Summary
Objectives
To characterize the seroepidemiological features of Pneumocystis jirovecii infection in healthy Chilean children using overlapping fragments (A, B, C) of the P. jirovecii major surface glycoprotein (Msg).
Methods
Serum antibodies to MsgA, MsgB, and MsgC were measured every 2 months by enzyme-linked immunosorbent assay (ELISA) in 45 Chilean infants from about age 2 months to 2 years.
Results
Peak antibody levels (usually reached at age 6 months) and the force (or rate) of infection were somewhat greater for MsgC than for MsgA. Significant seasonal variation in antibody levels was only found with MsgA. Respiratory infections occurred in most children, but nasopharyngeal aspirates were of limited value in detecting the organism. In contrast, serological responses commonly occurred, and higher levels only to MsgC were significantly related to the number of infections.
Conclusions
Serological responses to recombinant Msg fragments provide new insights into the epidemiological and clinical features of P. jirovecii infection of early childhood. MsgA, the amino terminus fragment, is more sensitive in detecting seasonal influences on antibody levels, whereas MsgC is better able to detect changes in antibody levels in response to clinical infection.
Keywords: Serological responses, Pneumocystis, Major surface glycoprotein (Msg), Children
Introduction
Pneumocystis jirovecii remains an important cause of serious pneumonia (‘Pneumocystis pneumonia’ or PcP) in HIV-infected patients and other immunocompromised hosts.1 Serological studies have shown that primary P. jirovecii infection (as defined by the development of antibody responses to Pc antigens) is acquired in early childhood; so by 2–3 years of age, 70–90% of healthy children have serum antibodies to the organism.2-5 It is thought that this infection is mild or asymptomatic.4,6-8 One study found that the presence of P. jirovecii DNA detected by the polymerase chain reaction (PCR) was more commonly associated with upper respiratory tract infection (URI) symptoms than lower respiratory tract infection (LRI) symptoms.9
These serological studies mainly used antigens that consisted of crude extracts obtained from Pneumocystis-infected human or rodent lung.10 Attention was focused on the time when antibodies were first detected and cumulative seropositivity over time. Little was known about the specific antigens that were recognized by serum antibodies, the rate or force of infection, or whether antibody levels varied over time.10
The development of recombinant P. jirovecii antigens has begun a new era in serology, with attention focused mainly on two moieties: the major surface glycoprotein (Msg) and kexin (Kex1).11,12 We selected Msg as our target antigen because it elicits a strong immune response, contains protective epitopes, and plays a major role in the interaction of Pneumocystis with its mammalian host.1,13-15 We developed three overlapping recombinant fragments (Msg A, B, and C) that span the entire length of a single Msg isoform: MsgA, the amino terminus, which is quite variable; MsgB, the mid portion; MsgC, the carboxyl terminus, which is the most conserved fragment. We analyzed reactivity with serum antibodies in prevalence studies and in HIV patients and other adult populations, and found that MsgC was best able to distinguish (1) HIV patients hospitalized with PcP from patients with pneumonia due to other causes, (2) HIV patients who had previous PcP from patients who never had PcP, and (3) healthcare workers who had clinical contact with patients from workers who did not.16-18 We then developed variants (Msg C3, C8, and C9) of the parent MsgC (C1) in order to better delineate the reactivity of this antigen.19-21
Similar to the prominent surface proteins of other eukaryotic pathogens, such as the variant surface antigen (VSA) of Plasmodium and the variant surface glycoprotein (VSG) of Trypanosoma,22,23 Msg is encoded by multiple genes and is thus capable of antigenic variation.24 Serological surveys of young children, who experience most of the malaria fatalities, and of adults in an endemic area provide information about the host immune response to VSA, which is important for vaccine development.25-27 Analysis of serum antibody responses of populations in different geographic areas, climates, and seasons can enhance understanding of the host and environmental effects on the expression of VSA epitopes and their recognition by host antibodies.28-30 Our studies so far have revealed geographic, but not seasonal differences in the serological antibody responses to Msg in adults.21
One of us (SLV) previously followed healthy infants during the first 2 years of life with regular visits and with nasopharyngeal aspirates (NPAs) taken when there were respiratory symptoms.6 [Au?1] P. jirovecii DNA was detected in NPAs in 32% of these episodes, and at a significantly younger age than the NPAs that were negative for P. jirovecii. Yet, detection of P. jirovecii did not identify a specific pattern of symptoms. Serum antibodies to Pneumocystis murina extracted from the mouse with PcP, developed in 53% of the infants at 8 months of age and in 85% of the infants by 20 months of age; seroconversion occurred in the presence of respiratory symptoms in 79% of the subjects. Thus, P. jirovecii or an immune response to the organism could be frequently detected in healthy infants with mild respiratory disease.
Little is known about the serological responses of infants and young children to recombinant P. jirovecii antigens. In the present study, we examined the sequential serum antibody responses to Msg A, B, and C over a 2-year period in 45 infants from this pediatric cohort. We wanted to compare the serological responses to these antigens for their value in determining the force or rate of infection; in looking for seasonal differences in antibody levels; and in examining the host immune response to respiratory infections.
