the first 4 week period.
Lamotrigine Dosing Over 14 weeks
Lamotrigine starting dose was 12.5 mg during the first week. It was increased at 12.5 mg per week for the first 4 weeks, 25 mg per week for the next 2 weeks, and titrated to 200 mg by 8 weeks. All patients remained on this fixed dose of 200 mg for the last 6 weeks of treatment prior to being scanned at week 14.
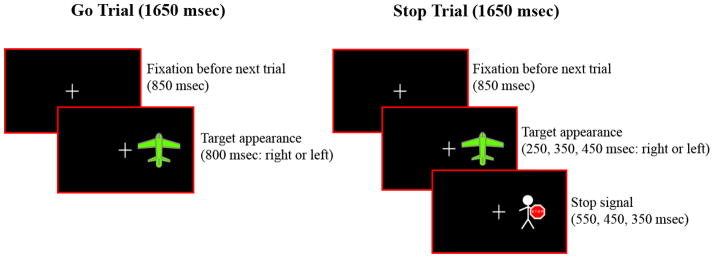
Response Inhibition Task
The fMRI behavioral paradigm was a block design task in which a motor response, already on the way’ from planning to execution, had to be voluntarily inhibited when a cue instructing subjects to stop an impending response was presented on some trials (Figure 1). Prior to the fMRI scanning session, subjects were trained to perform the task in a mock scanner. At the beginning of each trial a fixation cross appeared for 850 ms. On Go trials, a target stimulus (a green airplane) was presented for 800 ms with equal probability of being either to the left or right of a center crosshair. Subjects pressed a button with their right hand if the green plane appeared on the right side of the screen, or with their left hand if the plane appeared on the left. On Stop trials, a Stop signal (a man holding a Stop signal in his hands) replaced the airplane with equal probability 250, 350, or 450 ms after the airplane appeared and subjects had to inhibit their response. Button press response latencies in paradigms of this nature are approximately 650 ms in pediatric studies which led us to choose the 250–450 ms range of stop signal delays.27 Varying the Stop signal delays also ensured that subjects paid attention on each trial and did not habituate or learn fixed trial conditions. The task lasted 6.11 minutes and consisted of six experimental blocks, three of which were Go blocks (G) and three of which were Stop blocks (S), and there were7 resting blocks (F) of 10 sec fixation each. Each experimental block had 30 trials and lasted 49.5 sec. The experimental and fixation blocks were pseudo-randomly interspersed as follows: (F) G (F) S (F) S (F) G (F) S (F) G (F). In Go blocks (G), 70% of the trials were Go trials, and 30% were Stop trials. Conversely, in Stop blocks (S) 70% of the trials were Stop trials and 30% were Go trials. We adopted this 70/30 proportion of trials in Go and Stop blocks so that subjects would not habituate to fixed trial presentation within a certain block. Within each block, Go and Stop trials were pseudo-randomly presented.
Figure 1. Stop and Go Trials in Response Inhibition Task.
Appearance of airplane must be followed by immediate response in a Go trial; and must stop from responding when airplane is replaced by man with the stop sign’ in a Stop trial.
MRI Protocol
MRI studies were performed using a 3.0 Tesla whole body scanner (Signa, General Electric Medical System, Milwaukee, WI). Functional images were acquired using echo-planar imaging which is sensitive to regional alterations in blood flow via blood oxygenation level dependent (BOLD) contrast effects. Twenty-five axial slices were acquired. Parameters for functional scans were: TE=25ms; flip angle=90°; field of view=20×20cm2; acquisition matrix=64×64; TR=2.5s; 5mm slice thickness with 1mm gap. Anatomical images were acquired in the axial plane (three-dimensional spoiled gradient recalled [SPGR], 1.5mm thick contiguous axial slices) to coregister and normalize the functional data.
