Abstract
The peripheral taste system uses multiple signaling pathways to transduce a stimulus into an output signal that activates afferent neurons. All of these signaling pathways depend on transient increases in intracellular calcium but the current understanding of these calcium signals is not well-developed. Using molecular and physiological techniques, this study establishes that ryanodine receptors (RyRs), specifically isoform 1, are expressed in taste cells and that their physiological function differs among cell types employing different signaling pathways. RyR1 contributes to some taste-evoked signals that rely on calcium release from internal stores but can also supplement the calcium signal that is initiated by opening VGCCs. In taste cells expressing both signaling pathways, RyR1 contributes to the depolarization-induced calcium signal but not to the calcium signal that depends on calcium release from stores. These data suggest that RyR1 is an important regulator of calcium signaling and that its physiological role in taste cells is dictated by the nature of the calcium signaling mechanisms expressed.
Keywords: taste cells, ryanodine receptors, calcium imaging, CICR, voltage gated calcium channels
The sense of taste provides an organism with the ability to identify potential nutrients for consumption as well as the ability to detect and thus, avoid potentially harmful compounds. The peripheral taste system is comprised of taste receptor cells which are housed in taste buds and detect chemicals in the oral cavity. Ionic taste stimuli, which include salty and sour, interact directly with ion channels on taste cells while more chemically complex tastants (bitter, sweet, and umami stimuli) are detected by G protein–coupled receptors (GPCRs). Even though ionic and chemically complex stimuli activate distinct signaling pathways, both stimulus types utilize cytosolic calcium increases to cause neurotransmitter release. Sour stimuli rely on the activation of voltage-gated calcium channels (VGCCs) (Richter et al., 2003) to stimulate neurotransmitter release via chemical synapses while taste-activated GPCRs initiate a second messenger cascade that causes calcium release from internal stores (Akabas et al., 1988). These taste cells lack VGCCs and chemical synapses but elevate cytosolic calcium to mediate neurotransmitter release through a hemichannel (Huang et al., 2007; Romanov et al., 2007; Dando & Roper, 2009). Therefore, regardless of the signaling mechanism used by the cell, calcium is a critical second messenger in taste transduction, making its release and regulation by taste cells essential for normal signaling to occur.
Earlier studies established that chemically complex taste stimuli activate the phospholipase Cβ 2/IP3 receptor 3 (PLCβ 2/IP3R3) signaling pathway (Zhang et al., 2003; Zhao et al., 2003). However, behavioral studies using PLCβ 2-knockout mice found reduced but not abolished responses to bitter tastants (Dotson et al., 2005) indicating that the PLCβ 2 pathway is not solely responsible for transducing bitter stimuli. We also reported that some bitter-sensitive taste cells express VGCCs and that these bitter-evoked signals are mediated by PLCβ 3/IP3R1 instead of PLCβ 2/IP3R3 (Hacker et al., 2008). These studies suggest there is more diversity in the taste-dependent signal transduction mechanisms than has been previously appreciated and led us to hypothesize that other calcium-release mechanisms may also contribute to taste transduction.
Ryanodine receptors (RyRs) are calcium-gated calcium-release channels that mediate calcium release from the internal calcium stores in the endoplasmic reticulum (ER) (Zalk et al., 2007). There are three known RyR isoforms (1–3) that have well-characterized expression patterns in multiple cell types, including neurons (Baumann, 2000; Grant et al., 2006; Zalk et al., 2007; Shimizu et al., 2008). Studies in other organisms have not detected ryanodine receptors in taste cells (Ogura et al., 1997; Ogura & Kinnamon, 1999; Zhao et al., 2002), but RyR expression in mouse taste cells has not been investigated.
In this study, we used molecular, immunocytochemical, and physiological techniques to characterize RyR expression in mouse taste receptor cells. We found that RyR1 is widely expressed in multiple sub-populations of taste receptor cells and that these ryanodine receptors differentially contribute to stimulus-evoked calcium responses. Since neurotransmitter release depends on cytosolic calcium elevations in all taste cells, these data identify an important role for ryanodine receptors in the transduction of taste-evoked responses within the peripheral taste system.
MATERIALS AND METHODS
Taste receptor cell isolation
Taste receptor cells were harvested from the circumvallate taste buds of C57Bl/6 mice obtained from Jackson Labs (Bar Harbor, ME, USA) as previously described (Hacker et al., 2008). Both sexes of mice were used and mice ranged in age from 1 to 6 months. Mice were euthanized with carbon dioxide and cervical dislocation. Tongues are removed from animals and injected under the lingual epithelium with an enzymatic solution containing 0.7 mg of collagenase B (Roche, Indianapolis, IN, USA), 3 mg of dispase II (Roche), and 1 mg of trypsin inhibitor (Sigma, St. Louis, MO, USA) per mL of Tyrode’s solution (140mM NaCl, 5mM KCl, 1mM MgCl2, 3mM CaCl2, 10mM HEPES, 10mM glucose, and 1mM pyruvic acid, pH 7.4). Tongues are incubated in oxygenated Tyrode’s solution for 20 min before the epithelium is peeled from the connective and muscular tissue. The peeled epithelium is incubated for 30 min in Ca2+-Mg2+ free Tyrode’s solution (140mM NaCl, 5mM KCl, 10mM HEPES, 2mM BAPTA, 10mM glucose and 1mM pyruvic acid, pH 7.4) before taste cells are removed with a capillary pipette and plated onto glass cover slips coated with Cell-Tak (Biopolymers, Inc). Taste cells are viable for several hours. Animals were cared for in compliance with the University at Buffalo Animal Care and Use Committee.
Reverse transcription (RT) and PCR amplification
Isolated taste buds from the circumvallate and foliate papillae were lysed, and RNA was purified using the RNeasy Mini Kit from Qiagen (Valencia, CA, USA) according to the manufacturer’s instructions. Total RNA isolated from brain tissue was used as a positive control. mRNA from isolated taste buds was reversed transcribed using Superscript III (Invitrogen, Carlsbad, CA, USA). PCR analysis for GAPDH was performed on each sample to determine sample quality and to check for genomic contamination. Samples were discarded if any genomic contamination was detected. cDNA from samples that lacked genomic contamination was analyzed for the presence of ryanodine receptor isoforms 1, 2 and 3. The identity of all PCR products was confirmed by DNA sequencing. Previously published primers which cross at least one intron were used for the detection of mRNA encoding for the ryanodine isoforms (Fitzsimmons et al., 2000).
Immunocytochemistry
For these experiments, we used either a C57Bl/6 mouse or a transgenic mouse that expresses GFP driven by the IP3R3 promoter to identify cells with the PLCβ 2/IP3R3 pathway (Hacker et al., 2008). Tongues were dissected, immersion fixed overnight in 4% paraformaldehyde/0.1 M phosphate buffer (PB), and cryo-protected in 20% sucrose/0.1 M PB. The tongue was then embedded in OCT and frozen for sectioning. Forty micron sections were cut and washed in phosphate buffered saline (PBS) three times for 10 min each at room temperature (RT). Sections were initially blocked for 2 hours at RT in blocking solution (0.3% Triton X-100, 1% normal goat serum, and 1% bovine serum albumin in 0.1 M PBS), incubated with primary antibody for 2 hours at RT, and then left overnight in primary antibody at 4°C. Two different anti-RyR 1 antibodies were used to characterize RyR1 expression: Rabbit anti-RyR1 (Millipore, Billerica, MA, USA) was diluted 1:500 in blocking solution while rabbit anti-RyR1 (Alomone labs, Jerusalem, Israel) was diluted 1:200 in blocking solution. Following over night incubation, sections were washed three times for 10 min in PBS and incubated with a goat anti-rabbit cy5 tagged antibody (1:200) (Jackson Immuno Research Laboratories, West Grove, PA, USA) at RT in the dark for 2 h. Following incubation with secondary antibodies, sections were washed again, and then mounted using Fluoromount-G (Southern Biotechnology Associates, Birmingham, AL, USA). A blocking peptide for anti-RyR1 (corresponding to amino acids 1371–1386) was pre-incubated with the antibody for one hour (1μg peptide with 1μg antibody) and staining was eliminated.
Sections were viewed with a three-channel laser scanning confocal with Krypton-Argon lasers on a Nikon Diaphot 200. Sequential scanning techniques were used. Images were captured with a cooled CCD camera, and Axiovision software was used for data acquisition. Images were processed using Adobe Photoshop CS software adjusting only brightness and contrast. Equivalent adjustments were made for experimental and control sections.