Materials and methods
Patient population
In 1997, a total of 107 healthy infants were enrolled by 1 month of age in a 2-year prospective cohort study in Santiago, Chile.6 [Au?2] Follow-up occurred monthly at a well-baby clinic and serum specimens were obtained every 2 months. Parents were asked to report infections via the telephone or to bring the child to the clinic. NPAs were obtained for P. jirovecii amplification when a respiratory tract infection was diagnosed, especially when cough was reported. An infection was diagnosed as a URI if a cold (rhinitis), nasopharyngitis, or pharyngitis were present. An LRI consisted of bronchitis, bronchiolitis, or pneumonia. Other manifestations such as tonsillitis, tonsillopharyngitis, or otitis media were considered separate disorders. Serum specimens and NPAs (which were mixed with saline) that were obtained were stored at −70 °C. Chest radiographs were obtained when clinically indicated. An informed consent form for study participation was signed by both parents and approval for the study was given by the University of Chile Ethics Commission.
The 45 subjects in the present report were chosen because they had many sequential serum specimens taken, reliable clinical data, and exhibited a broad range of antibody responses to P. murina extracted-purified antigen in the previous study. Serum samples from the previous study were available from 2 to 24 months of age. However, for the analysis of antibody peaks, rate or force of infection, seasonal variation, and episodes of respiratory infection, only serum specimens obtained at or after 6 months of age were used in order to control for maternal antibodies.
DNA amplification
NPAs were examined for P. jirovecii by nested-DNA amplification of the large subunit mitochondrial ribosomal RNA gene of P. jirovecii.4 NPA samples were digested with proteinase K (20 mg/ml) at 60 °C in the presence of 10 mM ethylenediaminetetraacetic acid (EDTA) and 0.5% sodium dodecyl sulfate (SDS). Total DNA was purified and concentrated with use of the Pharma-Gen ‘clean up’ system (Pharma-Gen) and recovered in a volume of 50 μl. Five microliters of this DNA preparation were used for DNA amplification with P. jirovecii oligonucleotide primers pAZ102-E (5′-GATGGCTG-TAGG-3′) and pAZ102-H (5′GTGTACGTTGC-AAAGTACTC-3′), which are internal to the first set of primers and specific for P. jirovecii, in a second round of PCR take 1 μl from the first-round PCR product [Au?3]. Amplified P. jirovecii had a predicted length of 267 bp. Negative controls with no added template DNA were included after each sample to monitor for cross-contamination.
ELISA
Serum antibody levels to the recombinant Msg fragments were analyzed in a blinded manner by an ELISA.16,17 We later made slight modifications to the ELISA.19-21 Analysis of the same specimens by both methods showed good correlation of the results. The small amounts of serum in the specimens available to us precluded repeating the analysis of the specimens in this study by the new method. For similar reasons, we were only able to measure IgG antibodies in this study.
All serum specimens and the standard reference serum were diluted 1/100 and tested in duplicate wells of a 96-well plate against the following antigens: MsgA, MsgB, and MsgC; Escherichia coli extract expressing the pET vector without insert (vector control); phosphate buffered saline (PBS) without antigen (negative control); and tetanus toxoid (TT) (positive control). The plates were washed, followed by the addition of horseradish peroxidase (HRP)-labeled goat anti-human IgG; the plates were washed again and tetramethylbenzidine (TMB) substrate was added. The reaction was stopped by the addition of 0.18 M H2SO4 and the plates were read at a wavelength of 450 nm. The reference serum specimen was obtained from a single individual with known reactivity to Msg. This serum was run each day as an additional control. HRP-labeled S-protein was used as a positive control and to correct for antigen loading. The antibody level or reactivity of each serum specimen to Msg was expressed as the ratio of reactivity: (mean OD Msgtest serum − the mean OD PBStest serum)/(mean OD pETtest serum − mean OD PBStest serum).
Statistics
Antibody levels to Msg A, B, and C (C1) across time were evaluated for each child. The median age at first visit and median duration of follow-up were calculated. Analysis of variance (ANOVA) was performed to determine the differences in serum antibody levels to MsgC and MsgA among seasons and among the number of respiratory infections. Season was defined according to the climate in Chile as follows: summer (December–February), fall (March–May), winter (June–August), and spring (September–November). The number of respiratory infections was categorized as 0 (none), <3 (1–2 illnesses), and ≥3 illnesses. The maximum (peak) antibody level to each antigen was obtained for each child. The median and 25th and 75th percentiles of the set of all peak values were calculated to describe the distribution of these values. Similar percentiles of the distribution of ages when antibody levels peaked were calculated. The average age when P. jirovecii infection occurred was defined serologically as the median age at which the maximum of peak antibody levels occurred. The force (rate or incidence) of infection was defined as 1/median age of infection. The relationship between peak antibody levels and age at time of peak was graphically assessed to provide visual summaries of trends over time. SAS for Windows, version 9.1 (SAS Institute, Cary, NC, USA) was used for all the analyses and the level of statistical significance was based on 5% level (two-sided) unless stated otherwise.