Image Processing and Data Analysis
We conducted whole brain analyses. For functional imaging data, FIASCO software (Functional Imaging Analysis Software-Computational Olio)28 was used to implement 3D motion estimation and correction, and to remove slow signal drift. Individual volumes were excluded from analysis if, relative to median head position, head displacement was greater than 1.5mm or head rotation was greater than 0.5 degrees. The number of volumes retained after discarding those with motion artifact did not significantly differ across groups. To evaluate subject-wise activation effects for statistical analyses, voxel-wise effect size (r) maps were calculated for each subject by contrasting activation for Stop and Go blocks, Go and Fixation Blocks, Stop and Fixation blocks. For the purposes of this paper, our analyses focused on the Stop vs. Go contrast, to examine the impact of Stop vs. Go Signal processing. A Fisher z transform was applied to the r values so they would more closely approximate a normal distribution (zr).29 Subjects’ zr-maps (effect size) and SPGR anatomical images were warped into Talairach space using AFNI’s (Analysis of Functional NeuroImages) automated procedure.30 Functional maps were re-sampled to an isotropic 3×3×3 mm grid to provide a voxel dimension similar to that of the in-plane resolution of the acquired data prior to statistical analysis.
The primary analysis of the fMRI data was a whole-brain, voxelwise ANOVA in AFNI, with the between-subjects factor of Group (ABD, HC) and the within subject factor of time (baseline, 14 weeks) carried out voxelwise on the zr-maps representing the difference in activation between the Stop and Go conditions. Significant clusters of activation were identified using a contiguity threshold (minimum cluster volume of 270 cubic mm) that maintained an experiment-wise Type 1 error rate of p<0.025, based on AFNI’s AlphaSim Monte Carlo simulations. A significant Group by Time interaction [F(1, 24)=5.34, p<.03] was followed by step-down comparisons (see Tables 3–4) to clarify findings.
Table 3.
Significant Group Differences at Baseline and at Follow-up
| Brain region | Talairach coordinates for peak activation | BA Area | Volume (mm3) | t value for peak activation |
|---|---|---|---|---|
| Baseline | ||||
| ABD > HC | ||||
| R Motor Cingulate | 14, −17, 30 | 31 | 729 | 4.37 |
| L Motor Cingulate | −12, −10, 29 | 31 | 675 | 3.87 |
| R Ventral Premotor Cortex | 50, −17, 18 | 43 | 513 | 2.85 |
| L Striatum | −17, 2, 12 | 9 | 1107 | 4.14 |
| HC > ABD | ||||
| R Medial Frontal Gyrus | 17, 68, 17 | 11 | 1512 | 2.33 |
| L Medial Frontal Gyrus | −11, 68, 18 | 11 | 405 | 3.28 |
| R Middle Frontal Gyrus | 8, 53, 18 | 9 | 1080 | 2.64 |
| L Inferior Frontal Gyrus | −53, 26, 12 | 46 | 648 | 3.42 |
| Follow-up | ||||
| ABD > HC | ||||
| L Primary Motor Cortex (M1) | −7, −34, 47 | 5,7 | 324 | 2.89 |
| HC >ABD | ||||
| R Putamen | 26, −22, 1 | 513 | 2.67 | |
| L Putamen | −23, −20, −4 | 297 | 2.76 | |
| R Thalamus | 26, −23, 9 | 459 | 3.02 | |
| L Thalamus | −2, −17, 6 | 324 | 3.37 | |
Table 2 shows Talairach coordinates and t values for peak activation representing significant group differences in activation at baseline and follow-up for the Response Inhibition Task (clusters with p<0.025 using a contiguity threshold); HC: healthy controls; ABD: Adolescent bipolar disorder; BA: Brodmann’s Area; L: Left; R: Right.
Table 4.
Regions with Significantly Different Change from Baseline to Follow-up Testing in Patients with ABD Relative to the HC Group
| Brain region | Talairach coordinates for peak activation | BA Area | Volume (mm3) | t value for peak activation |
|---|---|---|---|---|
| ABD > HC | ||||
| L Medial Frontal Gyrus | −10, 65, 14 | 11 | 594 | 3.24 |
| L Inferior Frontal Gyrus | −31, 2, −10 | 45 | 270 | 2.46 |
| L Middle Frontal Gyrus | −43,47,8 | 46 | 270 | 3.1 |
| R Medial Frontal Gyrus | 5, 50, 17 | 11 | 297 | 3.03 |
| L Superior Temporal Gyrus | −43, 20, −25 | 22 | 567 | 2.77 |
| R Middle Temporal Gyrus | 47, −70, 26 | 39 | 432 | 4.28 |
| HC > ABD | ||||
| R Putamen | 20, 5, 6 | - | 621 | 2.56 |
| R Thalamus | 23, −19, 11 | - | 459 | 3.74 |
| L Thalamus | −19, −22, −1 | - | 270 | 2.48 |
Table 3 shows Talairach coordinates and t values for peak activation in significant clusters (p<0.025 with contiguity threshold) representing group differences in change from baseline to follow up; HC: healthy controls; ABD: Adolescent bipolar disorder; BA: Brodmann’s Area; L: Left; R: Right.