Calcium imaging
Isolated taste receptor cells were plated into a laminar flow chamber and loaded at RT for 40 min with 2 μM fura 2-AM (Molecular Probes, Invitrogen, Carlsbad, CA, USA) containing the nonionic dispersing agent Pluronic F-127 (Molecular Probes, Invitrogen) (see example cell in Figure S1). Loaded cells were visualized using an Olympus IX71 microscope with a 40X oil immersion lens and images were captured using a SensiCam QE camera (Cooke, Romulus, MI, USA) and Workbench 5.2 (Indec Biosystems, Santa Clara, CA, USA). Excitation wavelengths of 340 and 380 nm were used with an emission wavelength of 510 nm. Fluorescence values were calibrated using the Fura-2 Calcium Imaging Calibration kit (Molecular Probes, Invitrogen). The effective dissociation constant, Kd, was calculated to be 255 nM, and calcium concentrations were determined using the formula outlined by Grynkiewicz et al. (1985).
Data analysis
Experiments were plotted and analyzed using OriginPro 7.5 software (OriginLab Corp., Northampton, MA). Calcium increases were calculated as [(peak -baseline)/baseline] * 100 and were reported as percent increases over baseline. An evoked response was defined as measurable if the increase in fluorescence was >2 SD above baseline. For some analyses, we integrated the area under the curve to obtain a proportional measure of the amount of calcium in a response. Taste cells with baseline values above 200 nM were considered unstable and were not included in the analysis (Hacker et al., 2008). Statistical comparisons were made using either an unpaired Student’s t-test or a one-way ANOVA with a Bonferroni’s post-hoc analysis. For all analyses, a significance level of p < 0.05 was used and standard errors of the mean were reported.
Solutions
All solutions were bath applied using a gravity flow perfusion system (Automate Scientific, San Francisco, CA, USA) and laminar flow perfusion chambers (RC-25F, Warner Scientific, Hamden, CT, USA). The following solutions were used during experiments: HiK (Tyrode’s with 50 mM NaCl replaced by 50 mM KCl), bitter (10 mM denatonium benzoate), umami (10mM L-Glutamic acid monopotassium salt monohydrate, MPG, (Maruyama et al., 2006)), sweet (2mM saccharin), 2μM thapsigargin (to block internal store refilling by SERCA pumps), 1μM and 20μM ryanodine (to activate and inhibit RyRs respectively, Tocris, Ellisville, MO, USA). All chemicals were purchased from Sigma unless otherwise noted.
RESULTS
Ryanodine receptors are expressed in mouse taste cells
Ryanodine receptors are known to be involved in the release of calcium from internal stores in a number of excitable and non-excitable cells (Giannini et al., 1995; Baumann, 2000; Grant et al., 2006). Since calcium is important in taste cell signaling, we hypothesized that ryanodine receptors may be expressed in taste cells and have a critical role in taste-evoked calcium signaling. While caffeine is a well known activator of ryanodine receptors (Zucchi & Ronca-Testoni, 1997), it is perceived as a bitter stimulus and has been shown to modulate potassium channel activity in rat taste cells (Zhao et al., 2002). Therefore, we did not use caffeine to physiologically identify ryanodine receptors in taste cells but instead used a low concentration of ryanodine (1μM) to activate these receptors. Low concentrations of ryanodine lock the ryanodine receptor open in a sub-maximal state and causes calcium release from internal calcium stores while high ryanodine concentrations (> 10μM) block the receptor activity (Zucchi & Ronca-Testoni, 1997). Initial calcium imaging studies were done on a mixed population of isolated taste cells. We observed that 1μM ryanodine (30s) increased intracellular calcium levels in some taste cells (Figure 1A). Thapsigargin, which prevents the refilling of internal calcium stores, eliminated the ryanodine-evoked calcium response (Figure S2) indicating that these ryanodine-evoked responses depend on calcium release from internal stores. These experiments suggested that RyRs can be functionally expressed in mouse taste cells.
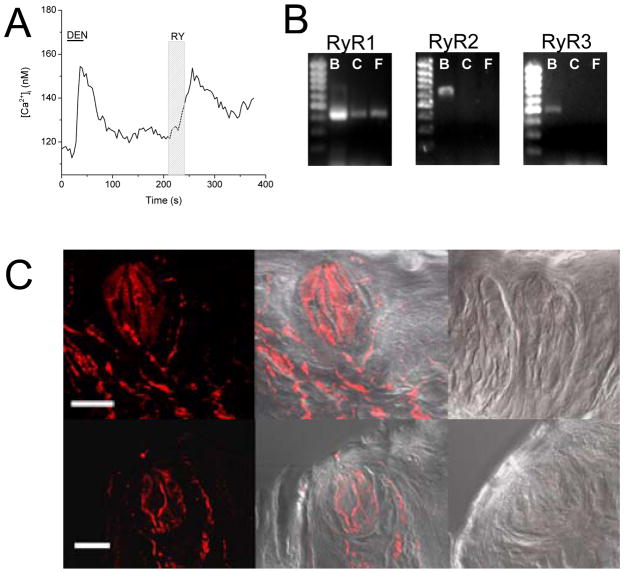
Figure 1. Ryanodine receptors are functionally expressed in some mouse taste cells.
A, Application of 1μM ryanodine (RY, 30s, light gray column) caused an increase in calcium in isolated mouse taste cells (n=18 out of 53 cells tested). This cell was determined to be a taste cell due to its responsiveness to 10mM denatonium (DEN, 30s, black line). B, RT-PCR analysis of mRNA isolated from circumvallate (C) and foliate (F) taste buds using specific primers for RyR isoforms. Brain mRNA (B) was used as a positive control. PCR amplicons of the correct size were amplified from the brain samples for all three RyR isoforms (RyR1-435 bp, RyR2-635bp, RyR3-505bp), but only RyR1 was amplified from the taste samples. PCR product identity was confirmed using sequence analysis. C, Application of anti-RyR1 to mouse taste buds was visualized using a cy5 secondary antibody. Anti-RyR1 labeling in Cv (top panel) and fungiform taste buds (bottom panel) is shown in the far left panels with an overlay on the bright field image in the middle panels. The negative controls consisting of antibody pre-incubated with the blocking peptide and overlaid on the bright field images are shown in the far right panels. Scale bar = 20μm.
To confirm these findings, we isolated mRNA from mousetaste buds that had been harvested from circumvallate (C) and foliate (F) taste papillae for RT-PCR analysis. Specific PCR primer pairs for all three RyR isoforms 1, 2 and 3 (Fitzsimmons et al., 2000) were used to amplify PCR products. Brain mRNA was used as a positive control since all three RyRs have been found in various regions of the brain (Giannini et al., 1995). Primers amplified products of the expected sizes in the positive control samples, but in taste cells, only a single product was amplified using the primers for RyR1 (Figure 1B). Sequence analysis of this 435-bp PCR product confirmed that it shared 100% identity with murine RyR1. Immunocytochemical analysis of taste buds in the circumvallate and fungiform papillae (Figure 1C) revealed specific anti-RyR1 labeling. Two different antibodies to RyR1 were used (see Methods) and results were similar for both antibodies. Application of the antibody that had been pre-incubated with the control peptide (shown on the far right panel) did not produce any labeling in the negative control sections. Based on these experiments, we concluded that RyR1 is functionally expressed in mouse taste cells.
Taste cell type definitions
The peripheral taste system is very heterogeneous with distinct sub-populations of taste cells that vary both in their morphology at the EM level and in the signaling pathways that they use. Some of these taste cell populations are selectively responsive to different tastants while others are more broadly tuned (Tomchik et al., 2007). Taste cells that release calcium from internal ER calcium stores in response to a taste stimuli via activation of the PLCβ 2/IP3R3 signaling pathway, are called type II or “receptor” cells. These taste cells detect and transduce a signal to afferent neurons in response to sweet, bitter, or umami compounds (Clapp et al., 2006; DeFazio et al., 2006). Cells in which membrane depolarization causes calcium influx through VGCCs are commonly referred to as type III or “presynaptic” cells and these cells have conventional chemical synapses (Yang et al., 2000). We have also identified a third population of taste cells that express VGCCs as well as a PLC signaling pathway that is activated by taste stimuli. In these cells, the bitter stimulus (10mM denatonium benzoate) is blocked by depleting internal calcium stores with thapsigargin and by the PLC blocker U73123 which indicates that the bitter stimulus is activating a similar signaling pathway as the one seen in type II cells. These cells are classified as “dual-responsive cells” and appear to use PLCβ 3 and IP3R1 to transduce a calcium signal when activated by taste stimuli (Hacker et al., 2008).