Results
General demographic and serological findings
The median age at first visit was 2 months, the visits were about 2 months apart, and the median follow-up time was 19 months. There were 37 and 42 infants included in the analysis of MsgA and MsgC distributions, respectively (Table 1). Although we had initially planned to examine serum specimens for all 45 subjects, we found that there were missing specimens or specimens with insufficient amounts of serum for analysis. Since antibody levels to MsgB were very low and showed few sequential changes, they were excluded from the analyses. The median peak of antibody level to MsgC was 48.5 vs. 21.2 to MsgA. As defined by peak antibody levels, the median age when primary P. jirovecii infection occurred was 10 months for MsgC vs. 12 months for MsgA. The force of infection measured by antibodies to MsgC was 10% per month compared with 8% per month for antibodies to MsgA. Thus, MsgC tended to elicit higher antibody levels and greater force of infection than MsgA, but the differences were not statistically significant.
Table 1.
Percentiles (%) of the distributions of maximum peak antibody levels to MsgA and MsgC and the age (months) when these levels were reached
| No. of peaks | Median (25th, 75th percentiles) | Age (25th, 75th percentiles) | |
|---|---|---|---|
| MsgA | 37 | 21.2 (11.0, 34.8) | 12 (8, 17) |
| MsgC | 42 | 48.5 (12.3, 110.7) | 10 (8, 14) |
Seasonal changes in antibody levels
The overall patterns of serological responses to MsgA and MsgC were quite different (Table 2). The lowest level of antibodies to MsgA was found in the fall (10.9), whereas the highest level was in the spring (33.2). Levels in the summer were in between. The seasonal differences in antibody levels to MsgA were significant (p = 0.03). In contrast to MsgA, high antibody levels to MsgC ranging from 46.8 to 53.7 were found in summer, fall and winter; the lowest antibody level was found in spring (28.3). However, these results were not statistically significant.
Table 2.
Percentiles (%) of the distributions of peak antibody levels to MsgA and MsgC by season; median peaks (25th, 75th percentiles)
| Spring (Sept–Nov) | Summer (Dec–Feb) | Fall (Mar–May) | Winter (Jun–Aug) | |
|---|---|---|---|---|
| MsgAa | 33.2 (12.0, 68.5); n = 14 | 19.3 (13.1, 27.6); n = 8 | 10.9 (3.9, 15.7); n = 10 | 22.9 (10.4, 77.8); n = 11 |
| MsgC | 28.3 (5.4, 139.4); n = 13 | 53.7 (10.0, 84.9); n = 10 | 46.8 (25.3, 80.0); n = 6 | 52.3 (27.3, 110.7); n = 13 |
n is the number of peaks.
Seasonal median estimates were significantly different (p = 0.03 by Chi-square test) among the seasons.
Antibody reactivity over the 2-year period
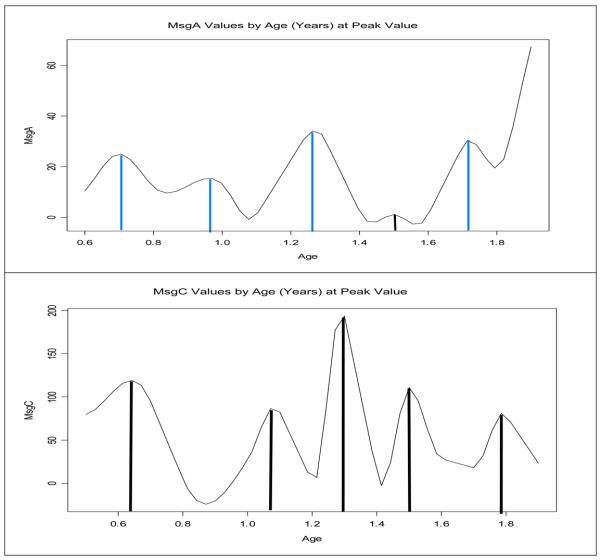
Most children had at least one major peak in their antibody levels to MsgA or MsgC, some had several peaks, while others had little or no peaks. The aggregate data also suggested that there were distinct antibody peaks in the antibody levels that tended to be grouped around specific ages rather than being uniformly distributed (Figure 1). Antibody peaks to MsgC were usually higher and had a shorter median interval between them (3.2 months) than the peaks to MsgA (3.7 months).
Figure 1.
MsgC and MsgA antibody levels by age. The vertical lines show the magnitude of the peaks. Note that the antibody peaks to MsgC are generally higher and have a shorter interval between them than the peaks to MsgA.
Antibody reactivity in individual subjects
Examples of the sequential antibody changes in individual children exhibited considerable variation in the responses to MsgC and MsgA (Figures 2 and 3). The most common pattern was one or more antibody peaks to MsgC after clearance of the maternal antibodies, which usually occurred at 6 months or the third clinic visit. Changes in antibody responses to respiratory infection were easy to find. Yet, since these episodes occurred in the presence or absence of detectable P. jirovecii DNA on the NPAs, and detection of P. jirovecii by PCR is highly dependent on the amount of sample (DNA content) analyzed,31 it was difficult to correlate these specific antibody changes with nested PCR results.