RESULTS
Clinical and demographic data are summarized in Table 1. Symptom control on mania (YMRS score <12) was achieved in 92% (n=12) of the patients. At week 14 the patients did not differ significantly from HC for YMRS scores, though they still differed on CDRS scores. At week 14 patients, although improved significantly, still had higher ratings of manic symptoms than HC, but they did not differ from HC on depressive symptoms (Table 1). There were three subjects (23%) with comorbid diagnosis of generalized anxiety disorder in the ABD group. Within the ABD group, the mean doses of SGAs received at the end point of first four weeks of acute symptom stabilization were: risperidone 1.2± 0.35 mg (n=5); aripiprazole 13.5± 2 mg (n=5); quetiapine 385± 75 mg (n=3). Benzotropine was required in 4 cases with a mean dose of 1.2± 0.6mg per day at the end of first 4 weeks of SGA therapy, and was weaned off subsequently, along with the SGAs. None of the subjects were on SGAs or any other psychotropic medications during the 6 week trial of fixed dose of lamotrigine at 200 mg.
Treatment Effects Using Response Inhibition Task
Behavioral Data
Response time and accuracy data are summarized in Table 2.
Table 2.
Response Time and Percent Accuracy Measures for Go and Stop trials at baseline and follow-up in the ABD and HC groups.
| ABD | HC | |
|---|---|---|
| Response Time in ms | Median (S.D.) | Median (S.D.) |
| Go trials at Baseline | 530 (114.5) | 567 (112.7) |
| Go trials at Follow-up | 570 (85.3) | 617 (69.3) |
| Incorrect Stop trials at Baseline | 573 (148.3) | 596 (126.1) |
| Incorrect Stop trials at Follow-up | 651 (127.3) | 677 (95.0) |
| Trial Average | 581 (118.9) | 614 (100.8) |
| Accuracy | % (S.D.) | % (S.D.) |
| Go trials at Baseline | 88% (8%) | 92% (8%) |
| Go trials at Follow-up | 86% (10%) | 88% (12%) |
| Stop trials at Baseline | 72% (16%) | 82% (13%) |
| Stop trials at Follow-up | 76% (17%) | 87%(8%) |
| Trial Average | 81% (12.8%) | 87% (10.3%) |
Separate repeated measures ANOVAs were conducted on Response Accuracy and Response Time (RT) data for the Response Inhibition Task. For Response Accuracy, Group (ABD, HC) was the between-subjects factor, and Testing Time (Baseline and Follow-up), “Trial Type” (Go trials in Go blocks and Stop trials in Stop blocks), and “Trial Block” (first, second, and third) were within-subjects factors. Our ANOVA on RT had the same factors, except that we included only mean RT for correct Go trials (because correct Stop trials had no key press).
Accuracy for Go and Stop Trials
Overall, on Stop Trials the HC group (87%) yielded more accurate performance then the ABD group (81%) [F(1,24)=7.01, p<.01]. There was no significant interaction of Group by Testing Time (p<.91), or Group by Testing Time by Trial Type (p<. 70), indicating that there were no group differences in accuracy performance from baseline to follow-up. The significant interaction of Testing Time by Trial Type [F(1, 24)=6.67, p<.02] revealed that, overall, both groups had improved performance accuracy on Stop trials at follow-up (82%) relative to baseline (77%) [F(1,24)=4.54, p<.04].