For this study, most cells were isolated from the circumvallate (Cv) papillae which has a preponderance of bitter-sensitive taste cells compared to other papillae types (Danilova & Hellekant, 2003). Therefore, to functionally identify a taste cell in this study, it was stimulated with the bitter compound denatonium followed by a 10s application of HiK (50mM KCl) to depolarize the cell and activate VGCCs. We have previously shown that applying 50mM KCl to isolated taste cells causes a calcium influx that is blocked by 200μM cadmium chloride (CdCl2) (Hacker et al., 2008) which is a known blocker of VGCCs (Beam & Knudson, 1988). For these experiments, all responses were measured in isolated taste receptor cells, ruling out a role for gap junctions and cell to cell communication through neurotransmitter release. If a cell responded to both stimuli, we considered it a dual-responsive cell, expressing the PLCβ 3/IP3R1 pathway and VGCCs (Hacker et al., 2008). If the cell only responded to denatonium and did not respond to HiK, we labeled it as type II cell which express the PLCβ 2/IP3R3 signaling pathway and lack VGCCs. Taste cells that only responded to HiK were assumed to express VGCCs and were identified as type III cells. While these classifications may be further refined as we learn more about the functional properties that distinguish different populations of taste cells, for this study, we are focused on understanding the role of ryanodine receptors in these three populations of taste cells: type II cells, type III cells and dual-responsive cells.
RyRs contribute to some bitter-evoked calcium signals
Since our initial studies indicated that RyR1 is functionally expressed, we began investigating its role in taste-evoked calcium signaling. For these experiments, we used a high concentration (20μM) of ryanodine to block RyRs (Zucchi & Ronca-Testoni, 1997) and tested the effect of blocking RyRs on the denatonium (bitter)-evoked calcium responses in Cv taste cells. We used two criteria to determine if ryanodine was inhibiting the denatonium-evoked calcium signal. First, there had to be a greater than 10% decrease in the peak amplitude of the response when ryanodine was applied compared to the initial evoked signal. Second, there had to be some recovery in the amplitude of the denatonium evoked calcium signal when ryanodine was removed. This ensured that we were not measuring what was actually a loss of signal as inhibition by ryanodine. While there was not always complete recovery of the responses from the ryanodine inhibition within the time frame of our experiments, there was some recovery of the evoked response amplitude after ryanodine was washed out for the cell to be considered ryanodine sensitive (Figure S3A).
While most ryanodine-insensitive cells had less than a 10% change in the signal amplitude when ryanodine was applied (Figure S3B), some denatonium-evoked signals decreased over time. There were also two cells that had a large increase in their amplitude when ryanodine was applied. To be conservative in our analysis, we considered both sets of these cells as ryanodine-insensitive (Figure S3C). These cells were included in our initial determination of cell sensitivity to ryanodine, but were excluded from the analysis shown in Figure 2C.
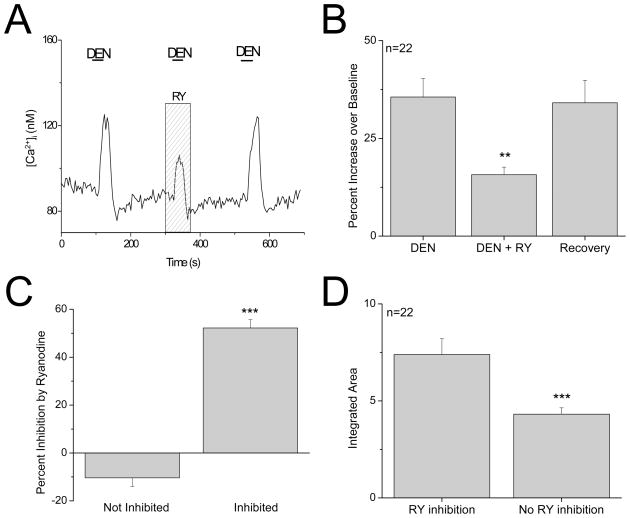
Figure 2. Ryanodine receptors contribute to bitter responses in a sub-population of type II taste cells.
A, Example trace showing 20μM ryanodine (RY, gray column) inhibiting the bitter-evoked calcium response (10mM denatonium benzoate, DEN, black line) in a type II taste cell that was sensitive to the bitter stimulus but did not express VGCCs. The bitter response recovered when ryanodine was washed out. B, Analysis using a one-way ANOVA with Bonferroni’s post-hoc analysis of the average denatonium-evoked peak responses found that 20μM ryanodine significantly reduced the denatonium-evoked response in these taste cells (n=22, **P=0.004). C, In type II taste cells that were sensitive to ryanodine, the evoked response was reduced an average of 50% by ryanodine (Inhibited). Compared to type II taste cells that were not sensitive to ryanodine (Not Inhibited), this was a significantly larger inhibition (Student’s t-test, n=22, ***P<0.0001). D, The area under the curve was integrated and compared for the denatonium-evoked calcium signals that were inhibited by ryanodine (RY effect) and the denatonium-evoked calcium responses that were not affected by ryanodine (No RY effect). An unpaired Student’s t-test found that the initial magnitude of the denatonium responses that were not inhibited by ryanodine was significantly smaller than the magnitude of the denatonium-evoked calcium signals that were inhibited by ryanodine (n=67, ***P=0.0002).
In type II cells, the denatonium response was significantly inhibited by ryanodine in 33% of cells (F2,63=6.17, P =0.004, n=67 cells tested, Figure 2A, B). There was an average 52% decrease in the amplitude of the response when 20μM ryanodine was applied which was significantly different from the effects of ryanodine on the insensitive taste cells (t50=12.04, P<0.0001, Figure 2C). The denatonium response was not completely eliminated by blocking RyRs, suggesting that while RyRs contribute to this calcium signal, they are not solely responsible for it. To determine if there were any differences in the type II cells’ denatonium-evoked calcium signals that were inhibited by ryanodine, we compared the integrated areas of the initial denatonium-evoked responses that were inhibited by ryanodine to the integrated areas of the initial denatonium-evoked responses that were not affected by ryanodine. This analysis revealed that the average integrated area of the ryanodine sensitive denatonium-evoked calcium signals responses was significantly larger than the area of the denatonium-evoked responses that were not inhibited by ryanodine (t65=4.01, P =0.0002; Figure 2D). These findings indicate that the ryanodine receptors are significantly contributing to the magnitude of the taste-evoked calcium signal in a sub-population of type II taste cells.
Ryanodine receptors are expressed in some type II cells
We looked at the expression of RyR1 in taste cells to see if it correlated with the data from our physiology experiments. Immunocytochemical analysis of 95 taste buds from 4 transgenic mice expressing IP3R3-GFP (which identify type II cells) revealed a partial overlap between IP3R3-GFP and RyR1-immunoreactivity (IR) in the Cv and fungiform papillae (Figure 3A and B). In the Cv, 31% of the IP3R3-GFP cells expressed RyR1 (n=145, Figure 3C) which is comparable to the percentage of bitter-evoked calcium responses in type II cells that were inhibited by ryanodine (33%). These data support the hypothesis that some type II cells functionally express RyR1 and that RyR1 is contributing to the formation of taste-evoked calcium signals in these cells.
Figure 3. RyR1 is expressed in a subpopulation of PLCβ 2/IP3R3 expressing cells.