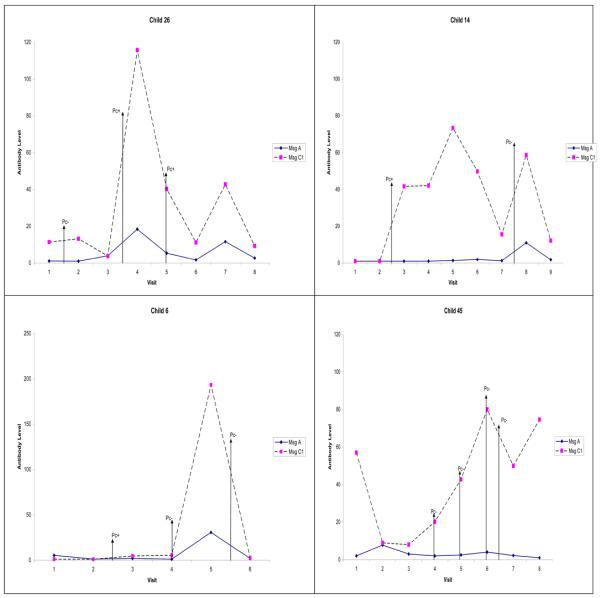
Figure 2.
Examples showing the changes in antibody levels to MsgC over time in four children. Pc+ indicates nasopharyngeal aspirate (NPA) positive for Pneumocystis jirovecii DNA, and Pc− indicates NPA negative for P. jirovecii DNA. The vertical arrows show the visits when the NPAs were obtained. Child 26 developed the first major peak at clinic visit 4 (8 months of age), followed by a smaller peak at clinic visit 7. The first respiratory illness that required medical attention occurred between clinic visits 1 and 2; NPA at that visit was negative for Pneumocystis (Pc−). Subsequent episodes of illness occurred between clinic visits 3 and 4 and at clinic visit 5; in both cases, the NPA was Pc+. Antibody levels to MsgC did not change after the first illness, increased after the second illness, and declined after the third illness. Child 14 developed the first MsgC antibody peak at clinic visit 5 and the second peak at clinic visit 8. Illness occurred between visits 2 and 3 (NPA Pc+), and between visits 7 and 8 (NPA Pc−). Serum antibody levels to MsgC increased after both illnesses. Child 6 developed the first and only MsgC antibody peak at visit 5. Illness occurred between visits 2 and 3 (NPA Pc+), at visit 4 (NPA Pc−), and between visits 5 and 6 (NPA Pc−). There was no change in antibody levels after the first illness, a rise after the second illness, and a fall after the third illness. Child 45 displayed the first antibody peak at visit 6 and second peak at visit 8. Illness occurred at visit 4 (NPA Pc−), visit 5 (NPA Pc−), visit 6 (NPA Pc−), and between visits 6 and 7 (NPA Pc−). Serum antibodies to MsgC rose after the first three illness episodes and fell after the fourth illness.
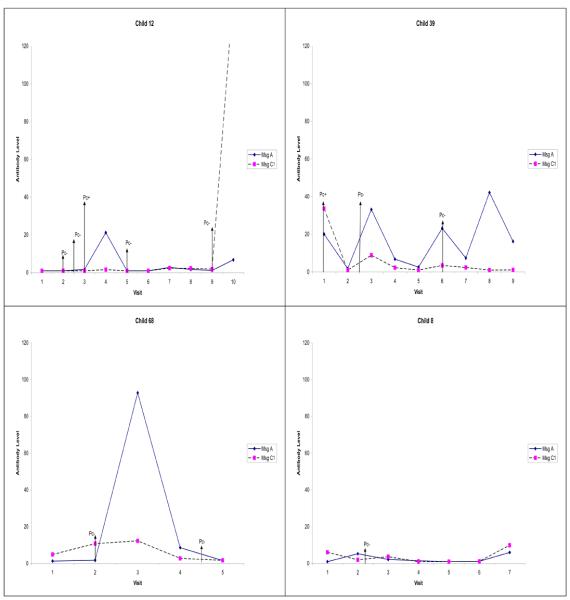
Figure 3.
Examples showing the changes in antibody levels to MsgA over time in four children. Pc+ indicates nasopharyngeal aspirate (NPA) positive for Pneumocystis jirovecii DNA, and Pc− indicates NPA negative for P. jirovecii DNA. The vertical arrows show the visits when the NPAs were obtained. Child 12 exhibited the first and only antibody peak to MsgA at visit 4. Illness occurred at visit 2 (NPA Pc−), between visits 2 and 3 (NPA Pc−), at visit 3 (NPA Pc+), and at visit 9 (NPA Pc−). Serum antibodies to MsgA rose after the illness at visit 3. Child 39 developed the first antibody peak at visit 3 and subsequent peaks at visit 6 and visit 8. Respiratory illness occurred at visit 1 (NPA Pc+), between visits 2 and 3 (NPA Pc−), and at visit 6 (NPA Pc−). Serum antibody levels rose after the first illness, fell after the second illness, and rose again after visit 7 though there was no illness reported. Child 68 exhibited the first and only MsgA antibody peak at visit 3. Illness occurred at visit 2 (NPA Pc−) and between visits 4 and 5 (NPA Pc−). The only serum antibody rise occurred after the first illness. Child 8 demonstrated no antibody peak to MsgA. Illness occurred between visits 2 and 3 (NPA Pc−).