RT for Go Trials
We obtained significant main effects of Testing Time [F(1,24)=8.32, p<.008] and Block [F(1,48)=9.20, p<.0004], but no other main or interaction effects were significant. There were no Group differences in reaction times (p<.20). While RT was faster at baseline than follow-up across groups (548 ms vs. 593 ms), there were no significant group differences in these changes from baseline to follow-up (p<.77).
fMRI Data
Group Differences at Baseline and Follow-up (Table 3)
ABD versus HC at Baseline
For the Stop vs. Go condition comparison at baseline, the ABD group showed greater activation than the HC group in bilateral motor cingulate, right ventral premotor cortex and striatum, but less activation in right and left PFC as specified in Table 3.
ABD versus HC at Follow-up
ABD group exhibited greater activation than the HC group in left motor cortex (M1), but less activation in bilateral thalamus and putamen. No group differences were found in PFC activation at follow-up.
Baseline vs. Follow-up in ABD and HC
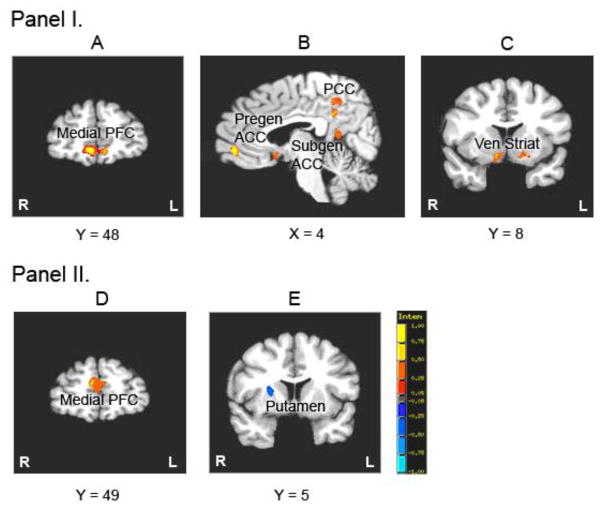
Within the ABD group, significantly greater activation was found at follow-up in bilateral medial frontal gyri, pregenual ACC, posterior cingulate and ventral striatum, and right subgenual ACC and left superior temporal gyrus. There were no regions of greater activation at baseline relative to follow-up (Figure 2- Panel I: Pictures A, B and C). The HC group exhibited greater activation at baseline compared to follow-up in left middle frontal gyrus/DLPFC and bilateral medial frontal gyri, and greater activation at follow-up in bilateral motor cingulate gyri and dorsal striatum. These results are presented in the Supplemental material.
Figure 2. Lamotrigine Treatment Effects.
PANEL I: Pictures A, B and C shows post-treatment activation relative to pre-treatment for Stop vs. Go Condition in patients with Adolescent Bipolar Disorder (ABD). Red indicates increased activation post-treatment relative to pre-treatment and blue indicates increased activation pre-treatment relative to post-treatment (not shown here) PFC = prefrontal cortex; Pregen/Subgen ACC = pregenual/subgenual anterior cingulate cortex; PCC = posterior cingulate cortex; Ven Striat= Ventral Striatum.
PANEL II: Pictures D and E show lamotrigine treatment effects over time on brain function in patients with ABD vs. Healthy Controls. Red indicates increased activation in patients with ABD relative to HC; and blue indicates increased activation in HC relative to patients with ABD for Stop vs. Go Condition over the 14 week period.
Degree of Change in Activation Over the Duration of Trial Period in ABD vs. HC group
The ABD group exhibited greater increases in activation than the HC group from baseline to follow-up in left PFC (medial, inferior and middle frontal gyri) and right PFC (medial frontal gyri) (Figure 2- Panel II: Picture D), as well as in temporal cortex (left superior temporal and right middle temporal gyri) (Table 4). The HC showed a larger increase in activation at follow-up than the ABD group in right putamen (Figure 2- Panel II: Pictures E) and bilateral thalamus.
Correlation Between Symptom Response and Brain Activation
We performed hypothesis-driven Pearson correlation analyses (2-tailed) to examine the relationship between improvement on YMRS and CDRS-R scores over the 14 week trial and change in activation from baseline to follow-up in selected ROI (medial, inferior, middle frontal gyri, cingulate cortex (subgenual, pregenual, dorsal and posterior cingulate), striatum (caudate and putamen), and temporal cortex (middle and superior temporal gyri)) in ABD. After correcting for multiple comparisons, we found positive correlation between the degree of improvement in YMRS scores and increase in activation from baseline to follow-up in right (r=.57, p<.01, corrected) and left (r=.54, p<.01, corrected) inferior frontal gyri/VLPFC. No other significant correlations were found.
