Abstract
The central nucleus of the amygdala (CeA) plays a critical role in regulating the behavioral, autonomic and endocrine response to stress. Dopamine (DA) participates in mediating the stress response and DA release is enhanced in the CeA during stressful events. However, the electrophysiological effects of DA on CeA neurons have not yet been characterized. Therefore, the purpose of this study was to identify and characterize the effect of DA application on electrophysiological responses of CeA neurons in coronal brain sections of male Sprague Dawley rats. We used whole cell patch clamp electrophysiological techniques to record evoked synaptic responses and to determine basic membrane properties of CeA neurons both before and after DA superfusion. DA (20–250μM) did not significantly alter membrane conductance over the voltage range tested. However, DA significantly reduced peak amplitude of evoked inhibitory synaptic currents in CeA neurons. Pretreatment with the D2 receptor antagonist eticlopride failed to significantly block the inhibitory effects of DA. In contrast, pretreatment with the D1 receptor antagonist SCH-23390 significantly reduced DA effects on evoked inhibitory neurotransmission in these neurons. Moreover, bath superfusion of the specific D1 receptor agonist SKF-39393, but not the D2 receptor agonist quinpirole, significantly reduced peak amplitude of evoked inhibitory synaptic events. DA reduced the frequency of miniature IPSCs without altering the amplitude, while having no effect on the amplitude of IPSCs elicited by pressure application of GABA. These results suggest that DA may modulate inhibitory synaptic transmission in CeA through D1 receptor activation primarily by a presynaptic mechanism.
Keywords: GABA, stress, electrophysiology, rat, inhibition
Introduction
The extended amygdala is an essential component of the neurocircuitry involved in mediation of the biological response to stress (Inglis and Moghaddam 1999; Inoue et al. 1994). The central nucleus of the amygdala (CeA) in particular has been implicated in the physiological response to stress and the associated changes in central nervous system dopamine levels. There is strong evidence to support dopaminergic mediation of the amygdala response to stress (Asan 1997; Guarraci and Kapp 1999) and although it is well documented that CeA dopamine release is enhanced in response to fear and stress-inducing stimuli, limited research has addressed specific effects of DA within the CeA. While the extended amygdala comprises the bed nucleus of stria terminalis, nucleus accumbens and the CeA, the CeA is the only component whose electrophysiological response to dopamine has not been characterized.
The CeA receives extensive innervation from mesolimbic DA-containing neurons (Freedman and Cassell 1994). Changes in amygdala DA levels are strongly related to changes in the stress response, as animal studies indicate that DA-releasing afferents are activated during stress conditioning (Coco et al. 1992) and that fear-arousing stimuli induces dopaminergic transmission in the amygdala (Yokoyama et al. 2005). Additionally, rats exposed to inescapable electrical footshock (Yokoyama et al. 2005) and chronic restraint stress (Torres et al. 2002) show elevations in amygdala DA levels for up to two hours (Yokoyama et al. 2005). Although there is no definitive agreement on specific DA receptor subtype localization (Boyson et al. 1986; Huang et al. 1992; Scibilia et al. 1992), the CeA may contain both D1 and D2 receptor subtypes. To date, there has been only one preliminary report characterizing the physiological responses mediated by the CeA DA receptor (Scheiss et al. 1988), however, several behavioral studies support a role for amygdala D1 receptor activation in the expression of anxiety (de la Mora et al. 2005) and fear in both the potentiated startle (Lamont and Kokkinidis 1998) and Pavlovian conditioned (Guarraci and Kapp 1999) paradigms.
As reviewed above, dopaminergic innervation and DA receptor density in the CeA is high. Combined behavioral and physiological evidence support alterations in DA levels during stress conditioning and particular drug states, and this suggests a strong modulatory role for DA within the CeA. Therefore, this study was undertaken to explore the physiological responses to DA receptor activation within the CeA.
Materials and Methods
Animals
Three to 4-week-old male Sprague-Dawley rats were housed in communal cages and given access to food and water ad libitum. All experimental procedures were approved and were in compliance with Duke University Medical Center and the Durham Veteran’s Affairs Medical Center (DVAMC) Institutional Animal Care and Use Committees. Duke and the DVAMC are AAALAC accredited institutions.
Slice Preparation
Rats were briefly anesthetized with isoflurane vapor and immediately decapitated. Skulls were opened and brains were removed and transferred to ice-cold artificial cerebral spinal fluid (ACSF) consisting of (in mM) 120 NaCl, 3.3 KCl, 1.23 NaH2PO4, 25 NaHCO3, 1.2 MgSO4, 1.8 CaCl2 and 10 D-Glucose equilibrated with 95% O2/5% CO2, pH=7.3. Coronal brain slices (350μM thickness) containing the central amygdala were cut on a vibratome (Vibratome 1000 Plus) and incubated in oxygenated ACSF at room temperature (21–23°C) for a minimum of one hour. Individual slices were transferred to the submersion recording chamber and perfused with oxygenated ACSF at a constant rate of 2–4 ml/min.