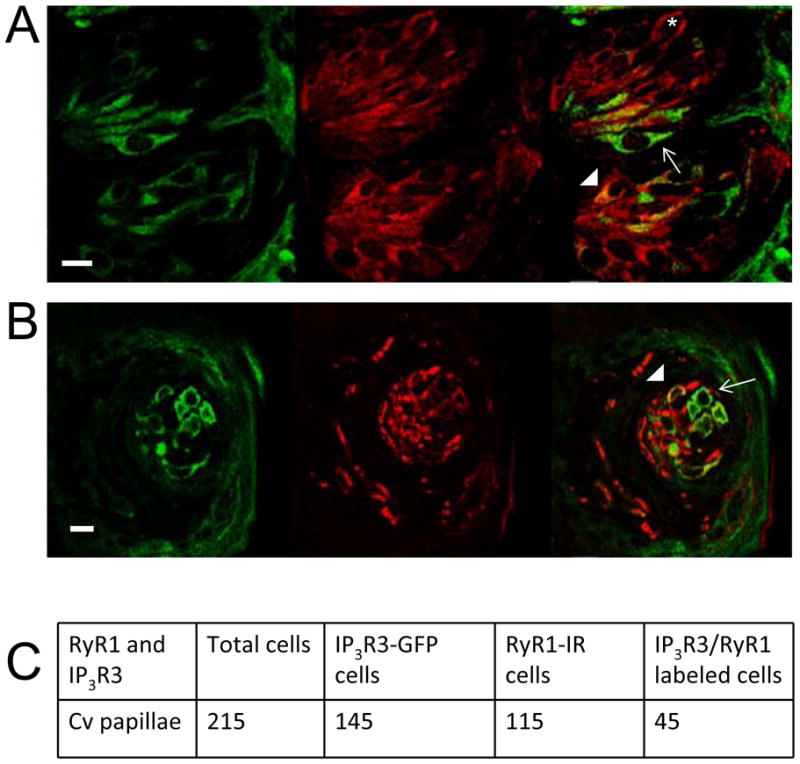
A sequentially captured Z-series stack of 20 laser scanning confocal micrographs (0.5 μm apart) show partial co-expression of IP3R3 (green, left) and RyR 1 (red, middle) in the A, Cv and B, fungiform. Co-localization of these two proteins is shown as yellow in the right panel. Arrows identify some IP3R3 cells which do not express RyR1. Arrowheads identify some taste cells that co-express IP3R3 and RyR1. An asterisk identifies an example taste cell that only expresses RyR1. Scale bar = 10μm. C, A table of IP3R3 and RyR1 expressing taste cells in the Cv taste buds analyzed from four mice.
Contribution of RyRs to other taste-evoked responses in type II cells
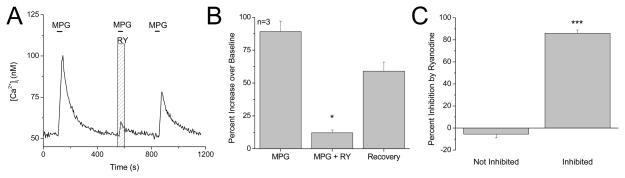
The effect of RyRs on the other taste stimuli that also use the GPCR pathways was investigated. According to Tomchik et al.(2007) , “receptor cells” or type II cells are narrowly tuned and the majority of type II cells in the Cv are bitter-sensitive. Our data support the conclusion that most type II cells in the Cv only respond to bitter stimuli which in our experiments were tested with the bitter compound, denatonium benzoate. We also used 10mM monopotassium glutamate (MPG) as an umami stimulus (Maruyama et al., 2006) but found very few taste cells that only respond to MPG. In these few MPG-only responsive taste cells that we located, the evoked response in 3 of the 5 cells was significantly inhibited by ryanodine (F2,6=37.43, P=0.0004, Figure 4). The same criteria that were used to evaluate the effects of ryanodine on the denatonium evoked responses were used to analyze ryanodine effects on the MPG-evoked signals. There was a significant difference between the effects of ryanodine on the MPG sensitive cells (t3=19.8, P=0.0003, Figure 4C), but this analysis was performed on a very limited number of MPG-responsive taste cells so the values may change if a larger number of cells are analyzed. All of the normalized values for the MPG-evoked responses are shown in Figure S3D, E.
Figure 4. Ryanodine receptors contribute to MPG responses in a sub-population of type II taste cells.
A, Representative trace showing inhibition of an umami-evoked calcium response (10mM L-Glutamic acid monopotassium salt monohydrate, MPG, black line) by 20 μM ryanodine (RY, gray column). B, Average RyR inhibition of the MPG-evoked calcium response is significantly different from control (n=3, ***P= 0.0004). C, In type II taste cells that were sensitive to ryanodine, the evoked response was reduced an average of 86% by ryanodine (Inhibited). Compared to type II taste cells that were not sensitive to ryanodine (Not inhibited), this was a significantly larger inhibition (Student’s t-test, n=5, ***P=0.0003).
We used 2mM saccharin to evoke a sweet response and found that only 29% of saccharin responses were somewhat inhibited by ryanodine, but this effect was not significant (F2,3=3.41, P=0.17, n=7 cells tested). For the umami responses (10mM MPG) and the sweet responses (2mM saccharin), control experiments were performed to ensure that these stimuli were activating a PLC signaling pathway that depends on calcium release from stores and not activating any calcium influx. Removing external calcium did not affect the umami and sweet responses but both 10mM MPG and 2mM saccharin evoked responses were abolished when either U73122 or thapsigargin was applied (data not shown), confirming that these calcium signals depend on calcium release from internal stores through the activation of a PLC signaling pathway. We concluded that RyR1 is important in the formation of some, but not all, taste-evoked calcium responses in type II taste cells.
RyRs do not affect taste responses in dual-responsive cells
Since only about 40% of the RyR1-IR was localized in type II cells (Figure 3C), we next determined if RyR1 had a physiological role in other taste cell populations. Since the dual-responsive taste cells also express a PLC signaling pathway that depends on calcium release from internal stores to generate a bitter response (Hacker et al., 2008), we assessed the possible contribution of RyRs to the denatonium-dependent calcium responses in these cells. None of the denatonium responses in dual-responsive taste cells were significantly inhibited by ryanodine (F2,84=2.23, P=0.11, Figure 5A, B), suggesting that RyRs do not contribute to the formation of the denatonium-evoked calcium responses in this population of taste cells.
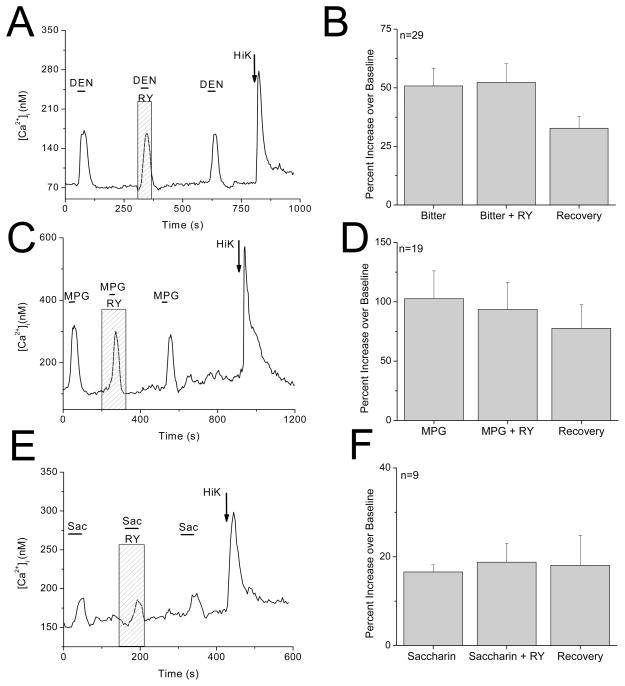
Figure 5. Ryanodine receptors do not contribute to taste-evoked responses in dual responsive taste cells.
Representative traces showing 20μM ryanodine having no effect on A, bitter (10mM denatonium benzoate) C, umami (10mM MPG) and E, sweet (2mM saccharin) evoked calcium responses in dual-responsive taste cells. Pooled results showing lack of significant inhibition by ryanodine in dual-responsive cells for B, denatonium (n=29, P=0.11), D, MPG (n=19, P=0.72) and F, saccharin (n=9, P=0.69)
We have found that many dual-responsive cells are broadly tuned and respond to multiple taste stimuli. In addition to HiK and denatonium, these individual, isolated taste cells may also respond to umami and sweet taste stimuli, so we tested if RyRs contribute to MPG- or saccharin-evoked calcium signals in the dual-responsive taste cells. Ryanodine had no effect on either the MPG responses (F2,54=0.328, P=0.72, Figure 5C, D) or the saccharin responses (F2,24=0.38, P=0.69, Figure 5E, F) which parallels the findings for the denatonium responses in these cells. We concluded that RyRs are not involved in the formation of the evoked taste signals that depend on calcium release from internal stores in the dual-responsive cells.