DISCUSSION
This is the first fMRI study to examine treatment effects on brain circuitry function underlying response inhibition in manic, mixed, and hypomanic patients with ABD. Our aim was to study the treatment effects on the ability to inhibit motor response in contrast to executive response function, instead of inhibiting a prepotent tendency to respond. The block design paradigm of Response Inhibition Task is utilized to measure the net behavioral inhibition and executive control by contrasting BOLD signal activity during Stop versus Go blocks in ABD patients relative to HC over a 14 week time period. Our aim was to understand the ability of patients with ABD to predominantly stop the context inappropriate behavior on treatment, rather than just the prepotent response generated by an occasional stop cue. The central finding is increased cortical activation in prefrontal and temporal regions after lamotrigine monotherapy in ABD relative to HC controls during performance of a Response Inhibition Task. The absence of significant group differences between ABD and HC groups in the 14 week data in prefrontal and temporal cortex suggests that these changes represent a normalization of function in these regions with lamotrigine therapy in patients. Further, within the ABD group, increased VLPFC activation with lamotrigine treatment is significantly correlated with reduction in manic and hypomanic symptoms. This association highlights the clinical significance of our findings as VLPFC is believed to be a higher cortical center associated with impaired affect regulation and cognitive-affective integration in PBD.5;31;32 With regard to behavioral data, both groups showed slowed RT and improved performance accuracy at follow-up relative to baseline, where there was no differential change in time by group. This suggests practice effects with the paradigms in both the groups, but no differential change in task performance that could account for the improvement in brain function in patients. However, it is important to note that patients are not slowed down by this medication regime and treatment did not lead to deterioration.
Understanding Circuitry Changes Over 14 Weeks of Lamotrigine Therapy in ABD
Reduced frontal function at Baseline in patients with ABD
Brain function abnormality in untreated patients with ABD relative to HC suggests pathophysiology associated with the illness and therefore constitutes a target of treatment. In patients with ABD at baseline, relative to HC, greater activation was observed during Stop trials in the “dorsal motor circuit” described by Alexander et al33 including motor cingulate and striatum. Greater activation in this system is consistent with reduced modulation by Stop Signal commands in motor circuitry.34 In parallel with this finding, patients with ABD showed a reduced engagement of prefrontal cortex during Stop trials that provides executive control to support voluntary motor response inhibition.35–37 This pattern of findings suggests that behavioral disinhibition seen in patients with ABD may in part be explained by dysregulation of motor circuitry secondary to reduced top-down control from prefrontal systems.
Our pretreatment findings are similar to those reported by Blumberg et al16 who used a block design Stroop paradigm to probe executive control and response inhibition. They showed increased striatal and thalamic activation and decreased prefrontal activation in ABD. A study of ABD by Leibenluft et al5 employed an event related Stop Signal Task that showed decreased VLPFC and striatal activation in patients relative to HC during failed inhibition trials. Our findings are consistent with these results in demonstrating abnormal VLPFC function in PBD and underscore the significance of treatment-related improvements in VLPFC. However, increased striatal activation at baseline, shown in our study, that decreases with treatment relative to HC during motor response inhibition is not comparable to the decreased striatal activation in failed trials reported by Leibenluft et al.5 These disparities need to be resolved in future studies comparing effects in successfully and unsuccessfully performed Stop trials across a range of Stop Signal delays, in addition to comparing the ability to inhibit with varying degrees of prepotent response. While there are no directly comparable fMRI treatment studies similar to our current study, our results are similar to that in euthymic adult BD patients treated with lamotrigine showing an increase in left prefrontal activation while performing an N-back working memory task.12 It is possible, however, that these changes of decreased BOLD signal in fronto-temporal regions may be specific to manic or hypomanic symptoms, and normalization may be due to stabilization of mood. Conversely, the changes in BOLD signal in patient group may not be specific to SGA and/or lamotrigine regime.
