Abstract
Persons with schizophrenia often appraise other individuals as threatening or persecutory. To evaluate social appraisal in schizophrenia, we probed brain networks with a task in which subjects judged whether or not they liked face stimuli with emotional expressions. We predicted that appraising negative expressions would engage patients, more than controls, and negative faces would be related to higher levels of negative affect and produce increased activity in the medial frontal cortex, an area involved in social appraisal. Twenty-one stable outpatients with chronic non-affective psychosis (16 schizophrenic, 5 schizoaffective) and 21 healthy subjects underwent functional magnetic resonance imaging. Compared with the control subjects, patients were slower to respond, but particularly slow when they judged negatively-valenced faces, a slowness correlated with negative affect in the psychosis patients. Appraisal activated the medial prefrontal cortex (mPFC) across all face valences. For negative expressions, patients exhibited greater activation of the dorsal anterior cingulate cortex (dACC). A psychophysiological interaction analysis of the dACC revealed co-modulation of the mPFC in controls, significantly less in patients, and a trend for co-modulation of occipital cortex in the patients. Activity in occipital cortex correlated with poor social adjustment and impaired social cognition, and co-modulation of the occipital gyrus by the dACC was correlated with poorer social cognition. The findings link appraisal of negative affect with aberrant activation of the medial frontal cortex, while early sensory processing of this social cognitive task was linked with poor social function, reflecting either top down or bottom up influences.
Keywords: schizophrenia, anterior cingulate cortex, faces, visual cortex, fMRI BOLD
Appraisal refers to processes that establish value and significance according to personal goals and needs (Ellsworth & Scherer, 2003; Frijda, 1986; Smith & Ellsworth, 1985), and one form of appraisal, relevant for schizophrenia, is social appraisal. As eminently social animals, humans have evolved critical processes for appraising others. First impressions form quickly, often automatically, to guide our behavior (Schiller, Freeman, Mitchell, Uleman, & Phelps, 2009; Willis & Todorov, 2006). Psychosis is often marked by aberrant appraisals of others, as in persecutory and referential delusions, or in social anxiety, a common co-morbid symptom in schizophrenia (Pallanti, Quercioli, & Hollander, 2004; Voges & Addington, 2005). The evaluation of social stimuli by schizophrenia patients has been approached through social cognition, generally using relatively objective tasks, such as the identification of facial emotion. Patients with schizophrenia, as well as relatives, have difficulty identifying emotional expressions (Gur et al., 2007), but important aspects of appraisal that demand the exercise of subjective preference have not been examined in schizophrenia.
Recent work has begun to identify some of the neural systems involved in social appraisal. Appraising a stimulus for personal preference reliably activates the medial prefrontal cortex (mPFC) and anterior cingulate cortex (ACC; Elliott & Dolan, 1998; Killgore et al., 2003; Zysset, Huber, Ferstl, & von Cramon, 2002), and social appraisal in particular has been linked to the dorsal aspect of the mPFC (Mitchell, Heatherton, & Macrae, 2002). The mPFC is also involved in appraisals of other types of emotional stimuli (Ochsner et al., 2004; Phan, Wager, Taylor, & Liberzon, 2002) and personal associations with stimuli (Phan et al., 2004), as well as self-related judgments and judgments about the mental state of other individuals (Amodio & Frith, 2006; Frith & Frith, 1999; Northoff & Bermpohl, 2004). Put more generally, it may be said that in social appraisal, the mPFC engages stimuli in a socio-emotional context, which includes an individual’s preferences, representations of the social world, and emotional valences elicited by that social stimulus. Posterior regions, such as the fusiform face area (FFA; Mitchell et al., 2002) and the superior temporal sulcus (Allison, Puce, & McCarthy, 2000), are thought to carry out perceptual categorization of social stimuli, whereas the amygdala, which has long been implicated in assessing value (Baxter & Murray, 2002; Costafreda, Brammer, David, & Fu, 2008; Sander, Grafman, & Zalla, 2003), appears to detect the relative salience of face stimuli (Gerber et al., 2008; Gobbini & Haxby, 2007; Sander et al., 2003). The posterior cingulate cortex has recently been implicated in the formation of first impressions of individuals (Schiller et al., 2009). Lastly, the network of regions described here exhibits complex interactivity, e. g. the amygdala has been suggested to up-regulate FFA activity when a salient stimulus appears (Vuilleumier & Pourtois, 2007). As with most complex processes, functionality likely emerges through distributed interactions, which probably involve other regions, not as well characterized. Nevertheless, identification of candidate regions that carry out social appraisal has enabled the not only a preliminary mapping of these functions, but an analysis of the dysfunction found in psychiatric conditions.
Many of the nodes involved in social appraisal have also been implicated in schizophrenia. Reduced activation in patients during emotional challenges has been reported in the mPFC (Lee et al., 2006; Taylor, Liberzon, Decker, & Koeppe, 2002; Williams et al., 2004), the ACC (Hempel, Hempel, Schonknecht, Stippich, & Schroder, 2003; Reske et al., 2007) and the amygdala (Gur et al., 2002; Rasetti et al., 2009; Schneider et al., 1998; Taylor, Phan, Britton, & Liberzon, 2005; Taylor, Welsh, Chen, Velander, & Liberzon, 2007), although other groups have found increased activation (Holt et al., 2006a; Kosaka et al., 2002) or tonically elevated activity in the amygdala (Taylor et al., 2005). When viewing faces, schizophrenia patients have exhibited less focused BOLD signal in visual cortex (Pinkham, Hopfinger, Pelphrey, Piven, & Penn, 2008; Seiferth et al., 2009), and electrophysiological studies of face processing have shown reduced amplitude of the N170, an early event-related potential localized to the occipito-temporal junction (Bediou et al., 2007; Turetsky et al., 2007). Abnormal early processing of visual stimuli has been tied to later, higher level processing (Butler & Javitt, 2005; Turetsky et al., 2007), highlighting the importance of interactions between these networks. Since some investigators have shown evidence for reduced connectivity in schizophrenia (Das et al., 2007; Fletcher, McKenna, Friston, Frith, & Dolan, 1999; Foucher et al., 2005; Friston & Frith, 1995), abnormal network interactions may be an important feature of aberrant appraisal in the brain of a person with schizophrenia.
The present study sought to evaluate neural correlates of social appraisal in schizophrenia to identify dysfunctional circuitry related to social processing deficits. In a social preference task, never before studied in schizophrenia patients, subjects viewed face stimuli, with negative, neutral or positive expressions, judging whether or not they liked each face. The preference judgment was contrasted with judging the gender of faces, controlling for basic perception and motor responses.
We had three objectives. The first objective focused on mPFC activity, since this node has been suggested to provide socio-emotional context for appraisals, as reviewed above. Although hypoactivation of the mPFC (Lee et al., 2006; Taylor et al., 2002; Williams et al., 2004) to neutral socio-emotional stimuli has been reported, emotionally negative stimuli have caused increased activity (Crespo-Facorro et al., 2001a; Fakra, Salgado-Pineda, Delaveau, Hariri, & Blin, 2008; Paradiso et al., 2003; Rajarethinam et al., 2005; Taylor et al., 2002; Taylor et al., 2007). Following up on this previous work, we predicted that patients with chronic psychosis would exhibit a hyperactive response in the mPFC when appraising negatively-valenced faces. Negative affect is a significant feature of schizophrenia. For example, behavioral studies show that schizophrenia patients engage more negative affective states, exhibited in higher levels of trait negative affect (Blanchard, Mueser, & Bellack, 1998; Horan, Blanchard, Clark, & Green, 2008), experience mildly stressful situations as particularly aversive (Docherty, 1996; Horan et al., 2005; Jones & Fernyhough, 2007) and appraise neutral and positive stimuli as more aversive in laboratory tests (Cohen & Minor, 2010). Since the mPFC is engaged both by social and emotional stimuli (Ochsner, 2008; Phan et al., 2002), a greater mPFC signal in patients may represent greater ‘resonance’ with negative stimuli, possibly connected with a tendency to misconstrue social situations, a compensatory response for impairments in other symptoms, or inefficient regulation of negative emotions.
Additional objectives explored behavioral correlations and network relationships of the mPFC. As not all groups have found that aversive content induces a hyperactive signal in the mPFC (Takahashi et al., 2004), it has been suggested that the presence of high positive symptoms might account for differences between studies, such that patients with more delusional interpretations of the environment may be more engaged by negative stimuli (Taylor et al., 2007). Thus, a second objective was to explore symptom correlations with appraisal-related activation and test the prediction that mPFC activity would correlate with positive symptoms of reality distortion when appraising negative faces. We also explored correlations with performance measures of social cognition and social adjustment, searching for relationships between appraisal-related regions and poor social function. Our third objective was to evaluate connectivity patterns during appraisal, predicting that patients would have less connectivity in medial frontal regions during appraisal and evaluating connectivity with posterior areas involved in early sensory processing. As will be shown, the findings revealed dysfunction of the mPFC during social appraisal, as well as the association of impaired social cognition with early sensory processing.
Methods
Subjects
From a university-staffed community mental health center, 22 stable, medicated outpatients were recruited with DSM-IV schizophrenia (17) or schizoaffective (5) disorder (American Psychiatric Association, 1994) established by a Structured Clinical Interview for Diagnosis (First, Spitzer, Gibbon, & Williams, 1995). All patients were without active depression, alcohol/substance abuse/dependence (> 6 months without abuse/dependence), and significant medical illness that could affect cerebral function (e. g. diabetes mellitus, hypertension). Because one schizophrenic subject failed to follow instructions during scanning, he was excluded from the analysis, leaving 21 subjects for analysis (Table 1). Subject assessment included clinical ratings by an experienced clinician (SFT) on the Brief Psychiatric Rating Scale (Overall & Gorham, 1962), Hamilton Scale for Depression, 17 item (HAM-D; Hamilton, 1960), and the Scale for the Assessment of Negative Symptoms (Andreasen, 1983).
Table 1.