Whole-cell patch-clamp recording
Whole cell recordings were obtained using standard whole-cell patch electrophysiological techniques that have been described elsewhere (Li et al. 2002). Briefly, a Zeiss microscope with a fixed stage was used to visualize the tissue. A low power objective (5X) was used to localize the central amygdaloid nucleus, and a high power water-immersion objective (40X) was used to visualize individual neurons. Whole cell patch pipettes were pulled from thin walled borosilicate glass capillaries (Sutter Instruments Company, Model P-97) to produce electrodes with a tip resistance of 6–10 MΩ. The internal solution for current clamp experiments consisted of (in mM): 130 K-gluconate, 7 KCL, 10 HEPES, 4 Mg-ATP, and 0.3 Tris-GTP. Ten to 15 depolarizing pulse currents (600 ms) were delivered to the clamped cell through the patch electrode at 10 pA steps. The internal solution for voltage clamp experiments consisted of (in mM): 135 CsCl, 10 HEPES, 4 MgATP, 0.3 GTP, 2 MgCl2, 0.5 EGTA, 5 QX-314, pH=7.23. Inhibitory events were pharmacologically isolated by bath superfusion of both APV and DNQX (20–30 μM). Signals were amplified with an Axopatch-200B amplifier and displayed on an oscilloscope (Nicolet 310). Signals were then digitized using a National Instruments BNC-2100 D/A converter and acquired via Strathclyde Software (Whole Cell program, V3.2.9) or pClamp 10.
Evoked IPSCs
To evoke synaptic events, a tungsten stimulating electrode was placed in the lateral division of the CeA. The stimulating electrode was placed approximately 100–200 μm from the recording electrode and current was applied at 1–50 mu;A for the duration of 0.2 ms at a rate of 1/20 s.
Miniature IPSCs
Miniature IPSCs (mIPSCs) were recorded from CeA neurons held at −70mV after adding 1μM tetrodotoxin (TTX) into the perfused solution to block Na-dependent action potentials. In order to facilitate spontaneous mIPSCs, the temperature in recording chamber was kept at 34°C throughout the experiments.
Drugs
DA, SKF-38393 and quinpirole were prepared daily immediately before use to minimize oxidation and were diluted in the bath superfusate to known concentrations. Stock solutions of apomorphine and WAY-100635 were aliquoted and stored in the freezer (−20° C). Stock solutions of the dopaminergic antagonists SCH-23390 and eticlopride were prepared weekly and stored in the refrigerator (4°C). Stock solutions of (−)- bicuculline methiodide (BMI), 6,7-dinitroquinoxaline-2,3(1H,4H)-dione (DNQX), DL-2-amino-5-phosphonopentanoic acid (APV) and tetrodotoxin (TTX) were prepared as needed and stored at room temperature. For measurement of drug effects DA receptor agonists were superfused for at least 10 minutes, and receptor antagonists were pre-applied for least 15 minutes. All chemicals were obtained from Sigma (St. Louis, MO). As an antioxidant, sodium metabisulfite (Na2S2O5, 50μM) was added into the bathing solution to prevent dopamine oxidation.
Exogenous GABA application
A glass pipette filled with 100 μM GABA was position near the soma of the recorded cell held at −70mV. Whole cell GABA-induced postsynaptic IPSCs were evoked every 1 minute by direct pressure application of GABA (8–20 psi, duration 20msec) using a pressure injecting apparatus (Picospritzer IID, General Valve, Fairfield, NJ USA). The perfused solution also contained TTX (1 μM), APV (50 μM), DNQX (20 μM) and CGP-55845(1μM)
Data analysis
All data are expressed as mean and standard error of the mean unless otherwise stated. Statistical analyses consist of analysis of variance (ANOVA) and appropriate t-tests. P values less than or equal to .05 are taken to be statistically significant.
Results
Dopamine inhibits inhibitory currents in CeA neurons
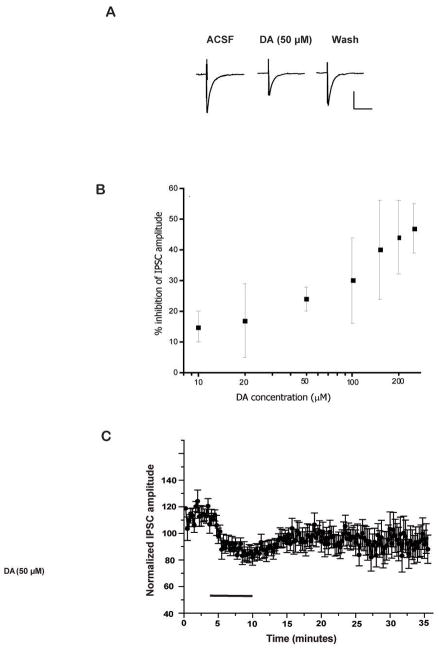
Postsynaptic currents were evoked by direct stimulation of the lateral division of the CeA and recorded from cells in the medial and lateral divisions of the CeA. IPSCs were evoked at a holding potential of −70 mV, and isolated by bath inclusion of the NMDA-and AMPA-specific antagonists, D-APV (50 μM) and DNQX (20 μM). Evoked IPSCs were completely blocked by inclusion of the GABAA receptor antagonist bicuculline methiodide. Superfusion of DA (Figure 1) significantly depressed inhibitory neurotransmission in the majority of CeA cells tested without altering decay kinetics (data not shown). DA (50 μM) reduced total peak IPSC amplitude by 30.58 ± 3.34% (Figure 1B & C; p<.01, n=14). There was no significant difference in the effect of DA between the medial and lateral divisions and therefore, inhibitory postsynaptic currents recorded from both divisions were pooled for data analysis. In addition, the non-specific dopaminergic agonist apomorphine also attenuated inhibitory neurotransmission in the CeA by 60.17 ± 13.55% (Figure 2D, n=3, p< .03).
Figure 1.
Representative waveforms of evoked inhibitory potentials during application of DA and specific DA antagonists and agonists. A. IPSC waveforms of baseline, DA (50 μM), and washout (scale 25 pA, 25 msec). B. Dose-response curve for DA on evoked IPSCs. C. Time course of DA (50 μM) effect (applied for the period of bar) on normalized evoked IPSC amplitudes (n=14).