RyRs contribute to the depolarization-induced calcium signal in dual-responsive taste cells
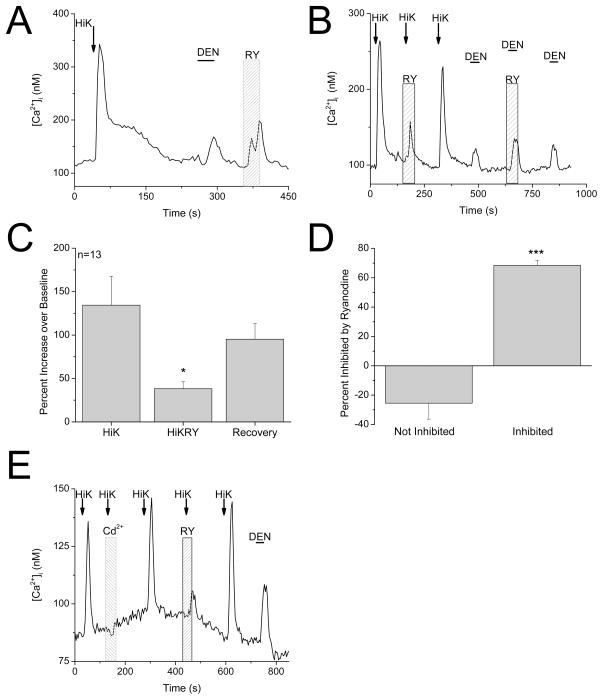
Since inhibiting RyRs did not affect the taste-evoked responses, we initially concluded that RyRs were not functionally expressed in dual-responsive taste cells. However, a low concentration of ryanodine (1μM) did cause an increase in intracellular calcium in these cells (Figure 6A), indicating that RyRs are functionally expressed. In skeletal muscle, VGCCs and RyRs are physically linked and work together to trigger muscle contraction (Niggli, 1999). There is also evidence of a functional coupling between RyRs and different L-type VGCC subunits in neurons (Chavis et al., 1996; Mouton et al., 2001; Ouardouz et al., 2003; Kim et al., 2007; Berrout & Isokawa, 2009). Since RyRs did not affect the taste-evoked calcium release from internal stores but are functionally expressed, we tested the effect of inhibiting RyRs on the calcium influx signal that is initiated by opening VGCCs. This calcium signal was significantly inhibited in 40% of dual-responsive cells (F2,36=4.66, P=0.02, n=32 cells tested, Figure 6B, C) while the denatonium-evoked calcium responses in the same cells were not affected (F2,12=0.19, P=0.83, Figure 6B). The previously described criteria were used to analyze ryanodine effects on the 50mM KCl-evoked responses. There was a significant difference between the effects of ryanodine on the calcium influx signal between the ryanodine-sensitive and ryanodine-insensitive taste cells (t30=7.42, P<0.0001, Figure 6D). All of the normalized values for the evoked responses, including the cells that were excluded from the analysis, are shown in Figure S4. We concluded that in some dual-responsive taste cells, ryanodine receptors contribute to the calcium signal that depends on calcium influx through VGCCs but do not contribute to taste-evoked calcium signals which depend on calcium release from internal stores.
Figure 6. Ryanodine receptors are functionally expressed in dual-responsive taste cells.
A, Application of 1μM ryanodine (RY, 30s) in a dual-responsive taste cell caused an increase in intracellular calcium (n=8 out of 23 cells tested). B, Example trace showing 20μM ryanodine inhibiting the calcium signal due to calcium influx through VGCCs but not the bitter-evoked calcium response in a dual-responsive taste cell (n=5 out of 8 cells tested). C, Pooled results showing the effects of 20μM ryanodine on the calcium signal due to VGCCs in dual-responsive cells (n=13, *P=0.02). D, In dual-responsive taste cells that were sensitive to ryanodine, the Hi K-evoked response was reduced an average of 68% by ryanodine (Inhibited). Compared to dual-responsive taste cells that were not sensitive to ryanodine (Not Inhibited), this was a significantly larger inhibition (Student’s t-test, n=32, ***P<0.0001). E, Calcium responses to 50mM KCl (HiK) were blocked with the VGCC blocker, 200μM CdCl2, to ensure that these elevations were due to opening VGCCs. Cadmium blocked the HiK calcium response that was subsequently inhibited by 20μM ryanodine (RY).
RyRs contribute to the VGCC-dependent calcium signal in type III taste cells
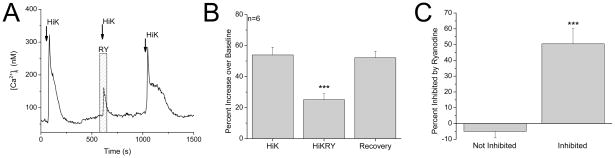
We also determined if the calcium signal due to opening VGCC was reduced by blocking RyRs in type III taste cells. These cells do not respond to bitter, sweet or umami taste stimuli but increase intracellular calcium in response to membrane depolarization with 50mM KCl. We found that in approximately 30% of type III cells, the calcium influx signal was significantly inhibited by ryanodine (F2,15=13.36, P = 0.0005, n=20 cells tested, Figure 7), indicating that RyRs are functionally coupled to VGCCs in some, but not all type III cells. Based on the criteria described above, we excluded one cell from our analysis (see Figure S5) but found that the level of ryanodine inhibition was significantly greater in the ryanodine sensitive taste cells compared to those that were not sensitive to ryanodine (t17=6.27, P=0.0001, Figure 7C).
Figure 7. RyRs contribute to depolarization-dependent calcium signal in type III taste cells.
A, Representative trace showing that 20μM ryanodine inhibits the calcium signal that is initiated by opening VGCC in a type III taste cell. B, Pooled results showing inhibition of the calcium signal due to opening VGCCs by 20μM ryanodine in type III cells (n=6, ***P= 0.0005). C, In type III taste cells that were sensitive to ryanodine, the evoked response was reduced an average of 50% by ryanodine (Inhibited). Compared to taste cells that were not sensitive to ryanodine (Not inhibited), this was a significantly larger inhibition (Student’s t-test, n=20, ***P=0.0001).
DISCUSSION
This study clearly demonstrates that RyR1 is functionally expressed in mouse taste cells and has unique physiological roles within different taste cell populations. In approximately 30% of type II taste cells, RyR1 contributes to the taste-evoked calcium responses that depend on calcium release from stores. Since the other type II cells do not appear to express RyR1, this finding suggests that within this taste cell population, there is more diversity in the signaling pathways that contribute to the taste-evoked calcium signals than has previously been recognized.
RyRs are known to be important in calcium-induced calcium release (CICR) in multiple cell types (Thayer et al., 1988; Gyorke & Fill, 1993; Llano et al., 1994) and CICR makes significant contributions to neurotransmitter release in some neurons (Narita et al., 1998; Brain et al., 2001; Carter et al., 2002). Since calcium is an essential second messenger in the transduction of taste stimuli (Akabas et al., 1988; Caicedo & Roper, 2001), its release from internal calcium stores and subsequent regulation is critical in the formation of a normal output signal that occurs in response to taste stimuli. We have now shown that RyRs can significantly contribute to the taste evoked-calcium signals initiated by IP3R3 receptor activation for the representative bitter, umami and sweet stimuli used in this study. Due to its widespread effects, it is likely that RyR1 also affects the evoked calcium signals generated by other taste stimuli that depend on calcium release from internal stores. Since the taste-evoked calcium signals were inhibited, but not completely abolished by high concentrations of ryanodine, we conclude that RyRs are likely activated via CICR that depends on the initial activation of IP3R3. These two calcium-release channels appear to work together to generate and sustain an appropriate taste-evoked calcium signal. Several reports in other neurons have documented an interdependent relationship between RyRs and IP3Rs in order to accomplish a full intracellular calcium mobilization (Haak et al., 2001; White & McGeown, 2002; McGeown, 2004; Morales-Tlalpan et al., 2005).