Demographic and clinical characteristics of subjects
| Mean ± SD | Psychosis patients (n = 21) | Healthy control subjects (n = 21) | t, χ2 | P = |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 40.7 ± 9.3 | 39.8 ± 10.3 | 0.31 | 0.76 |
| Males/females | 14/7 | 15/6 | 0.11 | 0.74 |
| Parental education, y | 15.7 ± 3.3 | 15.5 ± 3.0 | 0.15 | 0.88 |
| Subject education, y | 14.4 ± 2.6 | 16.5 ± 3.5 | 2.18 | 0.04 |
| Socio-economic status | 2.6 ± 0.7 | 2.5 ± 0.6 | 0.56 | 0.58 |
| Behavioral measures | ||||
| WRAT-R reading | 49.9 ± 5.4 | 51.2 ± 4.6 | 0.86 | 0.39 |
| MSCEITa | 0.39 ± 0.08 | 0.47 ± 0.06 | 3.55 | 0.001 |
| SAS | 2.13 ± 0.42 | 1.56 ± 0.26 | 5.36 | < 0.001 |
| Negative emotion (DES) | 2.43 ± 0.83 | 1.71 ± 0.44 | 3.47 | 0.001 |
| Clinical measures | ||||
| Duration ill, y | 19.5 ± 12.3 | |||
| Hospitalizations, # | 3.9 ± 2.4 | |||
| BPRS total | 34.0 ± 7.0 | |||
| BPRS positive | 10.1 ± 4.0 | |||
| BPRS negative | 7.7 ± 3.1 | |||
| BPRS anxiety | 2.7 ± 1.0 | |||
| SANS global sum | 6.2 ± 2.9 | |||
| HAM-D-17 | 5.2 ± 2.4 | |||
Abbreviations: WRAT-R: Wide Range Achievement Test, revised, Reading subtest; MSCEIT: Mayer-Salovey-Caruso Emotional Intelligence Test; SAS: Social Adjustment Scale (lower scores = better adjustment); DES: Differential Scale of Emotions; BPRS: Brief Psychiatric Rating Scale; SANS: Scale for the Assessment of Negative Symptoms, HAM-D-17: Hamilton Scale for Depression, 17 item
20 patients for the MSCEIT, standardized scores, adjusted for age and gender
Twenty-one healthy comparison subjects were recruited from community advertisements, selected to match the age range, gender distribution and family education level of the patients. They were without Axis I psychiatric disorders (Structured Clinical Interview for Diagnosis, non-patient version (First, Spitzer, Gibbon, & Williams, 1996), without first-degree relatives with psychosis, and not taking medication.
As a measure of educational achievement, subjects took the Wide Range Achievement Test, revised, Reading subtest (WRAT-R; Jastak & Wilkinson, 1984). For assessment of socio-emotional function, they completed the Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT), Managing Emotions branch (Mayer, Salovey, & Caruso, 1999) and the Social Adjustment Scale (SAS; Weissman, 1999). Because of our interest in negative emotions, subjects also completed the Differential Emotions Scale (Izard, 1977), as modified by Fredrickson and colleagues (Fredrickson, Tugade, Waugh, & Larkin, 2003), a 20-item scale assesses discrete emotions experienced in the past 2 weeks.
Prior to data collection, the purpose and risks of the study were explained to all subjects, who gave written, informed consent to participate, in a protocol as approved by the University of Michigan institutional review board for adherence to ethical standards of research (Declaration of Helsinki).
In addition to the results reported here, subjects also participated in additional scans and neuropsychological assessments, reported elsewhere (Stern, Welsh, Fitzgerald, & Taylor, 2008; Welsh, Chen, & Taylor, 2010), and a different analysis of the healthy control group has also been published (Chen, Welsh, Liberzon, & Taylor, 2010).
Task Design
The social appraisal task required a simple social judgment about face stimuli with differing emotional expressions. Sixty-fix face stimuli were derived from a set previously published (Kohler et al., 2003) and supplemented with images obtained under identical conditions. Twenty-two individuals portrayed 3 different facial expressions: positive (smiling), neutral or negative (fearful, sad, angry) expressions. Subjects saw each face for 3 sec, above a word directing them to indicate a preference (“Like?”) or identify the gender (“Gender?”) with a button press of the index or middle finger of the right hand (yes/no for preference, male/female for gender). For the appraisal task, subjects were instructed to judge the face quickly, with their first impression. Four faces appeared in a block, with the same valence and judgment, separated by rest periods from 4 to 8 seconds when a fixation cross appeared.
Subjects practiced the task, initially outside and again inside the scanner (viewing faces not used during data acquisition). In the scanner, stimuli were presented via MRI-compatible, high resolution LCD goggles (Resonance Technology, Northridge, CA). After the fMRI scan, subjects rated each face seen during the scan for preference on a 5-point Likert scale.
Functional MRI acquisition
MRI scanning occurred on a GE 3T Signa scanner (LX [8.3] release, General Electric Healthcare, Buckinghamshire, United Kingdom). A T1-weighted image was acquired in the same prescription as the functional images to facilitate co-registration. Functional images were acquired with a T2*-weighted, reverse spiral acquisition sequence (gradient recalled echo, TR=2000 msec, TE=30 msec, FA=90 degrees, field of view=20 cm, 40 slice, 3.0mm thick/0mm skip, equivalent to 64 × 64 voxel grid) sensitive to signal in ventral medial frontal regions (Yang et al., 2002). Subjects underwent 4 runs, each consisting of 166 volumes plus 4 initial, discarded volumes to allow for equilibration of scanner signal, for a total of 664 volumes, with isotropic voxels 3 mm on edge. Total duration of the task was approximately 22 min. After acquisition of functional volumes, a high resolution T1 scan was obtained for anatomic normalization.
fMRI Data Analysis
Data processing began with standard pre-processing steps. Sinc-interpolation, weighted by a Hanning kernel, in time, slice-by-slice, was corrected for the staggered sequence of slice acquisition, and all scans were realigned to the first image acquired during a scanning session (“mcflirt”, Jenkinson, Bannister, Brady, & Smith, 2002). Re-alignment parameters were inspected as a proxy for subject movement, in order to ensure that movement did not exceed either 1 voxel or 1 degree rotation within a run. The remainder of pre-processing and image analysis was performed using the Statistical Parametric Mapping SPM2 package (Wellcome Institute of Cognitive Neurology, London). Parameters for anatomic normalization (MNI152 brain) were derived from the high resolution T1 image and applied to the time series of co-registered, functional volumes, which were re-sliced and smoothed with a 6 mm isotropic Gaussian smoothing kernel. Time series of smoothed, normalized images were high pass filtered (128 sec), and regressors of interest, for each of the three face valences (NEG, NEUT, POS), crossed with the two tasks (PREF and GENDER), were constructed for each 12-second block in which face stimuli appeared, with the variable inter-block interval treated as an implicit baseline. Regressors were convolved with a canonical hemodynamic response function (HRF) at the subject level, yielding estimates for the magnitude (height) of the HRF. To evaluate the effect of explicit appraisal, contrasts for PREF-GENDER were calculated for each of the three valences. To evaluate the effect of valenced stimuli on appraisal, contrasts were calculated within the PREF task between the three face valences.
For group (schizophrenic/schizoaffective: ‘Schiz’; healthy controls: ‘Ctrl’) and between-group analyses, contrast images derived for each subject were smoothed again with a 6mm Gaussian kernel to stabilize variance properties, gray matter-masked and entered into a second level, random effects analysis. Behavioral data demonstrated increases in RT during PREF, compared to GENDER, and amongst the different face valences (see Results). This increase differed between the groups; thus, to remove BOLD effects that only reflected differences in RT, RT differences in the task contrast were entered as a co-variate of no-interest at the second level. By controlling for time-on-task between subjects, this approach focused the analysis on the process of appraisal -- evaluating the likeability of the face -- and not on the fact that some subjects spent more time on a decision. We derived clusters from t-maps for between-group effects, Z-transformed and thresholded at a Z-value of 2.58, and size > 30 voxels (810 μl). To minimize Type 2 errors, we report figures and tables with these uncorrected thresholds, but we limit our attention to clusters that exceeded a corrected probability of 0.05, using a Gaussian random field correction across the entire brain volume (Worsley & Friston, 1995). Since a priori hypotheses focused on the medial frontal cortex, a small volume correction was also used for findings in this region (−18 < x < 18, 0 < y < 70, −30 < z < 70).
A psychophysiological interaction (PPI) analysis was performed to identify regions interacting with a designated region of interest. PPI provides a within-subject measure of functional interactions (‘coupling’) between brain regions, interacting with tasks. Based on the assumption that structures involved in the same functional network ‘co-modulate’ activity while carrying out specific tasks, connectivity is inferred by significant changes in correlation, as a function of task manipulation, between the time courses of regional neuronal activity at the within-subject level (Friston et al., 1997). To calculate the interaction, the time-series of the first eigenvariate (from the primary model) for a seed ROI (9mm radius) was extracted, de-convolved with a canonical hemodynamic response function (Gitelman, Penny, Ashburner, & Friston, 2003), and multiplied by a binary vector coding for the task (‘psychological factor’), yielding an element-by-element product. This product was then convolved with the canonical hemodynamic response function and entered as the psycho-physiological interaction term (PPI.ppi) in the PPI model, with a high pass filter (128 sec) as well as AR1 temporal autocorrelation. Subsequently, positive and negative contrast weights were placed on the ‘PPI.ppi’ regressor to test for positive and negative psycho-physiological interactions, respectively. The contrast images generated were then entered into a random effect group analysis, testing for effects as described above. A significant effect in the psycho-physiological interaction term for a voxel implies that there is a change in correlation slope between neural activity in the voxel (‘coupled region’) and those in the seed ROI, as a function of task condition, e. g. PREF vs. GENDER.
We conducted exploratory correlations with socio-emotional functional measures and symptom ratings in separate AnCOVAs, for SAS, MSCEIT, SANS sum of global scores and BPRS positive symptoms (sum of: hallucinatory experience, unusual thought content, suspiciousness) and HAM-D scores. Scatter plots of significant foci were examined to rule out correlations driven by outliers (> 3 S. D. from mean). Because of the exploratory nature of this analysis, a more stringent threshold was adopted, such that only clusters significant after whole brain correction are reported.
Behavioral data analysis
The behavioral data was analyzed with SPSS, version 16, in mixed model ANOVAs, for accuracy (2-way for GENDER, with group and valence as fixed factors) and in-scan reaction time (3-way, with task, group and valence as fixed factors).
Results
Behavioral results
On measures of social cognition (managing emotion subtest of the MSCEIT) and social adjustment, patients were more impaired than the control groups (Table 1). Also, on the measure of self-assessed negative emotion, the psychotic patients reported more negative affects in the preceding two weeks than the control subjects.