Figure 2.
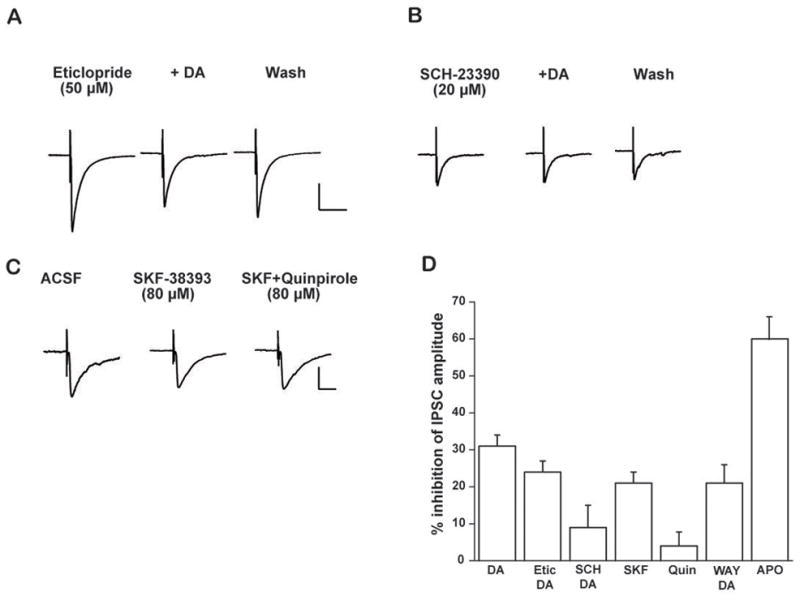
DA inhibits inhibitory currents through the D1 receptor subtype in CeA neurons. A. IPSC waveforms after pretreatment with eticlopride (50 μM), DA, and washout. B. IPSC waveforms after pretreatment with SCH-23390 (20 μM), DA, and washout. C. Representative waveforms of evoked IPSC activity during baseline, superfusion of SKF-38393 alone, and co-superfusion of SKF-38393 & quinpirole. Scale: 12 pA, 10 msec. D. Bar graph depicting percent change in IPSC peak amplitude during superfusion of the following chemicals (from left to right): DA (50 μM; 30.58 ± 3.34%, p<.01, n=14); DA following pretreatment with the D2 receptor antagonist eticlopride (50 μM) (24.44 ± 2.58%, p< .001, n=11); DA following pretreatment with the D1 receptor antagonist, SCH-23390 (20 μM) (8.85 ± 5.69%, n=9); D1 receptor agonist, SKF-38393 (80 μM; 21.00± 3.33%, p<.000, n=10); D2 receptor agonist quinpirole (80 μM, 3.83 ± 3.85%, n=6); co-application of 50 μM DA and WAY-100635 (50 nM) (21.21 ± 5.62, p<.02, n=5); apomorphine (50 μM); 60.17±13.55%, p<.03, n=3).
Dopamine modulates inhibitory currents through the D1 receptor subtype
DA and specific DA receptor antagonists and agonists were bath applied to slices in order to determine whether specific DA receptor subtypes mediate the inhibitory action of DA on inhibitory transmission in CeA cells. Independent superfusion of DA antagonists resulted in no significant change in baseline responses (data not shown). Pretreatment of the D2 receptor antagonist eticlopride (50 μM) failed to significantly block the inhibitory effects of DA (50 μM) on evoked inhibitory events (Figure 2A & D). In the presence of eticlopride, DA still reduced peak IPSC amplitude by 24.44 ± 2.58% (p<.001, n=11). In contrast, pretreatment with the D1 antagonist SCH-23390 (20 μM) prevented DA (50 μM) from significantly reducing evoked inhibitory neurotransmission. Pretreatment with SCH-29930 reduced the inhibitory effects of DA on IPSC amplitude, resulting in an 8.85 ± 5.69% reduction of peak amplitude (n=9; Figure 2B & D).
To further characterize the D1-receptor-mediated response of CeA neurons, the specific D1- receptor agonist SKF-38393 (80 μM) was bath applied. SKF-38393 also significantly attenuated evoked inhibitory neurotransmission in the CeA. Specifically, SKF-38393 reduced peak IPSC amplitude by 21.00 ± 3.33% (Figure 2C, p<.0001, n=10). The specific D2 agonist quinpirole (80 μM) was also bath applied on another subset of slices containing the CeA. Quinpirole did not significantly change peak amplitude of IPSCs (2.67 ± 1.36%, n=6) (Figure 2C & D). In order to determine if full expression of the DA effect required activation of both D1 and D2 receptors, quinpirole (80 μM) was co-applied with SKF-38393 in a separate series of neurons (Figure 2C & D). Subsequent superfusion of quinpirole did not significantly alter the attenuating effects of the D1 agonist, suggesting that the attenuating effect of DA is primarily mediated via the D1 receptor (SKF-38393 = 16.34 ± 4.17% inhibition; SKF-38393 & quinpirole= 15.25 ± 6.59, n=4). In order to rule out a non-specific effect of DA acting on 5HT1A receptors, Way-100635, a specific 5- HT1A antagonist, was co-applied in the presence of DA. Way-100635 (50 nM) failed to alter the attenuating effects of DA on IPSCs in CeA (Figure 2D), as application of DA (50 μM) still reduced IPSC amplitude by 21.21 ± 5.62% (p<.02, n=5). Therefore, it is highly unlikely that the inhibitory effects of DA on synaptic activity resulted from a non-specific action of DA on 5HT1A receptors.