Development of Automaticity in HC
There was a greater increase in putamen and thalamic activity over time in HC relative to patients during the Response Inhibition Task. Within group change analyses suggest that this group difference is primarily due to increased activation in striatum in controls at follow-up. This might reflect differential practice effects38 in HC, with more automatic task performance in HC supported by striatal function based on experience and skills gained from prior task performance. The positive correlation between maintenance of symptom control and increased activation in bilateral VLPFC from baseline to follow-up suggests that one mechanism of action of pharmacological treatment may involve normalization of function in VLPFC. In cross sectional studies, we previously showed reduced VLPFC activation in patients with ABD relative to HC while viewing angry faces compared to neutral faces,32 and also during cognitive processing under emotional challenge.31 As mentioned above Leibenluft et al5 previously reported reduced VLPFC activation during a Stop Signal Task condition in patients with ABD. The parallel changes in ABD symptoms and VLPFC activation during a cognitive paradigm indicate the critical role of VLPFC in relation to both emotional control and response inhibition deficits in ABD. Thus, enhanced function in VLPFC observed in the present study might facilitate positive clinical outcomes via modulation of downstream affective and motor system function. Given the glutamatergic projections that extend from VLPFC to amygdala, MPFC, and striatum,39 lamotrigine may exert effects on this circuitry to enhance behavioral self-control and affect modulation. It is important to note here that the study was specifically designed to allow SGAs for acute phase treatment and did not entirely rely on lamotrigine, although patients received lamotrigine as monotherapy in the 6-week stabilization phase. We do not suggest that lamotrigine is effective in acute mania as monotherapy especially given the long titration period. It was shown to be effective in maintenance in adult bipolar disorder7;8 as opposed to negative trials in acute mania.40 In line with the experience in adult studies, we utilized it only for symptom control after initial mood stabilization.
Limitations of the study are using a block design, as opposed to an event related design which would enable us to look at correct versus incorrect trials, and not being able to implement a placebo controlled trial. Given the acutely vulnerable and seriously ill patients, we considered it unethical to use placebo. Although the HC group does not replace the value of a placebo controlled group of patients, it accounts at least partially for practice effects. This is a preliminary study, leading to our future studies that are rapid event related fMRI designs which will parametrically vary and contrast the degrees of response inhibition across trials. Also, though not a limitation, it is important to note that the findings in this study are relevant to the treatment of manic, hypomanic, or mixed episodes of bipolar illness. Our patient findings may differ from those subjects in depressive episode or euthymic state receiving medications other than lamotrigine.
CONCLUSION
This is a “proof of concept” study using a block design fMRI paradigm to examine the pharmacological effects of initial SGAs followed by lamotrigine maintenance treatment on brain function in ABD. Strengths of this study include studying unmedicated patients at base line, prospective use of initial SGAs followed by lamotrigine monotherapy for 6 weeks prior to scanning, use of a HC group to account, at least in part, for practice effects in fMRI data, and the whole brain analyses adapting a neuroscience systems approach to study treatment effects on frontostriatal systems and their relation to clinical outcome. The study findings suggest that improved VLPFC function may be a promising treatment target, and thus provide a biomarker of clinical response in ABD to pharmacotherapy that may serve to normalize frontostriatal and frontolimbic systems to improve behavioral response inhibition and affect modulation respectively.
Acknowledgments
This research was funded by NARSAD, Marshall Reynolds Foundation, NIH K23RR018638, Mr. and Mrs. Susan and Walter Berger, GlaxoSmithKline and NIH-MO1-RR-13987
Footnotes
DISCLOSURE
Dr. Pavuluri’s work unrelated to this manuscript is supported by NIMH, NICHD, Dana foundation, AFSP, Abbott Pharmaceuticals (Study medication), and Johnson and Johnson (Study medication). Dr. Sweeney, also unrelated to this work, received support from NIH, GlaxoSmithKline, and Johnson and Johnson. The other authors have no financial relationships to disclose.