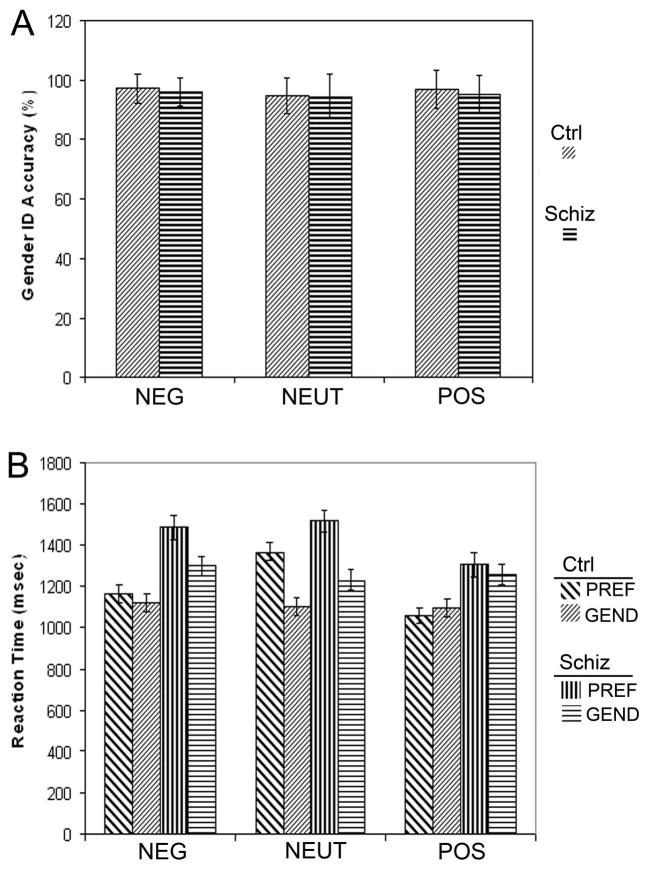
Comparing reaction time for the PREF and GENDER tasks, there was an overall effect of task (F[1,40]=28.4, p=0.000), such that RTs were slower for PREF, compared to GENDER (Figure 1), and a main effect of valence (F[2, 80]=30.2, p=0.000), as well as an interaction between valence and task (F[2, 80]=40.9, p=0.000), such that RT slowed for PREF in some valences, while RT for GENDER remained relatively unchanged across valence. As expected, the patients were slower overall, in both tasks (F[1, 40]=11.5, p=0.002), and there was a significant interaction between group and valence (F[2, 80]=5.77, p=0.005), which reflected greater slowing for the patients when they evaluated NEG faces. A three-way interaction between group, valence and task, exhibited trend significance (F[2, 80]=2.21, p=0.11). While both groups were quickest to the POS valence, and slowed equally for the NEUT condition, the patients had their longest response times to the NEG condition. For the POS faces, the difference between PREF and GENDER tasks was non-significant (p=0.3) for the patients, and the controls were nominally faster for PREF compared to GENDER.
Figure 1.
The figure indicates the performance data for social appraisal (PREF) and gender identification (GEND) tasks, for each valence of the faces (NEG, NEUT or POS): (A) Accuracy is depicted for gender identification, for the patients (Schiz) and the healthy control subjects (Ctrl); there were no effects of group. (B) Reaction time is shown for the GEND and PREF tasks for each group. See text for ANOVA results. Error bars indicate standard error.
We examined correlations between the differential reaction time for the preference task (PREF - GENDER), and measures of negative affect (BPRS anxiety, HAM-D, DES negative emotion), finding that reaction time increase correlated directly with BPRS anxiety and DES negative emotion for NEG faces (r = 0.56, p = 0.008; r = 0.45, p = 0.04) and NEUT faces (r = 0.60, p =0.004; r = 0.46, p = 0.04), but not POS faces (r = 0.26 p = 0.26; r = 0.30 p = 0.18). There were no significant correlations with HAM-D depression.
The patients and controls exhibited the same pattern of responses in preference, preferring POS > NEUT > NEG. There were no differences in accuracy for gender identification (Figure 1; F[1,40] = 0.17, p = .683). See supplementary materials for details of the reliability analysis for the appraisal task and emotion identification of the faces.
fMRI results -- Effect of appraisal
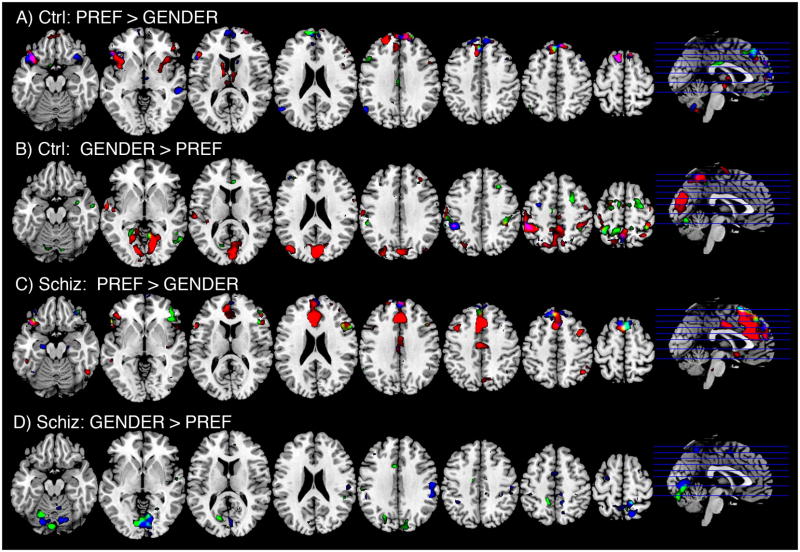
Appraising emotional face stimuli, compared to judging gender (PREF > GENDER), elicited activity in frontal regions, particularly the mPFC, as well as bilateral inferior frontal/insular areas, some lateral frontal regions and cerebellar areas (Figure 2, Tables 2, 3, 4). As predicted, the schizophrenic subjects engaged more of the medial frontal cortex, specifically a focus in the dorsal ACC when appraising NEG face stimuli (Table 2, and Figure 3). For NEUT stimuli, the patients exhibited a nominally larger cluster of activation, but not significantly different from controls, except for greater activation in the right lateral frontal cortex and the right insula. The only area where the control subjects exhibited greater activation than the patients occurred in the cerebellar vermis, when appraising POS faces.
Figure 2.
BOLD signal changes for the contrast of social appraisal (PREF) and gender identification (GEND), for each group. Contrasts are mapped onto a reference image at planes corresponding to the lines through the sagittal images on the far right (Z levels, from left to right, are −18, −2, 13, 23, 33, 43, 52, 62). Red = NEG faces; Green = NEUT faces; Blue = POS faces. Display is thresholded to display Z-scores > 2.59.
Table 2.
Negative faces: Preference > gender identification
| Regiona | Ctrl | Schiz | Schiz > Ctrl | Ctrl > Schiz | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (x, y, z)b | Clusterc | Zd | (x, y, z) | Cluster | Z | (x, y, z) | Cluster | Z | (x, y, z) | Cluster | Z | |
| Anterior cingulate g | −9, 30, 24 | 986 | 5.67 | −9, 30, 24 | 112 | 5.07 | no regions over threshold | |||||
| Superior medial frontal g | −9, 21, 63 | 449 | 4.70 | −3, 24, 45 | 5.08 | 9, 24, 39 | 44 | 3.95 | ||||
| −9, 57, 3 | 46 | 4.73 | ||||||||||
| R superior medial frontal g | 9, 60, 36 | 73 | 4.23 | |||||||||
| Medial orbital cortex | 6, 57, −15 | 43 | 3.38 | |||||||||
| R superior frontal g | 27, 63, 9 | 37 | 3.60 | |||||||||
| L inferior frontal/orbital | −36, 21, −15 | 372 | 4.08 | −36, 24, −18 | 230 | 3.92 | ||||||
| −48, 36, 3 | 3.90 | −54, 24, −3 | 3.84 | |||||||||
| R inferior frontal/triangular | 48, 36, 9 | 269 | 3.76 | 51, 36, 15 | 147 | 3.40 | ||||||
| R inferior frontal/orbital | 48, 24, −15 | 71 | 3.32 | |||||||||
| L middle frontal g | −42, 15, 42 | 63 | 3.83 | |||||||||
| L inferior frontal/triangular | −57, 18, 15 | 37 | 3.97 | |||||||||
| R middle frontal g | 36, 9, 51 | 47 | 4.27 | |||||||||
| L inferior temporal g | −51, 6, −42 | 66 | 3.84 | −45, 6, −33 | 39 | 3.47 | ||||||
| Mid cingulate g | −3, −12, 42 | 144 | 3.96 | 6, −21, −45 | 36 | 3.17 | ||||||
| R inferior parietal | 42, −57, 51 | 43 | 4.04 | 42, −57, 51 | 37 | 3.46 | ||||||
| R precuneus | 15, −69, 45 | 31 | 3.24 | 6, −66, 54 | 150 | 3.69 | ||||||
| R lingual g | 15, −69, −9 | 66 | 3.80 | |||||||||
| L caudate/putamen | −12, 9, 15 | 223 | 3.94 | |||||||||
| L hippocampus | −15, −39, 9 | 46 | 3.23 | |||||||||
| L cerebellum | −27, −78, −36 | 220 | 4.33 | −48, −66, −30 | 113 | 3.51 | −45, −57, 27 | 67 | 3.72 | |||
| −3, −57, −48 | 57 | 3.50 | ||||||||||
| R cerebellum | 48, −57, −39 | 101 | 3.70 | |||||||||
| Vermis | 6, −33, −36 | 50 | 3.90 | |||||||||
Regions from (Tzourio-Mazoyer et al., 2002), g = ‘gyrus’, L = ‘left’, R = ‘right’
Stereotactic coordinates from MNI152 reference, left/right, anterior/posterior and superior/inferior, respectively.
Cluster size in voxels; underline indicates p < 0.05, corrected for whole brain
Z-scores of maxima without cluster size are within cluster listed above; underlined values are p < 0.05, FDR corrected for whole brain (Genovese, Lazar, & Nichols, 2002)
Table 3.
Neutral faces: Preference > gender identification
| Regiona | Ctrl | Schiz | Schiz > Ctrl | Ctrl > Schiz | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (x, y, z)b | Clusterc | Zd | (x, y, z) | Cluster | Z | (x, y, z) | Cluster | Z | (x, y, z) | Cluster | Z | |
| Superior medial frontal g | −12, 63, 24 | 56 | 4.49 | |||||||||
| 3, 63, 24 | ||||||||||||
| −3, 36, 45 | 91 | 3.93 | 3, 18, 63 | 176 | 4.14 | |||||||
| 0, 21, 48 | 3.12 | 6, 33, 57 | 4.05 | |||||||||
| L inferior frontal/orbital | −45, 45, −15 | 65 | 3.98 | |||||||||
| R inferior frontal/orbital | 45, 30, −6 | 290 | 4.26 | |||||||||
| 45, 24, 12 | 3.79 | |||||||||||
| 57, 18, 21 | 3.71 | |||||||||||
| L middle frontal g | −48, 21, 39 | 73 | 3.63 | |||||||||
| R middle frontal g | 27, 6, 54 | 109 | 3.85 | |||||||||
| R insula | 39, 9, 6 | 95 | 3.44 | |||||||||
| L insula | −36, 3, 3 | 68 | 4.16 | |||||||||
| R inferior temporal g | 48, 0, −45 | 35 | 3.57 | |||||||||
| Mid cingulate g | 0, −18, 30 | 51 | 3.50 | −3, −24, 30 | 36 | 3.54 | ||||||
| L mid-temporal area | −45, −6, 30 | 75 | 3.83 | |||||||||
| L hippocampal area | −27, −15, −15 | 50 | 3.25 | |||||||||
| R parahippocampal area | 27, −27, −18 | 35 | 3.48 | |||||||||
Regions from (Tzourio-Mazoyer et al., 2002), g = ‘gyrus’, L = ‘left’, R = ‘right’
Stereotactic coordinates from MNI152 reference, left/right, anterior/posterior and superior/inferior, respectively.