Dopamine does not alter membrane conductance in CeA neurons
Current-voltage relationships (10 pA steps) of CeA neurons were examined both before and after DA application (Figure 3) At concentrations between 20–250 μM DA did not significantly increase steady state membrane conductance over the voltage range tested (although there was a slight tendency toward an increase in steady-state conductance, these results were not statistically significant). At the highest concentrations of DA used (500 μM) we observed variable membrane effects, although these effects were likely not attributable to specific actions at DA receptors. Apopmorphine (50 μM) did not significantly alter steady state membrane conductance of CeA neurons (data not shown).
Figure 3.
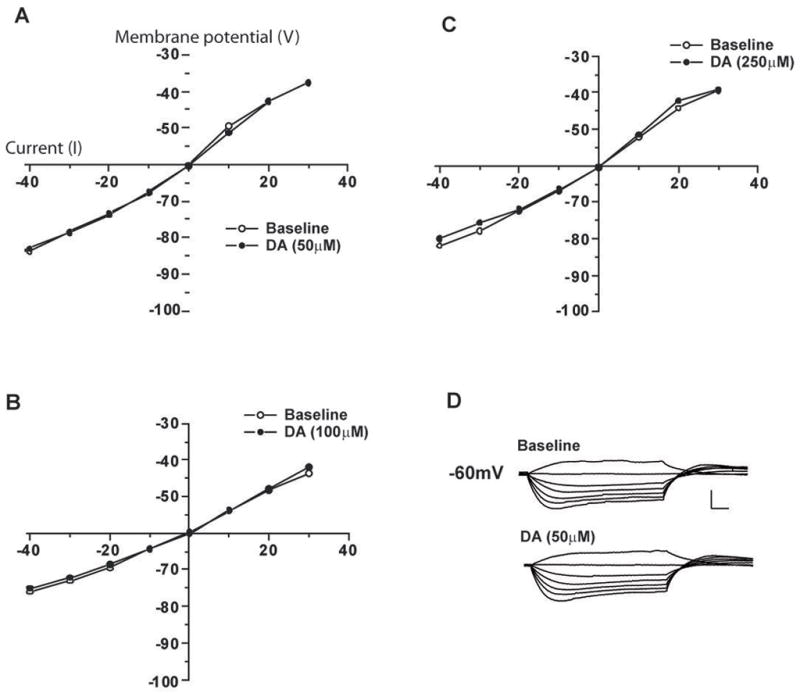
DA has little overall effect on steady state membrane conductance of CeA neurons even at very high concentrations. Current pulses (600 msec) were delivered at 10 pA steps. A. Graph of current-voltage relationship after application of 50 μM DA (n=7). B. 100 μM DA (n=7). C. 250 μM DA (n=7). D. Representative traces of current-voltage relationship before (upper) and after (lower) superfusion of 50 μM DA (scale 50 mV, 50 msec).
Dopamine reduces GABA release via a presynaptic mechanism
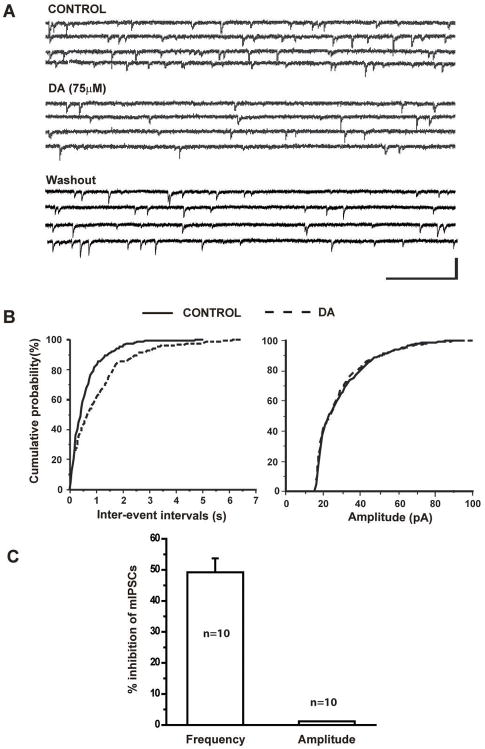
Previous results suggested the depressive effects of DA on IPSCS may involve either presynaptic or postsynaptic mechanism. We next determined if DA suppresses the amplitude of IPSCS via a reduction of presynaptic GABA release onto CeA neurons. We isolated mIPSCs from 10 CeA neurons held at −70mV in the presence of TTX and continuously monitored before and after bath application of DA (75uM). DA significantly reduced the frequency of mIPSCs but had no effect on their amplitude. Figure 4A shows representative traces of mIPSCs under control conditions and during bath application of DA. In this example, the frequency of mIPSCs was significantly reduced after addition of DA into the bath solution and recovered following washout (Figure 4B, left panel). However, the amplitude of mIPSCs recorded in this neuron remained unchanged (Figure 4B, right panel). In 10 CeA neurons tested, DA significantly prolonged inter-event intervals (Figure 4C, K-S test, p<0.05) but had no effects on amplitude (Figure 4C, K-S test p>0.05).
Figure 4.
DA reduced the frequency of spontaneous mIPSCs. A. TTX -insensitive mIPSCs were isolated from CeA neurons held at −70mV. Bath application of 75 μM DA reduced the frequency. B. Cumulative probability analysis indicated that DA attenuated the frequency of mIPSCs (K-S test, p<0.001) but had no effect on the amplitude (K-S test, p>0.05). C. Bar graph showing the mean percent inhibition by 75μM DA of the frequency (n=10, paired t-test, p<0.05) and amplitude of mIPSCs (paired t-test, p>0.05).