Reference List
- 1.Chang K, Adleman NE, Dienes K, et al. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: a functional magnetic resonance imaging investigation. Arch Gen Psychiatry. 2004;61:781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- 2.Dickstein DP, Treland JE, Snow J, et al. Neuropsychological performance in pediatric bipolar disorder. Biol Psychiatry. 2004;55:32–39. doi: 10.1016/s0006-3223(03)00701-7. [DOI] [PubMed] [Google Scholar]
- 3.Rich BA, Schmajuk M, Perez-Edgar KE, et al. Different psychophysiological and behavioral responses elicited by frustration in pediatric bipolar disorder and severe mood dysregulation. Am J Psychiatry. 2007;164:309–317. doi: 10.1176/ajp.2007.164.2.309. [DOI] [PubMed] [Google Scholar]
- 4.Pavuluri MN, Schenkel LS, Aryal S, et al. Neurocognitive function in unmedicated manic and medicated euthymic pediatric bipolar patients. Am J Psychiatry. 2006;163:286–293. doi: 10.1176/appi.ajp.163.2.286. [DOI] [PubMed] [Google Scholar]
- 5.Leibenluft E, Rich BA, Vinton DT, et al. Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. Am J Psychiatry. 2007;164:52–60. doi: 10.1176/ajp.2007.164.1.A52. [DOI] [PubMed] [Google Scholar]
- 6.Chang KD, Wagner C, Garrett A, et al. A preliminary functional magnetic resonance imaging study of prefrontal-amygdalar activation changes in adolescents with bipolar depression treated with lamotrigine. Bipolar Disord. 2008;10:426–431. doi: 10.1111/j.1399-5618.2007.00576.x. [DOI] [PubMed] [Google Scholar]
- 7.Calabrese JR, Suppes T, Bowden CL, et al. A double-blind, placebo-controlled, prophylaxis study of lamotrigine in rapid-cycling bipolar disorder. Lamictal 614 Study Group. J Clin Psychiatry. 2000;61:841–850. doi: 10.4088/jcp.v61n1106. [DOI] [PubMed] [Google Scholar]
- 8.Bowden CL. Lamotrigine in the treatment of bipolar disorder. Expert Opin Pharmacother. 2002;3:1513–1519. doi: 10.1517/14656566.3.10.1513. [DOI] [PubMed] [Google Scholar]
- 9.Sitges M, Chiu LM, Guarneros A, et al. Effects of carbamazepine, phenytoin, lamotrigine, oxcarbazepine, topiramate and vinpocetine on Na+ channel-mediated release of [3H]glutamate in hippocampal nerve endings. Neuropharmacology. 2007;52:598–605. doi: 10.1016/j.neuropharm.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Remy S, Urban BW, Elger CE, et al. Anticonvulsant pharmacology of voltage-gated Na+ channels in hippocampal neurons of control and chronically epileptic rats. Eur J Neurosci. 2003;17:2648–2658. doi: 10.1046/j.1460-9568.2003.02710.x. [DOI] [PubMed] [Google Scholar]
- 11.Anand A, Charney DS, Oren DA, et al. Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: support for hyperglutamatergic effects of N-methyl-D-aspartate receptor antagonists. Arch Gen Psychiatry. 2000;57:270–276. doi: 10.1001/archpsyc.57.3.270. [DOI] [PubMed] [Google Scholar]
- 12.Haldane M, Jogia J, Cobb A, et al. Changes in brain activation during working memory and facial recognition tasks in patients with bipolar disorder with Lamotrigine monotherapy. Eur Neuropsychopharmacol. 2008;18:48–54. doi: 10.1016/j.euroneuro.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Buchsbaum BR, Greer S, Chang WL, et al. Meta-analysis of neuroimaging studies of the Wisconsin card-sorting task and component processes. Hum Brain Mapp. 2005;25:35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konishi S, Kawazu M, Uchida I, et al. Contribution of working memory to transient activation in human inferior prefrontal cortex during performance of the Wisconsin Card Sorting Test. Cereb Cortex. 1999;9:745–753. doi: 10.1093/cercor/9.7.745. [DOI] [PubMed] [Google Scholar]
- 15.Rubia K, Russell T, Overmeyer S, et al. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage. 2001;13:250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- 16.Blumberg HP, Martin A, Kaufman J, et al. Frontostriatal abnormalities in adolescents with bipolar disorder: preliminary observations from functional MRI. Am J Psychiatry. 2003;160:1345–1347. doi: 10.1176/appi.ajp.160.7.1345. [DOI] [PubMed] [Google Scholar]
- 17.Strakowski SM, Adler CM, Holland SK, et al. Abnormal FMRI brain activation in euthymic bipolar disorder patients during a counting Stroop interference task. Am J Psychiatry. 2005;162:1697–1705. doi: 10.1176/appi.ajp.162.9.1697. [DOI] [PubMed] [Google Scholar]