Cluster size in voxels; underline indicates p < 0.05, corrected for whole brain
Z-scores of maxima without cluster size are within cluster listed above; underlined values are p < 0.05, FDR corrected for whole brain (Genovese et al., 2002)
Table 4.
Positive faces: Preference > gender identification
| Ctrl | Schiz | Schiz > Ctrl | Ctrl > Schiz | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Regiona | (x, y, z)b | Clusterc | Zd | (x, y, z) | Cluster | Z | (x, y, z) | Cluster | Z | (x, y, z) | Cluster | Z |
| Superior medial frontal g | −9, 18, 63 | 660 | 4.06 | −9, 43, 54 | 496 | 4.30 | No regions over threshold | |||||
| 6, 42, 51 | 3.99 | 3, 54, 36 | 4.11 | |||||||||
| 12, 63, 6 | 4.04 | |||||||||||
| L inferior frontal/orbital superior temporal pole | −42, 24, 21 | 290 | 4.95 | |||||||||
| −54, 21, 12 | 3.91 | |||||||||||
| R inferior frontal/orbital insula | 48, 24, −12 | 97 | 4.13 | |||||||||
| 33, 24, −9 | 3.04 | |||||||||||
| R insula | 33, 24, −18 | 55 | 3.57 | |||||||||
| L middle temporal g/pole | −51, 3, −33 | 45 | 3.39 | −48, 18, −30 | 102 | 4.35 | ||||||
| −45, 18, −15 | 3.28 | |||||||||||
| R middle temporal g | 54, −36, −6 | 73 | 4.15 | |||||||||
| L angular g | −57, −66, 30 | 66 | 4.06 | |||||||||
| L hippocampus/amygdala | −21, −15, −18 | 36 | 3.68 | |||||||||
| L cerebellum | −33, −78, −36 | 60 | 3.85 | −12, −63, −33 | 49 | 3.40 | ||||||
| −15, −78, −24 | 37 | 3.08 | ||||||||||
| −48, −69, −39 | 42 | 3.25 | ||||||||||
| Vermis | −3, −60, −39 | 97 | 3.60 | −3, −54, −27 | 134 | 3.66 | ||||||
Regions from (Tzourio-Mazoyer et al., 2002), g = ‘gyrus’, L = ‘left’, R = ‘right’
Stereotactic coordinates from MNI152 reference, left/right, anterior/posterior and superior/inferior, respectively.
Cluster size in voxels; underline indicates p < 0.05, corrected for whole brain
Z-scores of maxima without cluster size are within cluster listed above; underlined values are p < 0.05, FDR corrected for whole brain (Genovese et al., 2002)
Figure 3.
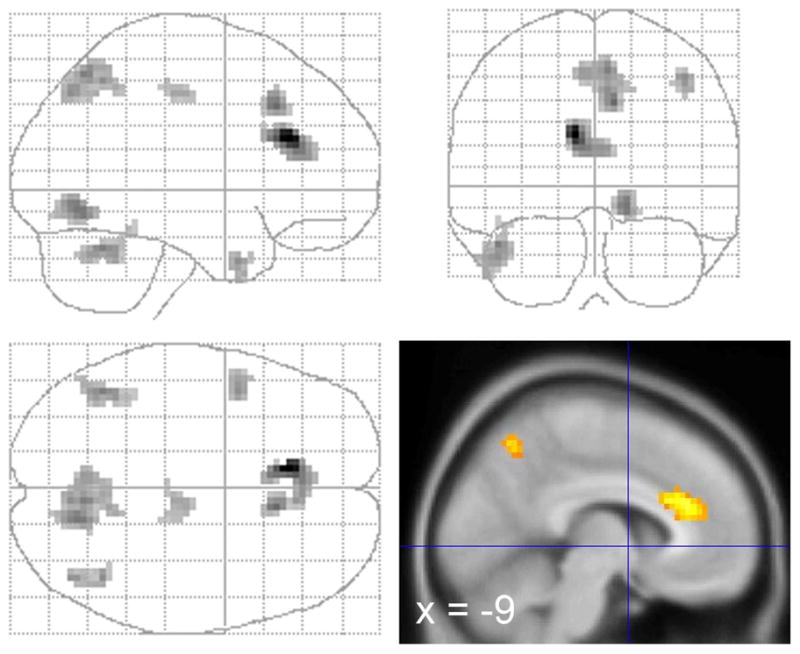
Activity where schizophrenic/schizoaffective patients showed a greater activation in the appraisal task (PREF > GENDER) than the controls, while viewing NEG faces. Clusters are displayed for Z > 2.59, voxel count > 30.
The patients also exhibited a significantly greater focus of activation in the precuneus while appraising the NEG faces. This contrast reached significance for a small focus for PREF > GENDER in the patients. However, a larger focus for GENDER > PREF in the control group demonstrated that the group difference reflected both effects, i. e. the patients increased activity, and the controls decreased activity for appraisal, relative to gender judgment (Figure 2, and supplementary materials, Tables S2, S3, S4). In general, both groups showed more posterior activity in visual cortical regions for GENDER > PREF, for both NEUT and POS faces, with the exception of the patients appraising the NEG faces. For the NEG faces, the patients exhibited no significant BOLD activity for GENDER > PREF in posterior regions engaged in visual processing.
The only sign of amygdala activity occurred for appraising POS faces for the patient group, but this was not significant after whole-brain correction, and not significantly different between groups. Even at a cluster threshold of 8 voxels, we found no amygdala activity during appraisal (PREF > GENDER).
fMRI results --Symptom and functional correlations
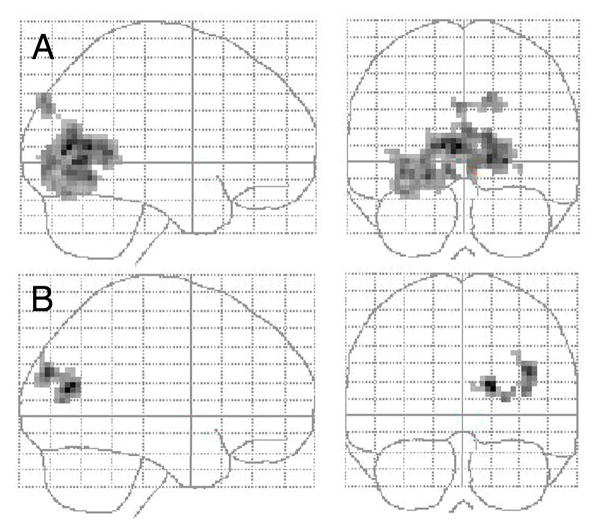
Correlations were calculated for the contrast images for NEG stimuli in the PREF-GENDER contrast, since this contrast figured in our primary hypothesis about increased salience of negative social stimuli for the patients and it exhibited the largest group differences. Correlations were conducted on extracted beta values from the dACC focus of group difference. There was a significant inverse correlation between HAM-D depression scores and ACC activity (r= −0.47, p=0.03), but no significant correlations with BPRS positive symptoms, negative symptoms, SANS scores, MSCEIT or SAS measures (p > 0.2). There were no significant correlations with chlorpromazine dose equivalents, gender or diagnosis (schizophrenia vs schizoaffective; all p’s > 0.2). Using voxel-by-voxel correlations, we did not find a correlation with BPRS positive symptoms in the medial cortex, even when we lowered the threshold to accept clusters as small as 8 voxels. We did not find correlations exceeding our threshold for negative symptoms, as measured by the SANS. However, significant correlation foci arose in the posterior occipital areas (see Figure 4) with MSCEIT and SAS measures, such that poorer performance on MSCEIT social cognition correlated with activity in the occipital lobe (right superior occipital gyrus, calcarine cortex; 18, −72, 15; k=123, p=0.004), and poor SAS social adjustment also correlated with occipital lobe activity (bilateral calcarine cortex, precuneus, lingual gyrus; −3, −72, 9 and 15, −66, 0; k=849, p=0.000). Examining the correlations in more detail, by extracting beta values, showed that patients with good social cognition and good social adjustment exhibited the pattern of GENDER > PREF, seen in the control subjects. In other words, poor social cognition and social adjustment was associated with greater activity during PREF, relative to GENDER, in the patients.
Figure 4.
BOLD signal in the occipital cortex of the patients during social appraisal (PREF - GENDER) to aversive face stimuli directly correlates with (A) poor SAS social adjustment and (B) poor MSCEIT social cognition. Only clusters at the whole-brain corrected significance level of p > 0.05 are displayed.
fMRI results -- Connectivity analysis with medial frontal cortex
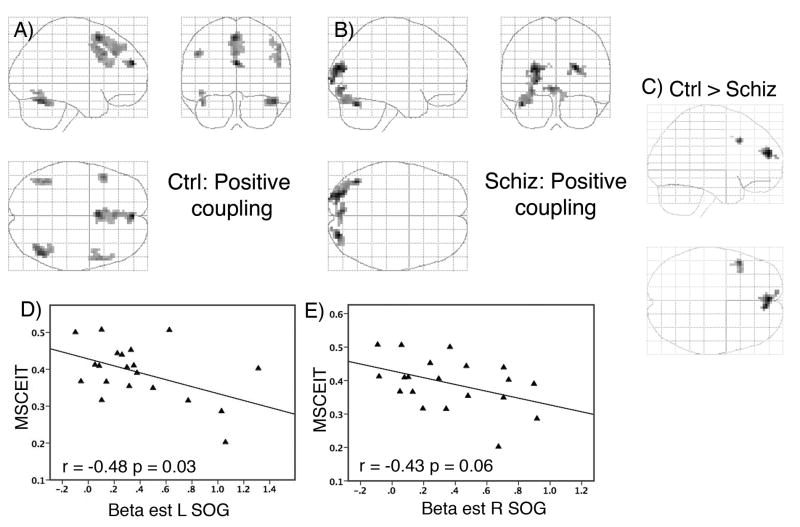
To evaluate interactions between the medial cortex and other brain areas, we performed a PPI analysis, which analyzed co-variation of BOLD activity, at the level of each subject, which interacted with the GENDER and PREF tasks. To ensure adequate statistical power, all faces were included and a dACC focus (−12, 30, 27) from the full analysis (all faces combined), which matched the dACC focus (−9, 30, 24) for appraising NEG faces, was selected as a seed for analysis. Results are shown in Figure 5 and Table 5. In the control subjects, positive coupling was found between the dACC and the mPFC, including superior aspects of the superior medial frontal gyrus, as well as bilateral frontal cortex. Coupling was also observed with both fusiform face areas, although below the threshold for whole-brain correction. The patients exhibited a different pattern of positive coupling, with significantly less mPFC coupling than the control group (ctrl > schiz at 0, 54, 21; Z = 3.96, k=30, p = 0.04, small volume corrected). On the other hand, the patients exhibited trend-level coupling with different areas of the occipital cortex: bilateral superior/middle occipital gyrus, left lingual gyrus, and the left cerebellum. The control subjects exhibited negative coupling with a cluster in the left superior temporal region, whereas the patients showed no negative coupling (ctrl > schiz at −36, −12, 12; Z=4.99, k= 190, p=0.006).