Dopamine does not suppress postsynaptic response to direct application of GABA
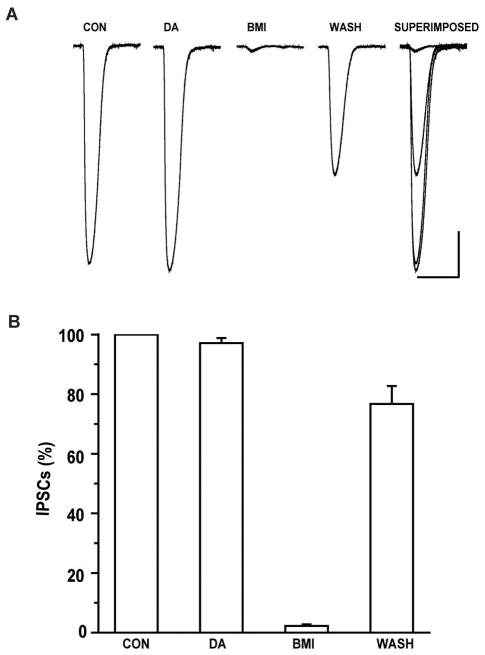
We further designed experiments to rule out post-synaptic GABA responses in the presence of DA. Whole-cell GABA currents were elicited in the presence of TTX (1 μM), APV (50 μM), DNQX (20 μM) and CGP-55845 (1μM) by puffing GABA (100μM) onto the soma of the recorded CeA neurons. When ACSF was directly pressure-applied to the soma of the recorded neurons no responses were observed. At a holding potential of −70mV, application of GABA (100μM) at intervals of 60s elicited inward currents. As shown in Figure 5A, the amplitude of whole cell currents elicited by GABA were not affected by bath application of DA (75μM) for 10 minutes but were blocked by BMI (20μM) and recovered following washout. A total of 10 cells were examined for their responses to DA and the results from these cells were summarized in Figure 5B. DA had no effect on the amplitude of GABAergic whole-cell currents (n=10, p>0.05). This result suggests that dopamine is not altering postsynaptic responses evoked by pressure application of GABA and dopamine suppression of GABA IPSCs likely occurs at a presynaptic locus.
Figure 5.
DA did not affect the post-synaptic GABA currents elicited by direct pressure application of GABA. A. Representative traces of whole cell currents evoked by puffing 100 nM GABA on the soma of CeA neurons before and during application of DA are shown. BMI reversibly blocked GABA-evoked whole cell currents. (Scale bar 1sec/100pA). B. There was no significant change in mean amplitude of GABA-evoked currents between baseline and DA application (one-way ANOVA, p>0.05).
Discussion
To our knowledge, this study is the first to characterize the effects of DA on inhibitory synaptic responses of neurons within the central nucleus of the amygdala. Our findings indicate that DA reduces evoked inhibitory neurotransmission of CeA neurons, likely through a D1 receptor-mediated mechanism. Furthermore, changes in mIPSC frequency and the absence of the effect on postsynaptic responses to exogenous GABA application suggest a presynaptic mode of action. Similar findings have been previously reported in the lateral (Bissiere et al. 2003; Loretan et al. 2004) and basolateral amygdala nuclei (Kroner et al. 2005); however, these nuclei are structurally and neurochemically dissimilar to the “striatal-like” CeA (Swanson and Petrovich 1998).
Delaney and Sah (1999, 2001) reported the presence of a GABAC receptor-mediated response in Ce that is negatively modulated by benzodiazepines and only weakly sensitive to bicuculline. However, under our experimental conditions, we were unable to elicit an apparent GABAC-like response. The reason for this discrepancy between these results remains unclear, but may be due to technical differences such as the internal solutions, difference in cell types, differences in stimulation pathways, different slice orientation or different rat strains (Sprague Dawley vs. Wistar). It seems possible that GABAC-like responses, which might be smaller than GABAA-mediated IPSCs, may require more stringent conditions to be revealed.
The previous research regarding DA receptor localization in CeA is conflicting (Boyson et al. 1986; Dawson et al. 1986; Dubois et al. 1986; Huang et al. 1992; Scibilia et al. 1992; Weiner et al. 1991; Jacobsen et al. 2006), however our data strongly support a D1 receptor-mediated effect of DA. Pretreatment with the specific D2 receptor antagonist eticlopride failed to attenuate the inhibitory effects of DA on inhibitory responses. In contrast, pretreatment with the specific D1 receptor antagonist, SCH-23390 resulted in a substantial reduction of DA-mediated inhibition of inhibitory neurotransmission.
Additional support for a D1-mediated effect is our finding that superfusion of the specific D1 receptor agonist SKF-38393 significantly attenuated inhibitory neurotransmission in the CeA, while independent superfusion of the D2 agonist quinpirole was without significant effect. Specifically, antagonism of the D1 receptor resulted in an almost complete blockade of the DA effect, while antagonism of the D2 receptor failed to significantly block the DA effect. Moreover, the attenuation resulting from superfusion of the D1 receptor agonist accounts for the majority of the inhibition produced by independent application of DA.