The absence of RyRs in approximately 70% of type II cells indicates that RyRs do not contribute to all of the evoked taste responses that depend on calcium release from internal stores. These findings support the hypothesis that there are unique calcium signals generated in distinct taste cell populations (Hacker et al., 2008) which likely confer specificity to the subsequent output signal. Type II taste cells lack chemical synapses and release neurotransmitter through a mechanism that relies on calcium release from internal stores and membrane depolarization (Huang et al., 2007; Romanov et al., 2007). In type II taste cells, TRPM5 channels are functionally “downstream” of the PLCβ2/IP3R3 signaling pathway and are activated by an increase in intracellular calcium which is due to calcium release from internal stores (Liu & Liman, 2003; Prawitt et al., 2003; Zhang et al., 2003). When activated, TRPM5 channels cause a membrane depolarization which is needed, along with calcium release from internal stores, to activate pannexin hemichannels (Barbe et al., 2006; Locovei et al., 2006a; Locovei et al., 2006b). These hemichannels open and release the neurotransmitter ATP onto the afferent gustatory neurons (Huang et al., 2007; Romanov et al., 2007; Dando & Roper, 2009). Therefore, calcium release from internal stores in type II cells directly impacts the synaptic activity of these cells. In a variety of synapses, inhibition of ryanodine receptors reduces neurotransmitter release (Mothet et al., 1998; Galante & Marty, 2003; Lelli et al., 2003; Liu et al., 2005), indicating that calcium release via RyRs is important for synaptic function in multiple types of neurons. Our data suggest a similar role for RyRs in some type II taste cells.
RyRs were also found in some dual-responsive taste cells which are taste cells that express VGCCs but also use a PLC signaling pathway to generate calcium responses to taste stimuli (Hacker et al., 2008). Contrary to what was seen in type II cells, RyRs did not significantly contribute to any taste-evoked calcium response that depends on calcium release from internal stores in these cells. Instead RyRs supplemented the calcium signal that is initiated by opening VGCCs. This effect was seen in some dual-responsive taste cells as well as some type III taste cells which also express VGCCs but do not respond to bitter, sweet or umami taste stimuli. In skeletal muscles, it is well-known that RyRs participate in muscle contraction during excitation-contraction (EC) coupling (Rios & Brum, 1987) through a physical linkage between RyRs and L-type VGCCs (Rios & Brum, 1987; Zalk et al., 2007). This functional coupling between RyR1 and L-type VGCCs has also been reported in the central nervous system. Chavis et al.(1996) first reported a functional coupling between RyRs and L-type calcium channels in cerebellar neurons and later studies found similar interactions between L-type calcium channels and RyR1 in cerebrum, hippocampus, and spinal cord (Mouton et al., 2001; Ouardouz et al., 2003; Kim et al., 2007; Berrout & Isokawa, 2009). These pairings of RyRs and VGCCs are believed to generate a depolarization-induced calcium release from internal stores which is important in regulating neuronal electrical activity as well as linking the membrane activity to subsequent gene expression affecting synaptic plasticity. Our finding that some mouse taste cells express RyR1 and the fact that some taste cells express L-type calcium channels (DeFazio et al., 2006; Roberts et al., 2009) suggests that a functional coupling between RyR1 and L-type VGCCs may exist in these cells. While it still unknown if RyR1 and VGCCs are physically linked in taste cells, our data clearly show that activation of RyRs in some type III and dual responsive cells significantly contributes to the calcium responses evoked by membrane depolarization.
One intriguing aspect of our findings is that a single ryanodine receptor isoform (RyR1) has two completely different physiological roles within the taste cells, either contributing to taste-evoked calcium release from stores or depolarization-induced calcium signal that is initiated by VGCCs. The respective role for RyR1 appears to be determined by the other calcium signaling pathways that are expressed in the taste cells. In addition to its participation in EC-coupling which controls RyR1 in a voltage-dependent and calcium-independent manner (Shoshan-Barmatz & Ashley, 1998), RyR1 can be activated by calcium and participate in CICR when not coupled to L-type VGCCs (Margreth et al., 1993; Anderson et al., 1994; Fill & Copello, 2002) which may explain why RyR1 has multiple physiological roles in taste cells. In type II cells which lack VGCCs, RyR1 cannot couple to any VGCCs and is available to participate in CICR. However, in type III and dual responsive taste cells, RyRs appear to become preferentially associated with VGCCs. In the dual responsive taste cells, this association prevents RyR1 from contributing to the taste-evoked calcium signals that depend on calcium release from internal stores. We have shown that RyR1 is physiologically associated with VGCCs in a preferential manner and it is possible that RyR1 may be physically coupled to VGCCs in taste cells. Future studies using co-immunoprecipitation or other protein interaction assays are needed to address this question.
The results from this current study point to a complex relationship between the cellular mechanisms that initiate the calcium signal and the downstream effectors that contribute to the formation of these calcium signals in taste cells. Since different taste cell populations selectively express different calcium signaling mechanisms, future studies can take advantage of this unique feature of the peripheral taste system to understand how distinct calcium signaling processes contribute to different calcium-dependent events including neurotransmitter release and gene expression.
Supplementary Material
An example of an isolated taste receptor cell loaded with fura 2-AM and viewed in epifluorescence (left panel) and bright field illumination (right panel).
Example trace showing that the ryanodine (1μM, 30s) evoked calcium signal was eliminated by thapsigargin (2μM, 120s), which prevents the refilling of calcium stores. The bitter-evoked calcium response which depends on internal stores was also eliminated (n=5).
Normalized data of the effects of ryanodine on the response amplitudes of the taste evoked signals in ryanodine sensitive and ryanodine insensitive type II taste cells. A–C, The response amplitude of the denatonium-evoked calcium signals were measured before, during, and after the application of ryanodine. Values were normalized to the initial response amplitude. A, Normalized denatonium responses from type II taste cells that were sensitive to ryanodine are shown in red (n=22). B, Normalized denatonium responses from type II cells that were not sensitive to ryanodine are shown in black (n=31). C, Normalized denatonium responses from type II taste cells that were categorized as ryanodine-insensitive but were not included in the statistical analyses in Figure 2 because the response amplitude continued to decrease even after ryanodine was washed out (n=12). There were two cells that had a large increase in their response amplitude when ryanodine was applied that were also excluded from the statistical analysis. These cells are shown in blue. D, Normalized MPG responses from type II taste cells that were sensitive to ryanodine are shown in red (n=3). E, Normalized MPG responses from type II cells that were not sensitive to ryanodine are shown in black (n=2).
Normalized data of the effects of ryanodine on the response amplitudes of the 50mM KCl-evoked signals in ryanodine sensitive and ryanodine insensitive dual-responsive taste cells. The response amplitude of the evoked calcium signals were measured before, during, and after the application of ryanodine. Values were normalized to the initial response amplitude. A, Normalized responses from dual responsive taste cells that were sensitive to ryanodine are shown in red (n=13). B, Normalized Hi K-evoked responses from dual-responsive taste cells that were not sensitive to ryanodine are shown in black (n=12). C, Normalized responses that were labeled as ryanodine-insensitive but were not included in the statistical analyses in Figure 6 because the response amplitude continued to decrease even after ryanodine was washed out are shown in blue (n=7).
Normalized data of the effects of ryanodine on the response amplitudes of the 50mM KCl-evoked signals in ryanodine sensitive and ryanodine insensitive type III taste cells. The response amplitude of the 50mM KCl-evoked calcium signals were measured before, during, and after the application of ryanodine. Values were normalized to the initial response amplitude. A, Normalized responses from type III taste cells that were sensitive to ryanodine are shown in red (n=6). B, Normalized 50mM KCl-evoked responses from type III taste cells that were not sensitive to ryanodine are shown in black (n=13). C, The response from one cell was labeled as ryanodine-insensitive but was not included in the statistical analyses in Figure 7 because the response amplitude continued to decrease after ryanodine was washed out is shown in blue.
Acknowledgments
The authors wish to thank Drs. Evanna Gleason, Sue Kinnamon and Scott Medler for their insightful comments on this manuscript. This work was supported by NIH Grant DC006358 and NSF 0917893 to KM.