Figure 5.
Psychophysiological interaction (PPI) results show the positive coupling for the (A) healthy subjects and (B) the schizophrenic/schizoaffective patients with a seed region in the dACC, as well as (C) the greater connectivity in the mPFC for the healthy subjects. Clusters are displayed for Z > 2.59, voxel count > 30. The extent of positive coupling depicted in (B) is inversely related to social cognitive skill (MSCEIT), as depicted by extracted beta estimates from the left superior occipital gyrus (SOG; D) and right superior occipital gyrus (E).
Table 5.
Connectivity analysis for dACC -- Psychophysiological interaction
| Ctrl | Schiz | |||||
|---|---|---|---|---|---|---|
| Regiona | (x, y, z)b | Clusterc | Zd | (x, y, z) | Cluster | Z |
| Positive coupling | ||||||
| L superior medial frontal g | 0, 54, 24 | 39 | 4.67 | |||
| −3, 12, 57 | 193 | 4.24 | ||||
| −6, 21, 48 | 3.40 | |||||
| L middle frontal g | −48, 18, 36 | 33 | 3.73 | |||
| R middle frontal g | 54, 9, 45 | 99 | 3.41 | |||
| L fusiform g | −42, −57, −24 | 42 | 3.57 | |||
| R fusiform g | 45, −63, −21 | 75 | 4.07 | |||
| R superior/middle occipital g | 24, −87, 21 | 62 | 4.01 | |||
| L superior/middle occipital g | −27, −87, 18 | 110 | 3.91 | |||
| L calcarine cortex/lingual g | −6, −84, −6 | 59 | 3.52 | |||
| L cerebellum | −42, −66, −27 | 108 | 3.73 | |||
| Negative coupling | ||||||
| L superior temporal g | −42, −24, 6 | 200 | 3.61 | |||
Regions from (Tzourio-Mazoyer et al., 2002), g = ‘gyrus’, L = ‘left’, R = ‘right’
Stereotactic coordinates from MNI152 reference, left/right, anterior/posterior and superior/inferior, respectively
Cluster size in voxels; underline indicates p < 0.05, dashed underline p < 0.10, corrected for whole brain,
Z-scores of maxima without cluster size are within cluster listed above; underlined values are p < 0.05, FDR corrected for whole brain (Genovese et al., 2002)
The PPI signals in the occipital lobe exhibited some overlap with the MSCEIT and SAS correlations with the preference task (regions corresponding to Brodman areas 17 and 18). To determine if the PPI coupling was associated with social functioning, we extracted the beta estimates, averaged across the clusters, for the PPI term from the three foci in the occipital cortex (from Table 5). Analysis showed that poor performance on the MSCEIT was associated with more modulation of the superior/middle occipital gyrus by the dACC (L occipital gyrus: r = −0.48, p=0.03; R occipital gyrus: r = −0.43, p=0.06; see Figure 5D & E). Other correlations were not significant (p’s > 0.10).
Discussion
The explicit social appraisal task employed here was designed to tap neurocircuits in a disorder with well-known social impairments. The task was not meant to elicit correct or incorrect judgments; rather, it served to engage medial frontal cortex, which it did across the three face valences, and within each group. Behaviorally, the psychosis patients had difficulty with the negative faces, taking more time to appraise these faces, a slowing which correlated with negative emotions in the patients. The patients also showed aberrant activity in the mPFC for the negative faces, exhibiting a hyperactive BOLD signal and reduced connectivity of the dACC with the mPFC. These findings highlight how negatively-valenced social stimuli elicit aberrant activity in the brain. The results also pointed to the involvement of early visual processing regions in social information processing and adjustment. The social appraisal task required visual processing of the stimuli, and deficits in complex social behavior (managing emotion and social adjustment) correlated with this early visual processing. Furthermore, the connectivity analysis demonstrated that co-modulation of the dACC with early sensory processing, not observed in the control subjects, was associated with poor social cognition. Taken together, the findings describe a pattern of aberrant medial cortical activity and aberrant connectivity of the medial cortex with early sensory areas during social appraisal in schizophrenia/schizoaffective disorder.
Processing negative affect in chronic psychosis
Behaviorally, the patients were more engaged by the negative stimuli, and this extra engagement was associated with negative affect in the patients. The negative socio-emotional stimuli were predicted to engage the schizophrenic subjects, based on several lines of work indicating greater negative affect in the experience of patients with schizophrenia. For example, persons with schizophrenia tend to have more negative experiences in everyday life (Myin-Germeys, Delespaul, & deVries, 2000) and in laboratory settings (Docherty, 1996; Horan et al., 2005; Jones et al., 2007). They also exhibit more negative trait affectivity (Horan et al., 2008) and exhibit high sensitivity to minor stresses (Horan et al., 2005). Laboratory studies have shown more speech disorganization in response to negatively-valenced questions in schizophrenia (Burbridge & Barch, 2002), and delusional patients have exhibited a negative bias in classifying words as pleasant or unpleasant, compared to non-delusional patients (Holt et al., 2006b). A recent meta-analysis of laboratory studies of emotional experience concluded that patients with schizophrenia tend to appraise neutral and positive stimuli as more negative than controls (Cohen et al., 2010). In our study, requiring patients to appraise an angry or sad face was intended to have a particularly strong resonance with socio-emotional neurocircuits. Accordingly, although they showed the identical pattern of preference, the patients took longer to appraise the negative faces, relative to judging the gender of these faces, whereas the controls found it relatively easy to reject the stimuli (which they did in the majority of cases), with latencies comparable to the judgment of gender. The slower responses of the patients was not due to perceptual difficulties with negative faces, which has been reported (Gur et al., 2007; Kohler et al., 2003), because the groups did not differ in their ability to identify the emotional expressions. We interpret this slowing as behavioral evidence of more engaged appraisal processing, related to the valence of the stimuli.
It was also notable that this slowing in the preference task for negative faces correlated with negative affect, both on clinician-rated (BPRS anxiety) and self-report scales (DES negative), suggesting that the task tapped psychological systems involved in emotion. Correlations with negative affect also occurred with the neutral faces, which took the patients longer to process, relative to gender identification, although in this respect they did not differ from the controls. The neutral faces may have evoked a small amount of negative experience in the psychotic patients, as has been reported in the literature (Cohen et al., 2010), resonating with their own experience of negative affect. From these data, it is not possible to say exactly why this apparently greater engagement with the stimuli associated with the experience of negative affect, although the linkage deserves additional investigation, especially in light of data showing that the experience of negative affect is tied with social outcome and adjustment in schizophrenia (Huppert, Weiss, Lim, Pratt, & Smith, 2001; Narvaez, Twamley, McKibbin, Heaton, & Patterson, 2008; Tso, Grove, & Taylor, 2010).
Social appraisal and medial frontal cortex
The BOLD response during appraisal activated mPFC for all groups and across all valences, as predicted, but the hyperactive response of the patients occurred in the adjacent dACC. Although the mPFC has been associated with socio-emotional processing, the ACC is also co-activated in many socio-emotional tasks (Ochsner et al., 2004; Phan et al., 2002). Hence, the signal in the dACC likely reflects some relationship with mPFC function. A functional association between these medial frontal cortical regions was evident in the connectivity analysis, which used the dACC as a seed to examine interactions with task (PREF versus GENDER). The positive coupling seen in the controls meant that mPFC and lateral PFC changed their relationships with the dACC across the PREF - GENDER task contrast. The positive sign of this interaction could reflect an increase in a direct correlation from PREF to GENDER, or less of an inverse correlation. While we cannot distinguish between the two possibilities, the existence of a PPI signal may reflect effective connectivity between regions (Friston et al., 1997), and the patients showed significantly less integration of activity in the medial frontal cortex. The finding aligns with previous work demonstrating impaired connectivity, measured by fMRI BOLD correlations, of the medial frontal cortex in schizophrenia for cognitive tasks (Fletcher et al., 1999) and for face processing (Das et al., 2007).
Some interpretations of the hyperactivity in the dACC may be offered. In order to process the negative stimuli, the patients may have been inefficient, such that greater dACC activity was required for appraisal, either directly reflecting inefficiency or compensating for other brain areas. There is indirect support from the literature for this interpretation. Resting metabolism/blood flow in the dACC increases with antipsychotic pharmacotherapy (Lahti et al., 2004; Snitz et al., 2005), and has been associated with a positive response of depressed patients to treatment (Brody et al., 2001; Goldapple et al., 2004). In healthy subjects, activation of this region has been associated with re-appraisal of negative stimuli (Ochsner, Bunge, Gross, & Gabrieli, 2002), playing a central role in the integration of cognition, motivation and arousal (Critchley, 2005; Kennerley, Walton, Behrens, Buckley, & Rushworth, 2006). Thus, it may reflect activity involved in re-appraisal or another strategy to manage the negative stimuli. In support of this interpretation, we found that patients with lower HAM-D scores, which picked up more general signs of negative affect, since none of the patients were in depressive episodes at the time of scanning, had greater activity in the dACC. It is not possible to answer these questions from our data, but the findings clearly point to a role for medial frontal cortex in socio-emotional information processing in chronic psychosis.
We did not find the predicted correlation between positive symptoms and medial frontal BOLD signal during appraisal. While it is difficult to speculate about a negative finding, several points should be mentioned. In a previous report, we found an association between positive symptoms (reality distortion) and BOLD activity in dorsal mPFC (Brodman area 10), anterior of the dACC focus in the current report by 3 cm (Taylor et al., 2007). Although these medial frontal regions are co-activated in socio-emotional tasks, as mentioned above, they also carry out distinct functions. The dACC appears to have a more general role, monitoring performance and integrating information about value with response (Botvinick, Cohen, & Carter, 2004; Critchley, 2005; Walton, Croxson, Behrens, Kennerley, & Rushworth, 2007), whereas Brodman area 10 appears to be more activated by self and social knowledge, particularly with close personal associations (Amodio et al., 2006; Mitchell et al., 2002; Phan et al., 2003). The previous study used a weaker appraisal task of complex visual stimuli (subjects simply rated pictures as pleasant or unpleasant), which did not evoke medial frontal activity as robustly as the social appraisal paradigm used here. The weaker appraisal task may have permitted the patients with reality distortion more latitude to associate with the valenced stimuli. In other words, engaging more of medial frontal cortex in the current study may have reduced the variance of the individual responses to the stimuli, reducing the power to detect a correlation with symptoms. Other differences may have influenced the results, and further investigation will be necessary to understand what, if any, connection positive symptoms have with medial frontal activity elicited by appraisal.