Several lines of evidence indicate the presence of both D1 and D2 receptor subtypes in the CeA, and although these receptor subtypes have been classically characterized as having opposing effects on adenylate cyclase activity, the physiological significance of their combined effect is unclear. For example, some studies suggest that simultaneous activation of D1 and D2 receptor subtypes results in a synergistic increase in physiological activity, while other studies suggest these receptors work in direct opposition (Umemiya and Raymond 1997; West and Grace 2002). Moreover, Wachtel and colleagues found that activation of the D1 receptor is essential to achieve full post-synaptic expression of D2 effects (Wachtel et al. 1989). Therefore, in the present study, as a means to determine if D1 and D2 receptor subtypes work synergistically to enhance physiological activity, or in opposition, the D2 receptor agonist quinpirole was bath applied after a stable baseline was achieved in the presence of SKF-38393. Although the results were variable, the additional application of quinpirole did not produce significant changes in evoked inhibitory neurotransmission. Based on these results alone, we cannot rule out the possibility that D2 receptor subtypes are involved in the attenuation of inhibitory events in the CeA, although it appears that the effect is primarily mediated via the D1 receptor subtype. The most likely reason the the D1 receptor agonist SKF-38393 had a somewhat smaller effect that DA is that SKF-388393 is a partial agonist at D1 receptors, at least with regard to stimulation of adenyl cyclase (Andersen and Jansen, 1990).
We also considered the possibility that DA may have actions at sites other than DA receptors. 5HT1A receptors are also widely expressed throughout the amygdala (Ohuoha et al. 1993) and are presumably located both pre-and post-synaptically. Moreover, although DA has a low affinity for 5HT1A receptors, DA non-selectively binds at these receptors at high concentrations. However, it seems unlikely that the attenuating effects of DA resulted from activation of 5HT1A receptors, as the specific 5HT1-A antagonist WAY-100653 failed to block the inhibitory effects of DA on IPSCs. Moreover, the lower DA concentrations utilized in this experiment would be highly unlikely to activate 5-HT1A receptors. Higher concentrations of DA (500 μM) did begin to affect membrane properties resulting in slight shifts in membrane conductance and resting potential, although at this concentration DA would likely not act specifically on dopamine receptors.
Although this study supports a D1 receptor-mediated effect of DA in the CeA, it is possible that activation of the D1 receptor in the CeA fails to stimulate adenylate cyclase activity. In contrast to the classic adenylate cyclase-coupled D1 receptor responses characterized in several other brain areas, Leonard and colleagues (2003) found that the effects of D1 receptor activation in the amygdala are not mediated by cAMP and further reported that D1 receptor activation in the CeA sometimes produced a decrease in adenylate cyclase activity (Leonard et al. 2003). Moreover, the specific D1A receptor subtype appears to activate the adenylate cyclase pathway, which is distinct from other D1 receptor subtypes that activate inositol phosphate formation (Friedman et al. 1997). Dopaminergic stimulation of inositol phosphate formation is high in the amygdala (Undie and Friedman 1990) and taken together, these findings suggest that the inhibitory effects of DA on CeA neurons could result from D1 receptor-activated inositol phosphate formation, rather than adenylate cyclase.
Our findings that DA attenuates evoked inhibitory neurotransmission in the CeA are consistent with a preliminary study of dopaminergic responses in the central amygdala (Scheiss et al. 1988) and the DA response reported in several other brain areas. DA has been shown to decrease both IPSC peak amplitudes in the striatum by both pre- and postsynaptic mechanisms (Mercuri et al. 1985) and presynaptic D1 activation reduces inhibitory inputs to the nucleus accumbens shell (Pennartz et al. 1992). Additional reports indicate that the dopaminergic depression of inhibitory neurotransmission in the nucleus results from a reduction of presynaptic calcium influx (Nicola and Malenka 1997).
Our approximate ED50 concentration was 50 uM, which appears to correspond with DA effects diverse brain areas. In nucleus accumbens, DA depressed both excitatory and inhibitory synaptic transmission in a range of 60–100 uM (Nicola and Malenka, 1997). In prefrontal cortex, DA reduced evoked IPSCs at an ED50 of approximately 30 uM (Gonzalez-Islas and Hablitz, 2001). DA inhibits GABAergic responses in midbrain dopaminergic neurons with an IC50 of 54 uM (Federici et al., 2002).
The CeA has a well-established role in conditioned fear responses (Davis 1992; LeDoux 2000). Dopamine may have a critical role in fear conditioning (Fadok et al. 2009), but the role of dopaminergic input specifically to the CeA is unclear in this regard. Although DA D1 receptor antagonists reportedly produce anxiolytic effects when injected into the amygdala (de la Mora et al. 2005), DA D1 receptor activation in CeA may mediate anxiolytic effects of opiates (Rezayof et al. 2009).
The current study is the first to investigate the effects of DA on inhibitory synaptic responses of neurons within the CeA. Results indicate that DA functions to reduce evoked inhibitory neurotransmission primarily through the D1 receptor. The CeA is strongly implicated in mediating the body’s response to stress and drug use, and is heavily innervated by DA-releasing afferents sensitive to such stimuli. It is important that the dopaminergic response in CeA become better understood in order to further determine the CeA’s involvement in response and mediation of stress. Additional research is necessary to confirm whether DA attenuates evoked synaptic activity through a pre- and/or postsynaptic mechanism of action and to determine the particular membrane channels involved.
Acknowledgments
This project was funded by VA Merit Reviews (Scott D. Moore and Wilkie A. Wilson), NIH-NIDA grant #DA09079 (Cynthia Kuhn), the Institute for Medical Research (Jennifer C. Naylor), and the VISN 6 MIRECC.