- 18.Altshuler LL, Bookheimer SY, Townsend J, et al. Blunted activation in orbitofrontal cortex during mania: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:763–769. doi: 10.1016/j.biopsych.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Correll CU. Assessing and maximizing the safety and tolerability of antipsychotics used in the treatment of children and adolescents. J Clin Psychiatry. 2008;69(Suppl 4):26–36. [PubMed] [Google Scholar]
- 20.Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform. 1984;10:276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- 21.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders IV. 4. Washington DC: American Psychiatric Association; 1994. [Google Scholar]
- 22.Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 23.Psychological Corporation. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: Harcourt Brace & Company; 1999. [Google Scholar]
- 24.Geller B, Warner K, Williams M, et al. Prepubertal and young adolescent bipolarity versus ADHD: Assessment and validity using the WASH-U-KSADS, CBCL, and TRF. J Affect Disord. 1998;51:93–100. doi: 10.1016/s0165-0327(98)00176-1. [DOI] [PubMed] [Google Scholar]
- 25.Pavuluri M, Henry D, Moss M, et al. Effectiveness of lamotragine in maintaining symptom control. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poznanski E, Grossman J, Buchsbaum Y, et al. Preliminary studies of the reliability and validity of the children’s depression rating scale. J Am Acad Child Adolesc Psychiatry. 1984;23:191–197. doi: 10.1097/00004583-198403000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Rubia K, Overmeyer S, Taylor E, et al. Prefrontal involvement in “temporal bridging” and timing of movement. Neuropsychologia. 1998;36:1283–1293. doi: 10.1016/s0028-3932(98)00038-4. [DOI] [PubMed] [Google Scholar]
- 28.Eddy WF, Fitzgerald M, Genovese CR, et al. Functional image analysis software - computational olio. In: Prat A, editor. Proceedings in Computational Statistics. Vol. 12. Heidelberg: Physica-Verlag; 1996. pp. 39–49. [Google Scholar]
- 29.Rosenthal R. Meta-analytic procedures for social research. Newbury Park, CA: Sage; 1991. [Google Scholar]
- 30.Talairach J, Tournoux P. Co-Planar Stereotactic Atlas of the Human Brain. Stuttgart New York: Thieme Medical Publishers; 1988. [Google Scholar]
- 31.Pavuluri MN, O’connor MM, Harral EM, et al. An fMRI study of the interface between affective and cognitive neural circuitry in pediatric bipolar disorder. Psychiatry Res. 2008;162:244–255. doi: 10.1016/j.pscychresns.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pavuluri MN, O’Connor MM, Harral EM, et al. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry. 2007;62:158–167. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 33.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 34.Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol. 2001;11:663–672. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- 35.Durston S, Thomas KM, Worden MS, et al. The effect of preceding context on inhibition: an event-related fMRI study. Neuroimage. 2002;16:449–453. doi: 10.1006/nimg.2002.1074. [DOI] [PubMed] [Google Scholar]
- 36.Rubia K, Smith AB, Woolley J, et al. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp. 2006;27:973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liddle PF, Kiehl KA, Smith AM. Event-related fMRI study of response inhibition. Hum Brain Mapp. 2001;12:100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashby FG, Ennis JM, Spiering BJ. A neurobiological theory of automaticity in perceptual categorization. Psychol Rev. 2007;114:632–656. doi: 10.1037/0033-295X.114.3.632. [DOI] [PubMed] [Google Scholar]
- 39.Floresco SB, Tse MT. Dopaminergic regulation of inhibitory and excitatory transmission in the basolateral amygdala-prefrontal cortical pathway. J Neurosci. 2007;27:2045–2057. doi: 10.1523/JNEUROSCI.5474-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghaemi SN, Shirzadi AA, Filkowski M. Publication Bias and the Pharmaceutical Industry: The Case of Lamotrigine in Bipolar Disorder. Medscape J of Med. 2008;10:211. [PMC free article] [PubMed] [Google Scholar]