Occipital activity and socio-emotional function
An important aspect of our findings was the convergence of several analyses on posterior processing regions, and this was most notable for the negative stimuli. In response to the negative faces, the patients did not show the same pattern of difference (GENDER > PREF), and the deviant activity pattern correlated with poor social adjustment and poor social cognition in the occipital cortex. The connectivity analysis showed that whereas the control subjects exhibited a trend positive modulation between dACC and the FFA, the patients exhibited a trend co-modulation of visual cortical areas, more involved in early sensory processing (Van Essen, Anderson, & Felleman, 1992), which overlapped partially with the regions where socio-emotional dysfunction correlated with BOLD signal. Moreover, this co-modulation of occipital cortex with the dACC correlated with poor socio-emotional dysfunction. In other words, linkage between medial frontal cortex and early visual processing was pathological in our patients. Although one should interpret trend-level findings with caution, the convergence of BOLD signal findings with complex measures of social dysfunction compels consideration.
If the medial frontal cortex is important for the preference task, why did the correlations with social adjustment and social cognition occur in visual processing areas? Although most work on emotional and cognitive function in schizophrenia tends to emphasize prefrontal and limbic regions, early sensory cortex has been implicated in the disorder by several lines of investigation, including socio-emotional functioning. Structural and functional pathology of the visual cortex has been reported in schizophrenia, such as thinning in post-mortem samples (Selemon, Rajkowska, & Goldman-Rakic, 1995), thinning on MRI studies of first episode patients (Narr et al., 2005), poor visual discrimination (O’Donnell et al., 1996), greater susceptibility to visual masking (Green, Nuechterlein, & Mintz, 1994) and electrophysiological evidence of reduced early event-related potentials to face stimuli (Bediou et al., 2007; Turetsky et al., 2007; Yeap et al., 2006). In fMRI BOLD studies of socio-emotional processing, schizophrenia patients have exhibited deficient FFA activation for face stimuli (Pinkham et al., 2008; Seiferth et al., 2009). Deficits in processing non-verbal social cues are modulated by early visual processing deficits (Corrigan, Green, & Toomey, 1994; Kee, Kern, & Green, 1998; Sergi & Green, 2003), and early visual electrophysiological components have been correlated with social and community dysfunction (Butler et al., 2005; Sergi, Rassovsky, Nuechterlein, & Green, 2006). These associations may reflect the lack of appropriate top-down influence on early cortical processing, or they may reflect a primary deficit of cortical circuitry, as has been suggested by some groups (Butler et al., 2007).
The findings reported here may hint at some relationship between top-down and bottom up processing in schizophrenia, permitting some speculation. Early processing deficits as relatively primary impairments would be consistent with the correlations with socio-emotional function in our study. More downstream regions, such as the dACC, may have exhibited a compensatory response to aberrant processing of emotional stimuli, less likely to exhibit simple linear correlations with global functional measures, since the degree of compensation would differ between individuals, not necessarily related to their functional deficit. The PPI co-modulation of the occipital gyrus and the dACC might reflect this compensatory relationship (increased connectivity during the preference task). Alternatively, the PPI finding could reflect less negative coupling in the patients during the preference task, consistent with reports of negative coupling in normally observed in healthy individuals between the ACC and visual cortex (Margulies et al., 2007). These possibilities remain speculative and point to the need for further research. Nevertheless, the findings illustrate to the importance of studying early sensory processing to understand some of the complex functional impairments in schizophrenia.
Other regions
The only significant region where the controls exceeded the patients was in the cerebellum, while judging positive faces. The cerebellum plays a significant role in processing emotional stimuli (Schmahmann, Weilburg, & Sherman, 2007; Schutter & van Honk, 2005), and it is frequently under-activated in schizophrenic patients during cognitive and emotional challenges (Crespo-Facorro et al., 2001b; Honey et al., 2005; Stephan et al., 2001; Takahashi et al., 2004). In the appraisal task, the hypoactivation may reflect the failure of the patients to respond to the hedonically-positive valence of the happy faces. Overall, the reduced activity in the patients for the POS condition contrasts with the hyperactivation observed for the NEG and NEUT conditions, and it demonstrates that abnormal activity is specific to the valence of the faces, and not a global effect of poor attention or poor motivation.
Emotional face stimuli have elicited both hypoactivation (Gur et al., 2002; Russell et al., 2007; Schneider et al., 1998; Williams et al., 2004) and hyperactivation (Holt et al., 2006a; Kosaka et al., 2002) of the amygdala in schizophrenia, but we found no significant activation in the amygdala for the appraisal task, or between valences. Comparing our results to the literature, the absence of a difference between face valences has been reported several times by groups using relatively slow presentations of face stimuli (Fitzgerald, Angstadt, Jelsone, Nathan, & Phan, 2006; Winston, O’Doherty, & Dolan, 2003), and recent work has shown that the amygdala responds to the motivational importance of stimuli, rather than positive or negative valence (Cunningham, Van Bavel, & Johnsen, 2008; Schiller et al., 2009). However, it is not clear why social appraisal did not elicit more activity than gender judgment from the amygdala. It may have been that the face stimuli during gender identification were sufficiently salient that the appraisal task could not evoke more activity from the amygdala. Hence, the study was unable to reveal information about different activity in the amygdala between the patients and controls.
Limitations
Characteristics of the patient sample should be kept in mind when interpreting these results. All of the patients in this study were stable outpatients, with relatively long durations of illness and on stable antipsychotic regimens. It is possible that the increased medial frontal activity in the patients was an effect of medications, but an increased medial frontal signal in response to aversive pictures has been reported in unmedicated patients (Paradiso et al., 2003), although these authors were not looking for mPFC activity. We found no correlation of dACC BOLD signal with CPZ equivalents that might explain our findings (including the connectivity analyses), although CPZ equivalents are an insensitive proxy for medication effects. If the dACC response to NEG faces represents the effect of medication, it would have to be highly specific, since the patients and the controls showed a significant overlap of activation in other regions, and for the other conditions. In posterior regions, the correlations between BOLD activity and socio-emotional function cannot be explained by medication effects, and a hyperactive response of visual cortical regions, identified in our task, has also been reported in unmedicated schizophrenic patients (Andreasen, Calage, & O’Leary, 2008; Crespo-Facorro et al., 2001b; Siegel et al., 1993). Although these facts, together, argue that medication effects cannot account for our results, data from unmedicated patients would be an important step to expand upon findings reported here.
Conclusion
We have demonstrated in chronic psychosis patients the abnormal recruitment of networks involved in social appraisal. The data implicated medial frontal cortex and pointed to posterior regions involved in processing visual stimuli, which were correlated with poor social cognition and poor social adjustment. These findings identify some of the neurocircuits that may be involved in determining the functional outcome of this devastating illness.
Supplementary Material
Acknowledgments
The authors wish to acknowledge the assistance of Wendy Davis for subject recruitment and Keith Newnham for data acquisition. This work was supported the National Institute of Mental Health (R01 MH01258 to SFT), Scottish Rite Schizophrenia Research fund (ACC) and the Boledovitch Schizophrenia Research Fund.
Footnotes
This work has been presented in abstract form at the International Congress on Schizophrenia Research in Colorado Springs, Colorado, March 2007
Disclosures/Conflicts of Interest
All of the authors report no biomedical financial interests or potential conflicts of interest relevant to the subject matter of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends Cogn Sci. 2000;4:267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) Washington, D.C.: American Psychiatric Association; 1994. [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. The scale for the Assessment of Negative Symptoms. Iowa City: The University of Iowa; 1983. [Google Scholar]
- Andreasen NC, Calage CA, O’Leary DS. Theory of mind and schizophrenia: a positron emission tomography study of medication-free patients. Schizophr Bull. 2008;34:708–19. doi: 10.1093/schbul/sbn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3:563–73. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Bediou B, Henaff MA, Bertrand O, Brunelin J, d’Amato T, Saoud M, Krolak-Salmon P. Impaired fronto-temporal processing of emotion in schizophrenia. Neurophysiol Clin. 2007;37:77–87. doi: 10.1016/j.neucli.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Mueser KT, Bellack AS. Anhedonia, positive and negative affect, and social functioning in schizophrenia. Schizophrenia Bulletin. 1998;24:413–424. doi: 10.1093/oxfordjournals.schbul.a033336. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–46. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Brody AL, Saxena S, Mandelkern MA, Fairbanks LA, Ho ML, Baxter LR. Brain metabolic changes associated with symptom factor improvement in major depressive disorder. Biol Psychiatry. 2001;50:171–8. doi: 10.1016/s0006-3223(01)01117-9. [DOI] [PubMed] [Google Scholar]
- Burbridge JA, Barch DM. Emotional valence and reference disturbance in schizophrenia. J Abnorm Psychol. 2002;111:186–91. [PubMed] [Google Scholar]