Abbreviations
- DA
dopamine
- CeA
central nucleus of the amygdala
- GABA
gamma-aminobutyric acid
- IPSC
inhibitory postsynaptic current
- mIPSC
miniature inhibitory postsynaptic current
- BMI
(−)- bicuculline methiodide (BMI)
- DNQX
6,7-dinitroquinoxaline-2,3(1H,4H)-dione
- APV
DL-2-amino-5-phosphonopentanoic acid
References
- Andersen PH, Jansen JA. Dopamine receptor agonists: Selectivity and dopamine D1 receptor efficacy. Eur J Pharmacol. 1990;188:335–347. doi: 10.1016/0922-4106(90)90194-3. [DOI] [PubMed] [Google Scholar]
- Asan E. Ultrastructural features of tyrosine-hydroxylase-immunoreactive afferents and their targets in the rat amygdala. Cell Tissue Res. 1997;288:449–469. doi: 10.1007/s004410050832. [DOI] [PubMed] [Google Scholar]
- Bissiere S, Humeau Y, Luthi A. Dopamine gates LTP induction in lateral amygdala by suppressing feedforward inhibition. Nat Neurosci. 2003;6:587–592. doi: 10.1038/nn1058. [DOI] [PubMed] [Google Scholar]
- Boyson SJ, McGonigle P, Molinoff PB. Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J Neurosci. 1986;6:3177–3188. doi: 10.1523/JNEUROSCI.06-11-03177.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coco ML, Kuhn CM, Ely TD, Kilts CD. Selective activation of mesoamygdaloid dopamine neurons by conditioned stress: attenuation by diazepam. Brain Res. 1992;590:39–47. doi: 10.1016/0006-8993(92)91079-t. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Gehlert DR, McCabe RT, Barnett A, Wamsley JK. D-1 dopamine receptors in the rat brain: a quantitative autoradiographic analysis. J Neurosci. 1986;6:2352–2365. doi: 10.1523/JNEUROSCI.06-08-02352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Mora MP, Cardenas-Cachon L, Mariana Vazquez-Garcia M, Crespo-Ramirez M, Jacobsen K, Hoistad M, Agnati L, Fuxe K. Anxiolytic effects of intra-amygdaloid injection of the D1 antagonist SCH23390 in the rat. Neurosci Lett. 2005;377:101–105. doi: 10.1016/j.neulet.2004.11.079. [DOI] [PubMed] [Google Scholar]
- Jacobsen KX, Hoistad M, Staines WA, Fuxe K. The distribution of dopamine D1 receptor and mu-opioid receptor immunoreactivities in the amygdala and interstitial nucleus of the posterior limb of the anterior commissure: Relationships to tyrosine hydroxylase and opioid peptide terminal systems. Neuroscience. 2006;141:2007–2018. doi: 10.1016/j.neuroscience.2006.05.054. [DOI] [PubMed] [Google Scholar]
- Dubois A, Savasta M, Curet O, Scatton B. Autoradiographic distribution of the D1 agonist [3H]SKF 38393, in the rat brain and spinal cord. Comparison with the distribution of D2 dopamine receptors. Neuroscience. 1986;19:125–137. doi: 10.1016/0306-4522(86)90010-2. [DOI] [PubMed] [Google Scholar]
- Fadok JP, Dickerson TMK, Palmiter RD. Dopamine Is Necessary for Cue-Dependent Fear Conditioning. J Neurosci. 2009;29:11089–11097. doi: 10.1523/JNEUROSCI.1616-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federici M, Natoli S, Bernardi G, Mercuri NB. Dopamine selectively reduces GABAB transmission onto dopaminergic neurones by an unconventional presynaptic action. J Physiol. 2002;540:119–128. doi: 10.1113/jphysiol.2001.013938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman LJ, Cassell MD. Distribution of dopaminergic fibers in the central division of the extended amygdala of the rat. Brain Res. 1994;633:243–252. doi: 10.1016/0006-8993(94)91545-8. [DOI] [PubMed] [Google Scholar]
- Friedman E, Jin LQ, Cai GP, Hollon TR, Drago J, Sibley DR, Wang HY. D1-like dopaminergic activation of phosphoinositide hydrolysis is independent of D1A dopamine receptors: evidence from D1A knockout mice. Mol Pharmacol. 1997;51:6–11. doi: 10.1124/mol.51.1.6. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Islas C, Hablitz JJ. Dopamine inhibition of evoked IPSCs in rat prefrontal cortex. J Neurophysiol. 2001;86:2911–2918. doi: 10.1152/jn.2001.86.6.2911. [DOI] [PubMed] [Google Scholar]
- Guarraci FA, Kapp BS. An electrophysiological characterization of ventral tegmental area dopaminergic neurons during differential pavlovian fear conditioning in the awake rabbit. Behav Brain Res. 1999;99:169–179. doi: 10.1016/s0166-4328(98)00102-8. [DOI] [PubMed] [Google Scholar]
- Huang Q, Zhou D, Chase K, Gusella JF, Aronin N, DiFiglia M. Immunohistochemical localization of the D1 dopamine receptor in rat brain reveals its axonal transport, pre- and postsynaptic localization, and prevalence in the basal ganglia, limbic system, and thalamic reticular nucleus. Proc Nat Acad Sci. 1992;89:11988–11992. doi: 10.1073/pnas.89.24.11988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis FM, Moghaddam B. Dopaminergic innervation of the amygdala is highly responsive to stress. J Neurochem. 1999;72:1088–1094. doi: 10.1046/j.1471-4159.1999.0721088.x. [DOI] [PubMed] [Google Scholar]
- Inoue T, Tsuchiya K, Koyama T. Regional changes in dopamine and serotonin activation with various intensity of physical and psychological stress in the rat brain. Pharmacol Biochem Behav. 1994;49:911–920. doi: 10.1016/0091-3057(94)90243-7. [DOI] [PubMed] [Google Scholar]