Relationship of antibody levels to number of infections
In order to determine if there was an association between antibody levels to MsgC or MsgA and the number of respiratory infections developed by the children, we compared the geometric mean (95% confidence interval) of antibody levels of all visits for all subjects with the same number of respiratory infections (Table 3). The data showed that there was a progressive rise in mean antibody levels to MsgC with increasing number of respiratory infections; children experiencing three or more episodes had significantly higher antibody levels to MsgC than children who had experienced no episodes or one to two episodes. By contrast, no such difference was seen in the antibody levels to MsgA. We also examined these antibody levels without controlling for maternal antibodies, and again significantly higher antibody levels were found in the children with three or more respiratory infections (data not shown).
Table 3.
Serum antibody levels to MsgA and MsgC in the children by the number of episodes of respiratory infection detected during the study
| No. of episodes |
No. of serum specimens |
Mean ratio (percentiles 25–75) |
|
|---|---|---|---|
| MsgC | MsgA | ||
| 0 | 38 | 3.74 (2.18–6.40) | 3.92 (2.41–6.38) |
| <3 | 147 | 5.59 (4.25–7.35) | 3.21 (2.56–4.03) |
| ≥3 | 125 | 12.44 (9.46–16.35)a | 4.49 (3.59–5.61) |
Children who had three or more clinical diagnoses had significantly higher mean MsgC levels compared to those who had no diagnosis and those who had one or two episodes (p < 0.05 by ANOVA).
In addition, we attempted by several different approaches to correlate antibody levels to MsgC or MsgA with the specific types of respiratory infection (URI or LRI), but the results were inconclusive (data not shown).
Discussion
The present report is the first seroepidemiological study of P. jirovecii infection in children using recombinant antigen preparations. As in our previous studies of adults in the USA,16-19 the consistently higher antibody levels elicited by MsgC suggest that it is the predominant antigen recognized by serum antibodies in Chilean infants and young children. MsgC also tended to elicit higher and more rapid antibody peaks than MsgA, as illustrated in the force of infection, which is an epidemiological tool that has been helpful in studying the dynamics of infection, both in populations and in models of infection.32-34
Previous reports have noted geographic differences in the occurrence and P. jirovecii colonization in HIV patients.35,36 One characteristic of multi-gene antigens used in serological surveys is geographic variation in the antibody responses. Our serological study of HIV subjects enrolled in the Multicenter AIDS Cohort Study (MACS), which compared the reactivity of the four recombinant MsgC fragments, demonstrated significant differences in the antibody levels to these MsgC fragments among the cities (Baltimore, Pittsburgh, Chicago, and Los Angeles) where the study was conducted.21 We also found that MsgC8 was the predominant Msg fragment recognized in Seville, Spain, and that MsgA was the major fragment recognized in Cameroon.20,37
The impact of climate on the occurrence of Pneumocystis infection in humans as well as in animals has been a subject of considerable investigation.38-43 A complex and sometimes contradictory relationship has been found: some studies have shown an increased occurrence associated with warmer temperatures, whereas other studies have found an increased association with colder temperatures or no seasonal association at all. Our study of MACS subjects extended this debate to serological responses, but found no seasonal differences in serological responses to the MsgC fragments.21
The present report also found no significant seasonal differences in antibody responses to MsgC in young Chilean children; however, the report did reveal a different pattern of reactivity to MsgA that was associated with significant differences among the seasons. The reasons for these findings are not clear. Perhaps host recognition of MsgA, which is the most exposed part of Msg, is more easily influenced by changes in the environment (e.g., temperature, humidity) than recognition of MsgC. In addition, the exposures of the young children in this study differed from exposures in adults in that many of these exposures were the children's initial encounter with the organism. These findings support our previous data, which suggest that the Msg fragments are recognized independently by the host.19-21 The results also emphasize the need for further investigation of the effects of environmental factors on host responses to P. jirovecii.
While previous reports have focused on the time at which children first develop serum antibodies to P. jirovecii,4-6 the present study examined the sequential changes in antibody levels during the first 2 years of life. Most subjects developed at least one major peak to MsgC and/or MsgA, but there was considerable variation in the individual responses. Perhaps the tendency for the peaks to be grouped or clustered around specific ages was related to maturational changes in the immune response or to age-related environmental exposures. Although one study of P. jirovecii colonization in families did not find clustering,44 clustering of serological responses in other infectious diseases has been observed.28-30,45,46
Respiratory infections occurred in most of the children at some point during the study, and were accompanied by antibody responses to MsgC and/or MsgA. The children who had more infections had significantly higher mean antibody levels to MsgC (but not to MsgA) than individuals who had fewer infections. These data suggest that MsgC antibody levels are a more sensitive marker of more frequent or more intense clinical exposure to P. jirovecii. The results are also consistent with our previous reports of the value of MsgC in discriminating present from past infection or active disease from colonization.16,17,21
While the data in this report extend the results of the previous study of these children,6 [Au?4] both studies have limitations. The serological analysis was performed from an epidemiological point of view and the small quantities of serum prevented analysis of IgM antibodies; thus, the results should not be extrapolated to the diagnosis of P. jirovecii infection. NPAs to detect P. jirovecii were only performed when respiratory symptoms were present; testing for other pathogens by culture, PCR, or serology was not performed. Without this information, caution must be used in interpreting the results. In addition, recent data show compartmentalization of P. jirovecii colonization in the upper respiratory tract and that the yield of noninvasive respiratory sampling increases if more than one niche is sampled.31
In summary, this study shows that recombinant Msg fragments are sensitive new tools that can provide new information about the epidemiological and clinical features of P. jirovecii infection in early childhood. MsgA, the amino terminus fragment, appears to be more sensitive in detecting seasonal influences on immune responses, while MsgC, the carboxyl terminus fragment, is more effective in detecting changes in antibody levels in response to clinical infection.