- Butler PD, Javitt DC. Early-stage visual processing deficits in schizophrenia. Curr Opin Psychiatry. 2005;18:151–7. doi: 10.1097/00001504-200503000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Martinez A, Foxe JJ, Kim D, Zemon V, Silipo G, Mahoney J, Shpaner M, Jalbrzikowski M, Javitt DC. Subcortical visual dysfunction in schizophrenia drives secondary cortical impairments. Brain. 2007;130:417–30. doi: 10.1093/brain/awl233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AC, Welsh RC, Liberzon I, Taylor SF. ‘Do I like this person?’ A network analysis of midline cortex during a social preference task. Neuroimage. 2010:51. doi: 10.1016/j.neuroimage.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Minor KS. Emotional Experience in Patients With Schizophrenia Revisited: Meta-analysis of Laboratory Studies. Schizophr Bull. 2010;36:143–150. doi: 10.1093/schbul/sbn061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan PW, Green MF, Toomey R. Cognitive correlates to social cue perception in schizophrenia. Psychiatry Research. 1994;53:141–51. doi: 10.1016/0165-1781(94)90105-8. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, Fu CH. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Research Reviews. 2008;58:57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Crespo-Facorro B, Paradiso S, Andreasen NC, O’Leary DS, Watkins GL, Ponto LL, Hichwa RD. Neural mechanisms of anhedonia in schizophrenia: a PET study of response to unpleasant and pleasant odors. Jama. 2001a;286:427–35. doi: 10.1001/jama.286.4.427. [DOI] [PubMed] [Google Scholar]
- Crespo-Facorro B, Wiser AK, Andreasen NC, O’Leary DS, Watkins GL, Boles Ponto LL, Hichwa RD. Neural basis of novel and well-learned recognition memory in schizophrenia: a positron emission tomography study. Hum Brain Mapp. 2001b;12:219–31. doi: 10.1002/1097-0193(200104)12:4<219::AID-HBM1017>3.0.CO;2-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. Journal of Comparative Neurology. 2005;493:154–66. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Van Bavel JJ, Johnsen IR. Affective flexibility: evaluative processing goals shape amygdala activity. Psychological Science. 2008;19:152–60. doi: 10.1111/j.1467-9280.2008.02061.x. [DOI] [PubMed] [Google Scholar]
- Das P, Kemp AH, Flynn G, Harris AW, Liddell BJ, Whitford TJ, Peduto A, Gordon E, Williams LM. Functional disconnections in the direct and indirect amygdala pathways for fear processing in schizophrenia. Schizophrenia Research. 2007;90:284–94. doi: 10.1016/j.schres.2006.11.023. [DOI] [PubMed] [Google Scholar]
- Docherty NM. Affective reactivity of symptoms as a process discriminator in schizophrenia. Journal of Nervous and Mental Disease. 1996;184:535–541. doi: 10.1097/00005053-199609000-00004. [DOI] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ. Neural response during preference and memory judgments for subliminally presented stimuli: a functional neuroimaging study. J Neurosci. 1998;18:4697–704. doi: 10.1523/JNEUROSCI.18-12-04697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth PC, Scherer KR. Appraisal processes in emotion. In: Davidson RJ, Scherer KR, Goldsmith HH, editors. Handbook of Affective Sciences. New York: Oxford University Press; 2003. [Google Scholar]
- Fakra E, Salgado-Pineda P, Delaveau P, Hariri AR, Blin O. Neural bases of different cognitive strategies for facial affect processing in schizophrenia. Schizophr Res. 2008;100:191–205. doi: 10.1016/j.schres.2007.11.040. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I disorders, patient edition (SCID-P), ver 2.0. New York: Biometrics Research; 1995. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I disorders, non-patient edition (SCID-I/NP), ver 2.0. New York: Biometrics Research; 1996. [Google Scholar]
- Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KL. Beyond threat: amygdala reactivity across multiple expressions of facial affect. Neuroimage. 2006;30:1441–8. doi: 10.1016/j.neuroimage.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Fletcher P, McKenna PJ, Friston KJ, Frith CD, Dolan RJ. Abnormal cingulate modulation of fronto-temporal connectivity in schizophrenia. Neuroimage. 1999;9:337–42. doi: 10.1006/nimg.1998.0411. [DOI] [PubMed] [Google Scholar]
- Foucher JR, Vidailhet P, Chanraud S, Gounot D, Grucker D, Pins D, Damsa C, Danion JM. Functional integration in schizophrenia: too little or too much? Preliminary results on fMRI data. Neuroimage. 2005;26:374–88. doi: 10.1016/j.neuroimage.2005.01.042. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL, Tugade MM, Waugh CE, Larkin GR. What good are positive emotions in crises? A prospective study of resilience and emotions following the terrorist attacks on the United States on September 11th, 2001. J Pers Soc Psychol. 2003;84:365–76. doi: 10.1037//0022-3514.84.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijda NH. The emotions. Cambridge: Cambridge University Press; 1986. [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–29. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds--a biological basis. Science. 1999;286:1692–5. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gerber AJ, Posner J, Gorman D, Colibazzi T, Yu S, Wang Z, Kangarlu A, Zhu H, Russell J, Peterson BS. An affective circumplex model of neural systems subserving valence, arousal, and cognitive overlay during the appraisal of emotional faces. Neuropsychologia. 2008;46:2129–39. doi: 10.1016/j.neuropsychologia.2008.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage. 2003;19:200–7. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- Gobbini MI, Haxby JV. Neural systems for recognition of familiar faces. Neuropsychologia. 2007;45:32–41. doi: 10.1016/j.neuropsychologia.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Goldapple K, Segal Z, Garson C, Lau M, Bieling P, Kennedy S, Mayberg H. Modulation of cortical-limbic pathways in major depression: treatment-specific effects of cognitive behavior therapy. Arch Gen Psychiatry. 2004;61:34–41. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Mintz J. Backward Masking in Schizophrenia and Mania. II. Specifying the Visual Channels. Arch Gen Psychiatry. 1994;51:945–951. doi: 10.1001/archpsyc.1994.03950120017004. [DOI] [PubMed] [Google Scholar]
- Gur RE, McGrath C, Chan RM, Schroeder L, Turner T, Turetsky BI, Kohler C, Alsop D, Maldjian J, Ragland JD, Gur RC. An fMRI study of facial emotion processing in patients with schizophrenia. Am J Psychiatry. 2002;159:1992–9. doi: 10.1176/appi.ajp.159.12.1992. [DOI] [PubMed] [Google Scholar]
- Gur RE, Nimgaonkar VL, Almasy L, Calkins ME, Ragland JD, Pogue-Geile MF, Kanes S, Blangero J, Gur RC. Neurocognitive endophenotypes in a multiplex multigenerational family study of schizophrenia. Am J Psychiatry. 2007;164:813–9. doi: 10.1176/ajp.2007.164.5.813. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel A, Hempel E, Schonknecht P, Stippich C, Schroder J. Impairment in basal limbic function in schizophrenia during affect recognition. Psychiatry Res. 2003;122:115–24. doi: 10.1016/s0925-4927(02)00126-9. [DOI] [PubMed] [Google Scholar]
- Holt DJ, Kunkel L, Weiss AP, Goff DC, Wright CI, Shin LM, Rauch SL, Hootnick J, Heckers S. Increased medial temporal lobe activation during the passive viewing of emotional and neutral facial expressions in schizophrenia. Schizophr Res. 2006a;82:153–62. doi: 10.1016/j.schres.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Holt DJ, Titone D, Long LS, Goff DC, Cather C, Rauch SL, Judge A, Kuperberg GR. The misattribution of salience in delusional patients with schizophrenia. Schizophr Res. 2006b;83:247–56. doi: 10.1016/j.schres.2005.12.858. [DOI] [PubMed] [Google Scholar]
- Honey GD, Pomarol-Clotet E, Corlett PR, Honey RA, McKenna PJ, Bullmore ET, Fletcher PC. Functional dysconnectivity in schizophrenia associated with attentional modulation of motor function. Brain. 2005;128:2597–611. doi: 10.1093/brain/awh632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Blanchard JJ, Clark LA, Green MF. Affective traits in schizophrenia and schizotypy. Schizophr Bull. 2008;34:856–74. doi: 10.1093/schbul/sbn083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Ventura J, Nuechterlein KH, Subotnik KL, Hwang SS, Mintz J. Stressful life events in recent-onset schizophrenia: reduced frequencies and altered subjective appraisals. Schizophr Res. 2005;75:363–74. doi: 10.1016/j.schres.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Huppert JD, Weiss KA, Lim R, Pratt S, Smith TE. Quality of life in schizophrenia: contributions of anxiety and depression. Schizophr Res. 2001;51:171–80. doi: 10.1016/s0920-9964(99)00151-6. [DOI] [PubMed] [Google Scholar]
- Izard CE. Human Emotions. New York: Plenum Press; 1977. [Google Scholar]
- Jastak S, Wilkinson GS. Wide Range Achievement Test - Revised. Wilmington, DE: Jastak Associates, Inc; 1984. [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jones SR, Fernyhough C. A new look at the neural diathesis--stress model of schizophrenia: the primacy of social-evaluative and uncontrollable situations. Schizophr Bull. 2007;33:1171–7. doi: 10.1093/schbul/sbl058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee KS, Kern RS, Green MF. Perception of emotion and neurocognitive functioning in schizophrenia: what’s the link? Psychiatry Research. 1998;81:57–65. doi: 10.1016/s0165-1781(98)00083-3. [DOI] [PubMed] [Google Scholar]
- Kennerley SW, Walton ME, Behrens TE, Buckley MJ, Rushworth MF. Optimal decision making and the anterior cingulate cortex. Nat Neurosci. 2006;9:940–7. doi: 10.1038/nn1724. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Young AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd DA. Cortical and limbic activation during viewing of high- versus low-calorie foods. Neuroimage. 2003;19:1381–94. doi: 10.1016/s1053-8119(03)00191-5. [DOI] [PubMed] [Google Scholar]
- Kohler CG, Turner TH, Bilker WB, Brensinger CM, Siegel SJ, Kanes SJ, Gur RE, Gur RC. Facial emotion recognition in schizophrenia: intensity effects and error pattern. Am J Psychiatry. 2003;160:1768–74. doi: 10.1176/appi.ajp.160.10.1768. [DOI] [PubMed] [Google Scholar]
- Kosaka H, Omori M, Murata T, Iidaka T, Yamada H, Okada T, Takahashi T, Sadato N, Itoh H, Yonekura Y, Wada Y. Differential amygdala response during facial recognition in patients with schizophrenia: an fMRI study. Schizophr Res. 2002;57:87. doi: 10.1016/s0920-9964(01)00324-3. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Holcomb HH, Weiler MA, Medoff DR, Frey KN, Hardin M, Tamminga CA. Clozapine but not haloperidol Re-establishes normal task-activated rCBF patterns in schizophrenia within the anterior cingulate cortex. Neuropsychopharmacology. 2004;29:171–8. doi: 10.1038/sj.npp.1300312. [DOI] [PubMed] [Google Scholar]
- Lee KH, Brown WH, Egleston PN, Green RD, Farrow TF, Hunter MD, Parks RW, Wilkinson ID, Spence SA, Woodruff PW. A functional magnetic resonance imaging study of social cognition in schizophrenia during an acute episode and after recovery. Am J Psychiatry. 2006;163:1926–33. doi: 10.1176/ajp.2006.163.11.1926. [DOI] [PubMed] [Google Scholar]
- Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007;37:579–88. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Mayer JD, Salovey P, Caruso DR. Mayer-Salovey-Caruso Emotional Intelligence Test. North Tonawanda, N: Multi-Health Systems Inc; 1999. [Google Scholar]
- Mitchell JP, Heatherton TF, Macrae CN. Distinct neural systems subserve person and object knowledge. Proc Natl Acad Sci U S A. 2002;99:15238–43. doi: 10.1073/pnas.232395699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myin-Germeys I, Delespaul PA, deVries MW. Schizophrenia patients are more emotionally active than is assumed based on their behavior. Schizophr Bull. 2000;26:847–54. doi: 10.1093/oxfordjournals.schbul.a033499. [DOI] [PubMed] [Google Scholar]