- Kroner S, Rosenkranz JA, Grace AA, Barrionuevo G. Dopamine modulates excitability of basolateral amygdala neurons in vitro. J Neurophysiol. 2005;93:1598–610. doi: 10.1152/jn.00843.2004. [DOI] [PubMed] [Google Scholar]
- Lamont EW, Kokkinidis L. Infusion of the dopamine D1 receptor antagonist SCH 23390 into the amygdala blocks fear expression in a potentiated startle paradigm. Brain Res. 1998;795:128–136. doi: 10.1016/s0006-8993(98)00281-9. [DOI] [PubMed] [Google Scholar]
- Leonard SK, Anderson CM, Lachowicz JE, Schulz DW, Kilts CD, Mailman RB. Amygdaloid D1 receptors are not linked to stimulation of adenylate cyclase. Synapse. 2003;50:320–333. doi: 10.1002/syn.10272. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Ann Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Li Q, Clark S, Lewis DV, Wilson WA. NMDA receptor antagonists disinhibit rat posterior cingulate and retrosplenial cortices: a potential mechanism of neurotoxicity. J Neurosci. 2002;22:3070–3080. doi: 10.1523/JNEUROSCI.22-08-03070.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loretan K, Bissiere S, Luthi A. Dopaminergic moduation of spontaneous inhibitory network activity in the lateral amygdala. Neuropharmacology. 2004;47:631–639. doi: 10.1016/j.neuropharm.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Marowsky A, Yanagawa Y, Obata K, Vogt KE. A specialized subclass of interneurons mediates dopaminergic facilitation of amygdala function. Neuron. 2005;48:1025–1037. doi: 10.1016/j.neuron.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Mercuri N, Bernardi G, Calabresi P, Cotugno A, Levi G, Stanzione P. Dopamine decreases cell excitability in rat striatal neurons by pre- and postsynaptic mechanisms. Brain Res. 1985;358:110–121. doi: 10.1016/0006-8993(85)90954-0. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Malenka RC. Dopamine depresses excitatory and inhibitory synaptic transmission by distinct mechanisms in the nucleus accumbens. J Neurosci. 1997;17:5697–5710. doi: 10.1523/JNEUROSCI.17-15-05697.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohuoha DC, Hyde TM, Kleinman JE. The role of serotonin in schizophrenia: an overview of the nomenclature, distribution and alterations of serotonin receptors in the central nervous system. Psychopharmacology. 1993;112:S5–15. doi: 10.1007/BF02245003. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Dolleman-Van der Weel MJ, Kitai ST, Lopes da Silva FH. Presynaptic dopamine D1 receptors attenuate excitatory and inhibitory limbic inputs to the shell region of the rat nucleus accumbens studied in vitro. J Neurophysiol. 1992;67:1325–1334. doi: 10.1152/jn.1992.67.5.1325. [DOI] [PubMed] [Google Scholar]
- Rezayof A, Hosseini S, Zarrindast M. Effects of morphine on rat behaviour in the elevated plus maze: The role of central amygdala dopamine receptors. Behav Brain Res. 2009;202:171–178. doi: 10.1016/j.bbr.2009.03.030. [DOI] [PubMed] [Google Scholar]
- Scheiss MC, Asprodini E, Shinnick-Gallagher P. The effects of dopamine on central amygdala neurons, in vitro intracellular recording. Soc Neurosci. 1988:932. (abstract) [Google Scholar]
- Scibilia RJ, Lachowicz JE, Kilts CD. Topographic nonoverlapping distribution of D1 and D2 dopamine receptors in the amygdaloid nuclear complex of the rat brain. Synapse. 1992;11:146–154. doi: 10.1002/syn.890110208. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Torres IL, Gamaro GD, Vasconcellos AP, Silveira R, Dalmaz C. Effects of chronic restraint stress on feeding behavior and on monoamine levels in different brain structures in rats. Neurochem Res. 2002;27:519–525. doi: 10.1023/a:1019856821430. [DOI] [PubMed] [Google Scholar]
- Umemiya M, Raymond LA. Dopaminergic modulation of excitatory postsynaptic currents in rat neostriatal neurons. J Neurophysiol. 1997;78:1248–1255. doi: 10.1152/jn.1997.78.3.1248. [DOI] [PubMed] [Google Scholar]
- Undie AS, Friedman E. Stimulation of a dopamine D1 receptor enhances inositol phosphates formation in rat brain. J Pharmacol Exp Ther. 1990;253:987–992. [PubMed] [Google Scholar]
- Wachtel SR, Hu XT, Galloway MP, White FJ. D1 dopamine receptor stimulation enables the postsynaptic, but not autoreceptor, effects of D2 dopamine agonists in nigrostriatal and mesoaccumbens dopamine systems. Synapse. 1989;4:327–346. doi: 10.1002/syn.890040409. [DOI] [PubMed] [Google Scholar]
- Weiner DM, Levey AI, Sunahara RK, Niznik HB, O’Dowd BF, Seeman P, Brann MR. D1 and D2 dopamine receptor mRNA in rat brain. Proc Nat Acad Sci. 1991;88:1859–1863. doi: 10.1073/pnas.88.5.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AR, Grace AA. Opposite influences of endogenous dopamine D1 and D2 receptor activation on activity states and electrophysiological properties of striatal neurons: studies combining in vivo intracellular recordings and reverse microdialysis. J Neurosci. 2002;22:294–304. doi: 10.1523/JNEUROSCI.22-01-00294.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama M, Suzuki E, Sato T, Maruta S, Watanabe S, Miyaoka H. Amygdalic levels of dopamine and serotonin rise upon exposure to conditioned fear stress without elevation of glutamate. Neurosci Lett. 2005;379:37–41. doi: 10.1016/j.neulet.2004.12.047. [DOI] [PubMed] [Google Scholar]