Acknowledgements
This study was supported by the Medical Research Service, Department of Veterans Affairs NIH grants R01AI-06492 and R01 HL-090335 (PDW); and by The Elizabeth Glaser Pediatric AIDS Foundation grant PG 51153-26 and The Fondo Nacional de Desarrollo Científico y Tecnológico, Santiago, Chile (FONDECYT) research grant 1060750 (SLV). We thank Diane Gillotte for excellent assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors have no conflict of interest.
References
- 1.Walzer PD, Smulian AG. Pneumocystis species. In: Mandell GL, Dolin R, Bennett JE, editors. Principles and practice of infectious diseases. Elsevier, Inc.; Philadelphia: 2010. pp. 3377–407. [Google Scholar]
- 2.Meuwissen JH, Tauber I, Leeuwenberg AD, Beckers PJ, Sieben M. Parasitologic and serologic observations of infection with Pneumocystis in humans. J Infect Dis. 1977;136:43–9. doi: 10.1093/infdis/136.1.43. [DOI] [PubMed] [Google Scholar]
- 3.Pifer LL, Hughes WT, Stagno S, Woods D. Pneumocystis carinii infection: evidence for high prevalence in normal and immunosuppressed children. Pediatrics. 1978;61:35–41. [PubMed] [Google Scholar]
- 4.Vargas SL, Hughes WT, Santolaya ME, Ulloa AV, Ponce CA, Cabrera CE, et al. Search for primary infection by Pneumocystis carinii in a cohort of normal, healthy infants. Clin Infect Dis. 2001;32:855–61. doi: 10.1086/319340. [DOI] [PubMed] [Google Scholar]
- 5.Peglow SL, Smulian AG, Linke MJ, Pogue CL, Nurre S, Crisler J, et al. Serologic responses to Pneumocystis carinii antigens in health and disease. J Infect Dis. 1990;161:296–306. doi: 10.1093/infdis/161.2.296. [DOI] [PubMed] [Google Scholar]
- 6.Stagno S, Pifer LL, Hughes WT, Brasfield DM, Tiller RE. Pneumocystis carinii pneumonitis in young immunocompetent infants. Pediatrics. 1980;66:56–62. [PubMed] [Google Scholar]
- 7.Nevez G, Totet A, Pautard JC, Raccurt C. Pneumocystis carinii detection using nested-PCR in nasopharyngeal aspirates of immunocompetent infants with bronchiolitis. J Eukaryot Microbiol. 2001;(Suppl):122S–123S. doi: 10.1111/j.1550-7408.2001.tb00479.x. [DOI] [PubMed] [Google Scholar]
- 8.Vargas SL, Ponce CA, Luchsinger V, Silva C, Gallo M, Lopez R, et al. Detection of Pneumocystis carinii f. sp. hominis and viruses in presumably immunocompetent infants who died in the hospital or in the community. J Infect Dis. 2005;191:122–6. doi: 10.1086/426451. [DOI] [PubMed] [Google Scholar]
- 9.Larsen HH, von Linstow ML, Lundgren B, Hogh B, Westh H, Lundgren JD. Primary Pneumocystis infection in infants hospitalized with acute respiratory tract infection. Emerg Infect Dis. 2007;13:66–72. doi: 10.3201/eid1301.060315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walzer PD. Immunological features of Pneumocystis carinii infection in humans. Clin Diagn Lab Immunol. 1999;6:149–55. doi: 10.1128/cdli.6.2.149-155.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garbe TR, Stringer JR. Molecular characterization of clustered variants of genes encoding major surface antigens of human Pneumocystis carinii. Infect Immun. 1994;62:3092–101. doi: 10.1128/iai.62.8.3092-3101.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kutty G, Kovacs JA. A single-copy gene encodes Kex1, a serine endoprotease of Pneumocystis jiroveci. Infect Immun. 2003;71:571–4. doi: 10.1128/IAI.71.1.571-574.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gigliotti F, Hughes WT. Passive immunoprophylaxis with specific monoclonal antibody confers partial protection against Pneumocystis carinii pneumonitis in animal models. J Clin Invest. 1998;81:1666–8. doi: 10.1172/JCI113503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Theus SA, Andrews RP, Steele P, Walzer PD. Adoptive transfer of lymphocytes sensitized to the major surface glycoprotein of Pneumocystis carinii confers protection in the rat. J Clin Invest. 1995;95:2587–93. doi: 10.1172/JCI117960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theus SA, Smulian AG, Steel P, Linke MJ, Walzer PD. Immunization with the major surface glycoprotein of Pneumocystis carinii elicits a protective response. Vaccine. 1998;16:1149–57. doi: 10.1016/s0264-410x(98)80113-8. [DOI] [PubMed] [Google Scholar]