- Narr KL, Toga AW, Szeszko P, Thompson PM, Woods RP, Robinson D, Sevy S, Wang Y, Schrock K, Bilder RM. Cortical thinning in cingulate and occipital cortices in first episode schizophrenia. Biol Psychiatry. 2005;58:32–40. doi: 10.1016/j.biopsych.2005.03.043. [DOI] [PubMed] [Google Scholar]
- Narvaez JM, Twamley EW, McKibbin CL, Heaton RK, Patterson TL. Subjective and objective quality of life in schizophrenia. Schizophr Res. 2008;98:201–8. doi: 10.1016/j.schres.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends in Cognitive Sciences. 2004;8:102–7. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- O’Donnell BF, Swearer JM, Smith LT, Nestor PG, Shenton ME, McCarley RW. Selective deficits in visual perception and recognition in schizophrenia. Am J Psychiatry. 1996;153:687–92. doi: 10.1176/ajp.153.5.687. [DOI] [PubMed] [Google Scholar]
- Ochsner KN. The social-emotional processing stream: five core constructs and their translational potential for schizophrenia and beyond. Biol Psychiatry. 2008;64:48–61. doi: 10.1016/j.biopsych.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–29. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, Mackey SC. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. Journal of Cognitive Neuroscience. 2004;16:1746–72. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. Brief psychiatric rating scale. Psychol Reports. 1962;10:799–812. [Google Scholar]
- Pallanti S, Quercioli L, Hollander E. Social anxiety in outpatients with schizophrenia: a relevant cause of disability. Am J Psychiatry. 2004;161:53–8. doi: 10.1176/appi.ajp.161.1.53. [DOI] [PubMed] [Google Scholar]
- Paradiso S, Andreasen NC, Crespo-Facorro B, O’Leary DS, Watkins GL, Boles Ponto LL, Hichwa RD. Emotions in unmedicated patients with schizophrenia during evaluation with positron emission tomography. Am J Psychiatry. 2003;160:1775–1783. doi: 10.1176/appi.ajp.160.10.1775. [DOI] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Decker LR, Noll DC, Nichols TE, Britton JC, Liberzon I. Activation of the medial prefrontal cortex and extended amygdala by individual ratings of emotional arousal: a fMRI study. Biol Psychiatry. 2003;53:211–5. doi: 10.1016/s0006-3223(02)01485-3. [DOI] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, Liberzon I. Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. Neuroimage. 2004;21:768–80. doi: 10.1016/j.neuroimage.2003.09.072. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Pinkham AE, Hopfinger JB, Pelphrey KA, Piven J, Penn DL. Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophr Res. 2008;99:164–75. doi: 10.1016/j.schres.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajarethinam R, Peethala R, Keshavan MS, Rosenberg DR, Diwadkar VA, LPK Enhanced dorsal medial prefrontal cortex responses to social threat perception in schizophrenia: A preliminary fMRI study at 4 Tesla. Neuropsychopharmacology. 2005;29:S193. [Google Scholar]
- Rasetti R, Mattay VS, Wiedholz LM, Kolachana BS, Hariri AR, Callicott JH, Meyer-Lindenberg A, Weinberger DR. Evidence that altered amygdala activity in schizophrenia is related to clinical state and not genetic risk. American Journal of Psychiatry. 2009;166:216–25. doi: 10.1176/appi.ajp.2008.08020261. [see comment] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reske M, Kellermann T, Habel U, Jon Shah N, Backes V, von Wilmsdorff M, Stocker T, Gaebel W, Schneider F. Stability of emotional dysfunctions? A long-term fMRI study in first-episode schizophrenia. Journal of Psychiatric Research. 2007;41:918–27. doi: 10.1016/j.jpsychires.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Russell TA, Reynaud E, Kucharska-Pietura K, Ecker C, Benson PJ, Zelaya F, Giampietro V, Brammer M, David A, Phillips ML. Neural responses to dynamic expressions of fear in schizophrenia. Neuropsychologia. 2007;45:107–23. doi: 10.1016/j.neuropsychologia.2006.04.026. [DOI] [PubMed] [Google Scholar]
- Sander D, Grafman J, Zalla T. The human amygdala: an evolved system for relevance detection. Reviews in the Neurosciences. 2003;14:303–16. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- Schiller D, Freeman JB, Mitchell JP, Uleman JS, Phelps EA. A neural mechanism of first impressions. Nature Neuroscience. 2009;12:508–14. doi: 10.1038/nn.2278. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Weilburg JB, Sherman JC. The neuropsychiatry of the cerebellum - insights from the clinic. Cerebellum. 2007;6:254–67. doi: 10.1080/14734220701490995. [DOI] [PubMed] [Google Scholar]
- Schneider F, Weiss U, Kessler C, Salloum JB, Posse S, Grodd W, Muller GH. Differential amygdala activation in schizophrenia during sadness. Schizophrenia Research. 1998;34:133–42. doi: 10.1016/s0920-9964(98)00085-1. [DOI] [PubMed] [Google Scholar]
- Schutter DJ, van Honk J. The cerebellum on the rise in human emotion. Cerebellum. 2005;4:290–4. doi: 10.1080/14734220500348584. [DOI] [PubMed] [Google Scholar]
- Seiferth NY, Pauly K, Kellermann T, Shah NJ, Ott G, Herpertz_Dahlmann B, Kircher T, Schneider F, Habel U. Neuronal correlates of facial emotion discrimination in early onset schizophrenia. Neuropsychopharmacology. 2009;34:477–87. doi: 10.1038/npp.2008.93. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex: a morphometric analysis of prefrontal area 9 and occiptal area 17. Arch Gen Psychiatry. 1995;52:805–818. doi: 10.1001/archpsyc.1995.03950220015005. [DOI] [PubMed] [Google Scholar]
- Sergi MJ, Green MF. Social perception and early visual processing in schizophrenia. Schizophr Res. 2003;59:233–41. doi: 10.1016/s0920-9964(01)00405-4. [DOI] [PubMed] [Google Scholar]
- Sergi MJ, Rassovsky Y, Nuechterlein KH, Green MF. Social perception as a mediator of the influence of early visual processing on functional status in schizophrenia. Am J Psychiatry. 2006;163:448–54. doi: 10.1176/appi.ajp.163.3.448. [DOI] [PubMed] [Google Scholar]
- Siegel BV, Buchsbaum MS, Bunney WE, Gottschalk LA, Haier RJ, Lohr JB, Lottenberg S, et al. Cortical-strial-thalamic circuits and brain glucose metablic activity in 70 unmedicate male schizophrenic patients. Am J Psychiatry. 1993;150:1325–1336. doi: 10.1176/ajp.150.9.1325. [DOI] [PubMed] [Google Scholar]
- Smith CA, Ellsworth PC. Patterns of cognitive appraisal in emotion. J Pers Soc Psychol. 1985;48:813–38. [PubMed] [Google Scholar]
- Snitz BE, MacDonald A, 3rd, Cohen JD, Cho RY, Becker T, Carter CS. Lateral and medial hypofrontality in first-episode schizophrenia: functional activity in a medication-naive state and effects of short-term atypical antipsychotic treatment. American Journal of Psychiatry. 2005;162:2322–9. doi: 10.1176/appi.ajp.162.12.2322. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Magnotta VA, White T, Arndt S, Flaum M, O’Leary DS, Andreasen NC. Effects of olanzapine on cerebellar functional connectivity in schizophrenia measured by fMRI during a simple motor task. Psychol Med. 2001;31:1065–78. doi: 10.1017/s0033291701004330. [DOI] [PubMed] [Google Scholar]
- Stern ER, Welsh RC, Fitzgerald KD, Taylor SF. Topographic analysis of individual activation patterns in medial frontal cortex in schizophrenia. Hum Brain Mapp. 2008 doi: 10.1002/hbm.20657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Koeda M, Oda K, Matsuda T, Matsushima E, Matsuura M, Asai K, Okubo Y. An fMRI study of differential neural response to affective pictures in schizophrenia. Neuroimage. 2004;22:1247–54. doi: 10.1016/j.neuroimage.2004.03.028. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Liberzon I, Decker LR, Koeppe RA. A functional anatomic study of emotion in schizophrenia. Schizophr Res. 2002;58:159–72. doi: 10.1016/s0920-9964(01)00403-0. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Phan KL, Britton JC, Liberzon I. Neural response to emotional salience in schizophrenia. Neuropsychopharmacology. 2005;30:984–95. doi: 10.1038/sj.npp.1300679. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Welsh RC, Chen AC, Velander AJ, Liberzon I. Medial frontal hyperactivity in reality distortion. Biol Psychiatry. 2007;61:1171–8. doi: 10.1016/j.biopsych.2006.11.029. [DOI] [PubMed] [Google Scholar]
- Tso IF, Grove TB, Taylor SF. Emotional experience predicts social adjustment independent of neurocognition and social cognition in schizophrenia. Schizophr Res. 2010 doi: 10.1016/j.schres.2009.12.007. ePub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Kohler CG, Indersmitten T, Bhati MT, Charbonnier D, Gur RC. Facial emotion recognition in schizophrenia: when and why does it go awry? Schizophr Res. 2007;94:253–63. doi: 10.1016/j.schres.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Anderson CH, Felleman DJ. Information processing in the primate visual system: an integrated systems perspective. Science. 1992;255:419–423. doi: 10.1126/science.1734518. [DOI] [PubMed] [Google Scholar]
- Voges M, Addington J. The association between social anxiety and social functioning in first episode psychosis. Schizophr Res. 2005;76:287–92. doi: 10.1016/j.schres.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia. 2007;45:174–94. doi: 10.1016/j.neuropsychologia.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Walton ME, Croxson PL, Behrens TE, Kennerley SW, Rushworth MF. Adaptive decision making and value in the anterior cingulate cortex. Neuroimage. 2007;36(Suppl 2):T142–54. doi: 10.1016/j.neuroimage.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM. Social Adjustment Scale – Self-Report. North Tonawanda, N: Multi-Health Systems Inc; 1999. [Google Scholar]
- Welsh RC, Chen AC, Taylor SF. Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in schizophrenia. Schizophr Bull. 2010;36:713–22. doi: 10.1093/schbul/sbn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Das P, Harris AW, Liddell BB, Brammer MJ, Olivieri G, Skerrett D, Phillips ML, David AS, Peduto A, Gordon E. Dysregulation of arousal and amygdala-prefrontal systems in paranoid schizophrenia. American Journal of Psychiatry. 2004;161:480–9. doi: 10.1176/appi.ajp.161.3.480. [DOI] [PubMed] [Google Scholar]
- Willis J, Todorov A. First impressions: making up your mind after a 100-ms exposure to a face. Psychol Sci. 2006;17:592–8. doi: 10.1111/j.1467-9280.2006.01750.x. [DOI] [PubMed] [Google Scholar]
- Winston JS, O’Doherty J, Dolan RJ. Common and distinct neural responses during direct and incidental processing of multiple facial emotions. Neuroimage. 2003;20:84–97. doi: 10.1016/s1053-8119(03)00303-3. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited--again. Neuroimage. 1995;2:173–81. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Yang Y, Gu H, Zhan W, Xu S, Silbersweig D, Stern E. Simultaneous perfusion and BOLD imaging using reverse spiral scanning at 3T: characterization of functional contrast and susceptibility artifacts. Magn Reson Med. 2002;48:278–289. doi: 10.1002/mrm.10196. [DOI] [PubMed] [Google Scholar]
- Yeap S, Kelly SP, Sehatpour P, Magno E, Javitt DC, Garavan H, Thakore JH, Foxe JJ. Early visual sensory deficits as endophenotypes for schizophrenia: high-density electrical mapping in clinically unaffected first-degree relatives. Arch Gen Psychiatry. 2006;63:1180–8. doi: 10.1001/archpsyc.63.11.1180. [DOI] [PubMed] [Google Scholar]
- Zysset S, Huber O, Ferstl E, von Cramon DY. The anterior frontomedian cortex and evaluative judgment: an fMRI study. Neuroimage. 2002;15:983–91. doi: 10.1006/nimg.2001.1008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.