ABBREVIATIONS
- CICR
Calcium-induced calcium release
- Cv
Circumvallate
- EC
Excitation-contraction coupling
- ER
Endoplasmic reticulum
- F
Foliate
- GPCRs
G protein–coupled receptors IP3R1, IP3 receptor 1
- IP3R3
IP3 receptor 3
- PLCβ 2
Phospholipase Cβ 2
- PLCβ 3
Phospholipase Cβ 3
- RyRs
Ryanodine receptors
- VGCCs
Voltage gated calcium channels
References
- Akabas MH, Dodd J, Al-Awqati Q. A bitter substance induces a rise in intracellular calcium in a subpopulation of rat taste cells. Science. 1988;242:1047–1050. doi: 10.1126/science.3194756. [DOI] [PubMed] [Google Scholar]
- Anderson K, Cohn AH, Meissner G. High-affinity [3H]PN200-110 and [3H]ryanodine binding to rabbit and frog skeletal muscle. Am J Physiol. 1994;266:C462–466. doi: 10.1152/ajpcell.1994.266.2.C462. [DOI] [PubMed] [Google Scholar]
- Barbe MT, Monyer H, Bruzzone R. Cell-cell communication beyond connexins: the pannexin channels. Physiology (Bethesda) 2006;21:103–114. doi: 10.1152/physiol.00048.2005. [DOI] [PubMed] [Google Scholar]
- Baumann O. Distribution of ryanodine receptor Ca(2+) channels in insect photoreceptor cells. J Comp Neurol. 2000;421:347–361. doi: 10.1002/(sici)1096-9861(20000605)421:3<347::aid-cne4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Beam KG, Knudson CM. Calcium currents in embryonic and neonatal mammalian skeletal muscle. J Gen Physiol. 1988;91:781–798. doi: 10.1085/jgp.91.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrout J, Isokawa M. Homeostatic and stimulus-induced coupling of the L-type Ca2+ channel to the ryanodine receptor in the hippocampal neuron in slices. Cell Calcium. 2009;46:30–38. doi: 10.1016/j.ceca.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain KL, Trout SJ, Jackson VM, Dass N, Cunnane TC. Nicotine induces calcium spikes in single nerve terminal varicosities: a role for intracellular calcium stores. Neuroscience. 2001;106:395–403. doi: 10.1016/s0306-4522(01)00280-9. [DOI] [PubMed] [Google Scholar]
- Caicedo A, Roper SD. Taste receptor cells that discriminate between bitter stimuli. Science. 2001;291:1557–1560. doi: 10.1126/science.291.5508.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AG, Vogt KE, Foster KA, Regehr WG. Assessing the role of calcium-induced calcium release in short-term presynaptic plasticity at excitatory central synapses. J Neurosci. 2002;22:21–28. doi: 10.1523/JNEUROSCI.22-01-00021.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavis P, Fagni L, Lansman JB, Bockaert J. Functional coupling between ryanodine receptors and L-type calcium channels in neurons. Nature. 1996;382:719–722. doi: 10.1038/382719a0. [DOI] [PubMed] [Google Scholar]
- Clapp TR, Medler KF, Damak S, Margolskee RF, Kinnamon SC. Mouse taste cells with G protein-coupled taste receptors lack voltage-gated calcium channels and SNAP-25. BMC Biology. 2006;4 doi: 10.1186/1741-7007-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dando R, Roper SD. Cell-to-cell communication in intact taste buds through ATP signalling from pannexin 1 gap junction hemichannels. J Physiol. 2009;587:5899–5906. doi: 10.1113/jphysiol.2009.180083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilova V, Hellekant G. Comparison of the responses of the chorda tympani and glossopharyngeal nerves to taste stimuli in C57BL/6J mice. BMC Neurosci. 2003;4:5. doi: 10.1186/1471-2202-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFazio RA, Dvoryanchikov G, Maruyama Y, Kim JW, Pereira E, Roper SD, Chaudhari N. Separate populations of receptor cells and presynaptic cells in mouse taste buds. J Neurosci. 2006;26:3971–3980. doi: 10.1523/JNEUROSCI.0515-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson CD, Roper SD, Spector AC. PLCb2-Independent Behavioral Avoidance of Prototypical Bitter-Tasting Ligands. Chemical senses. 2005;30:593–600. doi: 10.1093/chemse/bji053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev. 2002;82:893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- Fitzsimmons TJ, Gukovsky I, McRoberts JA, Rodriguez E, Lai FA, Pandol SJ. Multiple isoforms of the ryanodine receptor are expressed in rat pancreatic acinar cells. Biochem J. 2000;351:265–271. doi: 10.1042/0264-6021:3510265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galante M, Marty A. Presynaptic ryanodine-sensitive calcium stores contribute to evoked neurotransmitter release at the basket cell-Purkinje cell synapse. J Neurosci. 2003;23:11229–11234. doi: 10.1523/JNEUROSCI.23-35-11229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini G, Conti A, Mammarella S, Scrobogna M, Sorrentino V. The Ryanodine Receptor/Calcium Channel Genes Are Widely and Differentially Expressed in Murine Brain and Peripheral Tissues. The Journal of cell biology. 1995;128:893–904. doi: 10.1083/jcb.128.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant L, Slapnick S, Kennedy H, Hackney C. Ryanodine receptor localisation in the mammalian cochlea: an ultrastructural study. Hear Res. 2006;219:101–109. doi: 10.1016/j.heares.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A New Generation of Ca2+ Indicators with Greatly Improved Fluorescence Properties. The Journal of Biological chemistry. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Gyorke S, Fill M. Ryanodine receptor adaptation: control mechanism of Ca(2+)-induced Ca2+ release in heart. Science. 1993;260:807–809. doi: 10.1126/science.8387229. [DOI] [PubMed] [Google Scholar]
- Haak LL, Song LS, Molinski TF, Pessah IN, Cheng H, Russell JT. Sparks and puffs in oligodendrocyte progenitors: cross talk between ryanodine receptors and inositol trisphosphate receptors. J Neurosci. 2001;21:3860–3870. doi: 10.1523/JNEUROSCI.21-11-03860.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker K, Laskowski A, Feng L, Restrepo D, Medler K. Evidence for two populations of bitter responsive taste cells in mice. J Neurophysiol. 2008;99:1503–1514. doi: 10.1152/jn.00892.2007. [DOI] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci U S A. 2007;104:6436–6441. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Yun HM, Baik JH, Chung KC, Nah SY, Rhim H. Functional interaction of neuronal Cav1.3 L-type calcium channel with ryanodine receptor type 2 in the rat hippocampus. J Biol Chem. 2007;282:32877–32889. doi: 10.1074/jbc.M701418200. [DOI] [PubMed] [Google Scholar]
- Lelli A, Perin P, Martini M, Ciubotaru CD, Prigioni I, Valli P, Rossi ML, Mammano F. Presynaptic calcium stores modulate afferent release in vestibular hair cells. J Neurosci. 2003;23:6894–6903. doi: 10.1523/JNEUROSCI.23-17-06894.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Liman ER. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc Natl Acad Sci U S A. 2003;100:15160–15165. doi: 10.1073/pnas.2334159100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Chen B, Yankova M, Morest DK, Maryon E, Hand AR, Nonet ML, Wang ZW. Presynaptic ryanodine receptors are required for normal quantal size at the Caenorhabditis elegans neuromuscular junction. J Neurosci. 2005;25:6745–6754. doi: 10.1523/JNEUROSCI.1730-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I, DiPolo R, Marty A. Calcium-induced calcium release in cerebellar Purkinje cells. Neuron. 1994;12:663–673. doi: 10.1016/0896-6273(94)90221-6. [DOI] [PubMed] [Google Scholar]
- Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci U S A. 2006a;103:7655–7659. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006b;580:239–244. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Margreth A, Damiani E, Tobaldin G. Ratio of dihydropyridine to ryanodine receptors in mammalian and frog twitch muscles in relation to the mechanical hypothesis of excitation-contraction coupling. Biochem Biophys Res Commun. 1993;197:1303–1311. doi: 10.1006/bbrc.1993.2619. [DOI] [PubMed] [Google Scholar]
- Maruyama Y, Pereira E, Margolskee RF, Chaudhari N, Roper SD. Umami responses in mouse taste cells indicate more than one receptor. J Neurosci. 2006;26:2227–2234. doi: 10.1523/JNEUROSCI.4329-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeown JG. Interactions between inositol 1,4,5-trisphosphate receptors and ryanodine receptors in smooth muscle: one store or two? Cell Calcium. 2004;35:613–619. doi: 10.1016/j.ceca.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Morales-Tlalpan V, Arellano RO, Diaz-Munoz M. Interplay between ryanodine and IP3 receptors in ATP-stimulated mouse luteinized-granulosa cells. Cell Calcium. 2005;37:203–213. doi: 10.1016/j.ceca.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Mothet JP, Fossier P, Meunier FM, Stinnakre J, Tauc L, Baux G. Cyclic ADP-ribose and calcium-induced calcium release regulate neurotransmitter release at a cholinergic synapse of Aplysia. J Physiol. 1998;507 ( Pt 2):405–414. doi: 10.1111/j.1469-7793.1998.405bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton J, Marty I, Villaz M, Feltz A, Maulet Y. Molecular interaction of dihydropyridine receptors with type-1 ryanodine receptors in rat brain. Biochem J. 2001;354:597–603. doi: 10.1042/0264-6021:3540597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita K, Akita T, Osanai M, Shirasaki T, Kijima H, Kuba K. A Ca2+-induced Ca2+ release mechanism involved in asynchronous exocytosis at frog motor nerve terminals. J Gen Physiol. 1998;112:593–609. doi: 10.1085/jgp.112.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niggli E. Localized intracellular calcium signaling in muscle: calcium sparks and calcium quarks. Annu Rev Physiol. 1999;61:311–335. doi: 10.1146/annurev.physiol.61.1.311. [DOI] [PubMed] [Google Scholar]