- 16.Daly KR, Koch J, Levin L, Walzer PD. Enzyme-linked immunosorbent assay and serologic responses to Pneumocystis jiroveci. Emerg Infect Dis. 2004;10:848–54. doi: 10.3201/eid1005.030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daly KR, Huang L, Morris A, Koch J, Crothers K, Levin L, et al. Antibody response to Pneumocystis jirovecii major surface glycoprotein. Emerg Infect Dis. 2006;12:1231–7. doi: 10.3201/eid1208.060230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tipirneni R, Daly KR, Jarlsbert LG, Koch JV, Swartzman A, Roth BM, et al. Healthcare worker occupation and immune response to Pneumocystis jirovecii. Emerg Infect Dis. 2009;15:1590–7. doi: 10.3201/eid1510.090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daly KR, Koch JV, Shire NJ, Levin L, Walzer PD. Human immunodeficiency virus-infected patients with prior Pneumocystis pneumonia exhibit increased serologic reactivity to several major surface glycoprotein clones. Clin Vaccine Immunol. 2006;13:1071–8. doi: 10.1128/CVI.00140-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daly K, Koch J, Respaldiza N, de la Horra C, Montes-Cano MA, Medrano FJ, et al. Geographical variation in serological responses to recombinant Pneumocystis jirovecii major surface glycoprotein antigens. Clin Microbiol Infect. 2009;15:937–42. doi: 10.1111/j.1469-0691.2009.02716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walzer PD, Djawe K, Levin L, Daly KR, Koch J, Kingsley L, et al. Long-term serologic responses to the Pneumocystis jirovecii major surface glycoprotein in HIV-positive individuals with and without P. jirovecii infection. J Infect Dis. 2009;199:1335–44. doi: 10.1086/597803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sternberg JM. Human African trypanosomiasis: clinical presentation and immune response. Parasite Immunol. 2004;26:469–76. doi: 10.1111/j.0141-9838.2004.00731.x. [DOI] [PubMed] [Google Scholar]
- 23.Hviid L. The immuno-epidemiology of pregnancy-associated Plasmodium falciparum malaria: a variant surface antigen-specific perspective. Parasite Immunol. 2004;26:477–86. doi: 10.1111/j.0141-9838.2004.00733.x. [DOI] [PubMed] [Google Scholar]
- 24.Kovacs JA, Powell F, Edman JC, Lundgren B, Martinez A, Drew B, Angus CW. Multiple genes encode the major surface glycoprotein of Pneumocystis carinii. J Biol Chem. 1993;268:604–40. [PubMed] [Google Scholar]
- 25.Stirnadel HA, Beck HP, Alpers MP, Smith TA. Heritability and segregation analysis of immune responses to specific malaria antigens in Papua New Guinea. Genet Epidemiol. 1999;17:16–34. doi: 10.1002/(SICI)1098-2272(1999)17:1<16::AID-GEPI2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 26.Taylor RR, Egan A, McGuinness D, Jepson A, Drakely C, Riley E. Selective recognition of malaria antigens by human serum antibodies is not genetically determined but demonstrates some features of clonal imprinting. Int Immunol. 1996;8:905–15. doi: 10.1093/intimm/8.6.905. [DOI] [PubMed] [Google Scholar]
- 27.Vestergaard LS, Lusingu JP, Nielsen MA, Mmbando BP, Dodoo D, Akanmori BD, et al. Differences in human antibody reactivity to Plasmodium falciparum variant surface antigens are dependent on age and malaria transmission intensity in Northeastern Tanzania. Infect Immun. 2008;76:2706–14. doi: 10.1128/IAI.01401-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stirnadel HA, Al-Yaman F, Genton B, Alpers MP, Smith TA. [Au?5] Int J Epidemiol. 2000;29:579–86. [PubMed] [Google Scholar]
- 29.Nielsen MA, Vestergaard LS, Lusingu J, Kurtzhals JA, Giha HA, Grevstad B, et al. Geographical and temporal conservation of antibody recognition of Plasmodium falciparum variant surface antigens. Infect Immun. 2004;76:3531–5. doi: 10.1128/IAI.72.6.3531-3535.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Booth M, Dunne DW. Spatial awareness in parasite immuno-epidemiology. Parasite Immunol. 2004;26:499–507. doi: 10.1111/j.0141-9838.2004.00735.x. [DOI] [PubMed] [Google Scholar]
- 31.Vargas SL, Pizarro P, Lopez-Vieyra M, Neira-Aviles P, Bustamante R, Ponce CA. Pneumocystis colonization in older adults and diagnostic yield of single versus paired noninvasive respiratory sampling. Clin Infect Dis. 2010;50:e19–21. doi: 10.1086/649869. [DOI] [PubMed] [Google Scholar]