- Ogura T, Kinnamon SC. IP(3)-Independent release of Ca(2+) from intracellular stores: A novel mechanism for transduction of bitter stimuli. J Neurophysiol. 1999;82:2657–2666. doi: 10.1152/jn.1999.82.5.2657. [DOI] [PubMed] [Google Scholar]
- Ogura T, Mackay-Sim A, Kinnamon SC. Bitter taste transduction of denatonium in the mudpuppy Necturus maculosus. J Neurosci. 1997;17:3580–3587. doi: 10.1523/JNEUROSCI.17-10-03580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouardouz M, Nikolaeva MA, Coderre E, Zamponi GW, McRory JE, Trapp BD, Yin X, Wang W, Woulfe J, Stys PK. Depolarization-induced Ca2+ release in ischemic spinal cord white matter involves L-type Ca2+ channel activation of ryanodine receptors. Neuron. 2003;40:53–63. doi: 10.1016/j.neuron.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Prawitt D, Monteilh-Zoller MK, Brixel L, Spangenberg C, Zabel B, Fleig A, Penner R. TRPM5 is a transient Ca2+-activated cation channel responding to rapid changes in [Ca2+]i. Proc Natl Acad Sci U S A. 2003;100:15166–15171. doi: 10.1073/pnas.2334624100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter TA, Caicedo A, Roper SD. Sour taste stimuli evoke Ca2+ and pH responses in mouse taste cells. J Physiol. 2003;547:475–483. doi: 10.1113/jphysiol.2002.033811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios E, Brum G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature. 1987;325:717–720. doi: 10.1038/325717a0. [DOI] [PubMed] [Google Scholar]
- Roberts CD, Dvoryanchikov G, Roper SD, Chaudhari N. Interaction between the second messengers cAMP and Ca2+ in mouse presynaptic taste cells. J Physiol. 2009;587:1657–1668. doi: 10.1113/jphysiol.2009.170555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, Margolskee RF, Kolesnikov SS. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. Embo J. 2007;26:657–667. doi: 10.1038/sj.emboj.7601526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H, Fukaya M, Yamasaki M, Watanabe M, Manabe T, Kamiya H. Use-dependent amplification of presynaptic Ca2+ signaling by axonal ryanodine receptors at the hippocampal mossy fiber synapse. Proc Natl Acad Sci U S A. 2008;105:11998–12003. doi: 10.1073/pnas.0802175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoshan-Barmatz V, Ashley RH. The structure, function, and cellular regulation of ryanodine-sensitive Ca2+ release channels. Int Rev Cytol. 1998;183:185–270. doi: 10.1016/s0074-7696(08)60145-x. [DOI] [PubMed] [Google Scholar]
- Thayer SA, Hirning LD, Miller RJ. The role of caffeine-sensitive calcium stores in the regulation of the intracellular free calcium concentration in rat sympathetic neurons in vitro. Mol Pharmacol. 1988;34:664–673. [PubMed] [Google Scholar]
- Tomchik SM, Berg S, Kim JW, Chaudhari N, Roper SD. Breadth of tuning and taste coding in mammalian taste buds. J Neurosci. 2007;27:10840–10848. doi: 10.1523/JNEUROSCI.1863-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C, McGeown JG. Carbachol triggers RyR-dependent Ca(2+) release via activation of IP(3) receptors in isolated rat gastric myocytes. J Physiol. 2002;542:725–733. doi: 10.1113/jphysiol.2002.020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Crowley Hildegard H, Rock ME, Kinnamon JC. Taste Cells With Synapses in Rat Circumvallate Papillae Display SNAP-25- Like Immunoreactivity. The Journal of comparative neurology. 2000;424:205–215. doi: 10.1002/1096-9861(20000821)424:2<205::aid-cne2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Zalk R, Lehnart SE, Marks AR. Modulation of the Ryanodine Receptor and Intracellular Calcium. Annual Review of Biochemistry. 2007;76:367–385. doi: 10.1146/annurev.biochem.76.053105.094237. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Zuker CS, Ryba NJP. Coding of Sweet, Bitter, and Umami Tastes: Different Receptor Cells Sharing Similar Signaling Pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- Zhao FL, Lu SG, Herness S. Dual actions of caffeine on voltage-dependent currents and intracellular calcium in taste receptor cells. The American Journal of Physiology. 2002;283:115–129. doi: 10.1152/ajpregu.00410.2001. [DOI] [PubMed] [Google Scholar]
- Zhao GQ, Yifeng Z, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJP, Zuker CS. The Receptors for Mammalian Sweet and Umami Taste. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
- Zucchi R, Ronca-Testoni S. The sarcoplasmic reticulum Ca channel/ryanodine receptor: modulation by endogenous effectors, drugs and disease states. Pharmacological reviews. 1997;49:1–51. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
An example of an isolated taste receptor cell loaded with fura 2-AM and viewed in epifluorescence (left panel) and bright field illumination (right panel).
Example trace showing that the ryanodine (1μM, 30s) evoked calcium signal was eliminated by thapsigargin (2μM, 120s), which prevents the refilling of calcium stores. The bitter-evoked calcium response which depends on internal stores was also eliminated (n=5).
Normalized data of the effects of ryanodine on the response amplitudes of the taste evoked signals in ryanodine sensitive and ryanodine insensitive type II taste cells. A–C, The response amplitude of the denatonium-evoked calcium signals were measured before, during, and after the application of ryanodine. Values were normalized to the initial response amplitude. A, Normalized denatonium responses from type II taste cells that were sensitive to ryanodine are shown in red (n=22). B, Normalized denatonium responses from type II cells that were not sensitive to ryanodine are shown in black (n=31). C, Normalized denatonium responses from type II taste cells that were categorized as ryanodine-insensitive but were not included in the statistical analyses in Figure 2 because the response amplitude continued to decrease even after ryanodine was washed out (n=12). There were two cells that had a large increase in their response amplitude when ryanodine was applied that were also excluded from the statistical analysis. These cells are shown in blue. D, Normalized MPG responses from type II taste cells that were sensitive to ryanodine are shown in red (n=3). E, Normalized MPG responses from type II cells that were not sensitive to ryanodine are shown in black (n=2).
Normalized data of the effects of ryanodine on the response amplitudes of the 50mM KCl-evoked signals in ryanodine sensitive and ryanodine insensitive dual-responsive taste cells. The response amplitude of the evoked calcium signals were measured before, during, and after the application of ryanodine. Values were normalized to the initial response amplitude. A, Normalized responses from dual responsive taste cells that were sensitive to ryanodine are shown in red (n=13). B, Normalized Hi K-evoked responses from dual-responsive taste cells that were not sensitive to ryanodine are shown in black (n=12). C, Normalized responses that were labeled as ryanodine-insensitive but were not included in the statistical analyses in Figure 6 because the response amplitude continued to decrease even after ryanodine was washed out are shown in blue (n=7).
Normalized data of the effects of ryanodine on the response amplitudes of the 50mM KCl-evoked signals in ryanodine sensitive and ryanodine insensitive type III taste cells. The response amplitude of the 50mM KCl-evoked calcium signals were measured before, during, and after the application of ryanodine. Values were normalized to the initial response amplitude. A, Normalized responses from type III taste cells that were sensitive to ryanodine are shown in red (n=6). B, Normalized 50mM KCl-evoked responses from type III taste cells that were not sensitive to ryanodine are shown in black (n=13). C, The response from one cell was labeled as ryanodine-insensitive but was not included in the statistical analyses in Figure 7 because the response amplitude continued to decrease after ryanodine was washed out is shown in blue.