- 32.Mossong J, Putz L, Schneider F. Seroprevalence and force of infection of varicella-zoster virus in Luxembourg. Epidemiol Infect. 2004;132:1121–7. doi: 10.1017/s0950268804002754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith T, Maire N, Dietz K, Killeen GF, Vounatsou P, Molineaux L, et al. Relationship between the entomologic inoculation rate and the force of infection for Plasmodium falciparum malaria. Am J Trop Med Hyg. 2006;75(2 Suppl):11–8. doi: 10.4269/ajtmh.2006.75.2_suppl.0750011. [DOI] [PubMed] [Google Scholar]
- 34.Sutton AJ, Hope VD, Mathei C, Mravcik V, Sebakova H, Vallejo F, et al. A comparison between the force of infection estimates for blood-borne viruses in injecting drug user populations across the European Union: a modelling study. J Viral Hepat. 2008;15:809–16. doi: 10.1111/j.1365-2893.2008.01041.x. [DOI] [PubMed] [Google Scholar]
- 35.Beard CD, Carter JL, Keely SP, Huang L, Pieniazek NJ, Moura IN, et al. Genetic variation in Pneumocystis carinii isolates from different geographic regions: implications for transmission. Emerg Infect Dis. 2000;6:265–72. doi: 10.3201/eid0603.000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morris A, Kingsley LA, Groner G, Lebedeva IP, Bearch CB, Morris KA. Prevalence and clinical predictors of Pneumocystis colonization among HIV-infected men. AIDS. 2004;18:793–8. doi: 10.1097/00002030-200403260-00011. [DOI] [PubMed] [Google Scholar]
- 37.Nikinin SW, Daly KR, Walzer PD, Ndzi ES, Asonganyi T, Respaldiza N, et al. Evidence for high prevalence of Pneumocystis jirovecii exposure among Cameroonians. Acta Trop. 2009;112:219–24. doi: 10.1016/j.actatropica.2009.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoover DR, Graham NM, Bacellar H, Schrager LK, Kaslow R, Visscher B, et al. Epidemiologic patterns of upper respiratory illness and Pneumocystis carinii pneumonia in homosexual men. Am Rev Respir Dis. 1991;144:756–9. doi: 10.1164/ajrccm/144.4.756. [DOI] [PubMed] [Google Scholar]
- 39.Lubis N, Baylis D, Short A, Stebbing J, Teague A, Portsmouth S, et al. Prospective cohort study showing changes in the monthly incidence of Pneumocystis carinii pneumonia. Postgrad Med J. 2003;79:164–6. doi: 10.1136/pmj.79.929.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calderon E, de la Horra C, Medrano FJ, Lopez-Suarez A, Montes-Cano MA, Respaldiza N, et al. Pneumocystis jiroveci isolates with dihydropteroate synthase mutations in patients with chronic bronchitis. Eur J Clin Microbiol Infect Dis. 2004;23:545–9. doi: 10.1007/s10096-004-1151-3. [DOI] [PubMed] [Google Scholar]
- 41.Respaldiza N, Medrano FJ, Medrano AC, Varela JM, de la Horra C, Montes-Cano M, et al. High seroprevalence of Pneumocystis infection in Spanish children. Clin Microbiol Infect. 2004;10:1029–31. doi: 10.1111/j.1469-0691.2004.00974.x. [DOI] [PubMed] [Google Scholar]
- 42.Miller RF, Grant AD, Foley NM. Seasonal variation in presentation of Pneumocystis carinii pneumonia. Lancet. 1992;339:747–8. doi: 10.1016/0140-6736(92)90650-r. [DOI] [PubMed] [Google Scholar]
- 43.Sing A, Schmoldt S, Laubender RP, Heesemann J, Sing D, Wildner M. Seasonal variation of Pneumocystis jirovecii infection: analysis of underlying climatic factors. Clin Microbiol Infect. 2009;15:957–60. doi: 10.1111/j.1469-0691.2009.02804.x. [DOI] [PubMed] [Google Scholar]
- 44.Spencer L, Ukwu M, Alexander T, Valadez K, Liu L, Frederick T, et al. Epidemiology of Pneumocystis colonization in families. Clin Infect Dis. 2008;46:1237–40. doi: 10.1086/533449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Angeloni A, Heston L, Uccini S, Sirianni MC, Cottoni F, Masala MV, et al. High prevalence of antibodies to human herpesvirus 8 in relatives of patients with classic Kaposi's sarcoma from Sardinia. J Infect Dis. 1998;177:1715–8. doi: 10.1086/517429. [DOI] [PubMed] [Google Scholar]
- 46.Ng EK, Thompson SA, Perez-Perez GI, Kansau I, van der Ende A, Labigne A, et al. Helicobacter pylori heat shock protein A: serologic responses and genetic diversity. Clin Diagn Lab Immunol. 1999;6:377–82. doi: 10.1128/cdli.6.3.377-382.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]