Abstract
Purpose
A 2-arm, double-blinded, randomized trial to evaluate the effect of 0.1% mometasone furoate (MMF) on acute skin-related toxicity in patients undergoing breast or chest wall radiotherapy.
Methods and Materials
Patients with ductal carcinoma in situ or invasive breast carcinoma receiving external beam radiotherapy to breast or chest wall were randomly assigned to daily apply 0.1% MMF or placebo cream. Primary study end point was provider-assessed maximum grade of Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 radiation dermatitis. Secondary end points included provider-assessed CTCAE grade 3 or greater radiation dermatitis and adverse-event monitoring. Patient-reported outcome (PRO) measures included the Skindex-16, the Skin Toxicity Assessment Tool, a Symptom Experience Diary, and quality of life self-assessment. Assessment was performed at baseline, weekly during radiotherapy, and for 2 weeks after radiotherapy.
Results
In total, 176 patients were enrolled from September 21, 2007 through December 7, 2007. The provider-assessed primary end point showed no difference in mean maximum grade of radiation dermatitis by treatment arm (1.2 for MMF vs 1.3 for placebo; P=.18). CTCAE toxicity was greater in placebo group (P=.04), primarily from pruritus. For PRO measures, the maximum Skindex-16 score for MMF group showed less itching (P=.008), less irritation (P=.01), less symptom persistence or recurrence (P=.02), and less annoyance with skin problems (P=.04); the group's maximum Skin Toxicity Assessment Tool score showed less burning sensation (P=.02) and less itching (P=.002).
Conclusion
Patients receiving daily MMF during radiotherapy may experience reduced acute skin toxicity in comparison to placebo.
Keywords: breast neoplasms, mometasone furoate, radiotherapy, skin manifestations, toxicity
Introduction
Radiation dermatitis is a common adverse effect of radiotherapy in patients receiving irradiation of the breast and chest wall. It is the most common treatment-related toxicity for patients undergoing radiotherapy for early stage breast cancer (1). Although many topical agents are currently used in clinical practice for prevention and treatment of radiation dermatitis, randomized clinical trials have not consistently indicated the superiority of any single agent; however, a recent randomized clinical trial of mometasone furoate (MMF) in combination with an emollient cream versus an emollient cream alone showed a reduction in dermatitis and patient symptoms in the MMF arm (2-6). The present clinical trial was conducted as a confirmatory trial to assess the value of MMF in decreasing treatment-related skin toxicity of patients receiving adjuvant therapy for breast cancer.
Methods and Materials
The North Central Cancer Treatment Group performed a 2-arm, double-blinded, randomized trial designed to evaluate the effect of MMF on skin-related toxicity in breast cancer patients undergoing radiotherapy to the breast (breast conservation therapy) or chest wall (postmastectomy radiotherapy). This study was approved by the Mayo Clinic Institutional Review Board and was independently approved by the institutional review board of the participating institutions. Written informed consent was required for enrollment in the trial.
Patient Selection Criteria
Patients eligible for enrollment in this trial were adults (age, ≥18 years) with histologic proof of a primary invasive breast carcinoma or ductal carcinoma in situ who were to undergo a planned course of continuous, definitive, or adjuvant external beam radiotherapy to the whole breast as part of breast conservation therapy or to the chest wall as part of postmastectomy irradiation (minimum prescription dose, 50.0 Gy). Treatment of regional lymph nodes, including the axillary, supraclavicular, and internal mammary lymph nodes, was permitted. The daily treatment dose was between 1.75 Gy and 2.12 Gy. Patients could enter the trial before receiving the third radiotherapy fraction. An Eastern Cooperative Oncology Group performance status of 0, 1, or 2 was required.
Ineligibility criteria included the presence of inflammatory carcinoma of the breast or a known allergy or hypersensitivity to mometasone and furoate, to imidazolidinyl urea, or to formaldehyde. Additional ineligibility criteria included use of leukotriene inhibitors or use of a prescription or over-the-counter medication that contained hydrocortisone or any other cortisone- or corticosteroid-containing preparation. Patients were not eligible for the trial if they had preexisting loss of skin integrity or prior radiotherapy to the area being treated. Also excluded were women who were pregnant or breastfeeding and women of child-bearing age who were unwilling to use adequate contraception during the study period. Patients with bilateral breast carcinoma were ineligible, as were patients receiving partial (<75%) breast treatment.
Randomization
Patients were randomly assigned, in a double-blind manner using a dynamic allocation procedure, to either 0.1% MMF cream or an identical-appearing placebo cream (Dermabase; Paddock Laboratories, Inc, Minneapolis, Minnesota). Randomization was performed through the operations office of the North Central Cancer Treatment Group in Rochester, Minnesota. Stratification factors included whole-breast radiotherapy after lumpectomy versus chest wall radiotherapy after mastectomy, treatment versus no treatment of regional lymph nodes, and total radiation dose of 50.0 to 55.0 Gy versus more than 55.0 Gy.
Treatment
Patients were instructed to apply 3 mL of MMF cream or placebo cream lightly once daily to the area under treatment at no less than 4 hours before or after radiotherapy until completion of the prescribed course of irradiation. They were instructed to vary the amount of cream on the basis of body habitus and to cover the entire treated area. No other topical agents were allowed to be used in the radiotherapy field while the patient was receiving the study medication. If, in the judgment of a patient's clinician, radiation dermatitis necessitated initiation of an agent other than the study medicine, the patient was to discontinue the study medication and continue with evaluations in accordance with study protocol.
Study Evaluation
Patients were evaluated at baseline and at weekly intervals during their radiotherapy by their treatment providers (Table 1). Evaluation consisted of 1) provider-assessed toxicity assessment using the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 (7) and 2) patient-reported symptoms and quality of life (QOL) noted in patient-completed assessment forms. Additionally, after completion of radiotherapy, patients filled out a patient questionnaire booklet for the 2 weeks immediately after radiotherapy completion. Patient-reported outcomes were measured using the Skindex-16, the CTCAE Symptom Experience Diary, and the Skin Toxicity Assessment Tool.
Table 1.
Evaluation Schedule of the Phase 3 Trial
| Tests and Procedures | Baseline | Weekly During Radiotherapya | Observation and Follow-upb |
|---|---|---|---|
| Provider-assessed toxicity | |||
| CTCAE dermatitis assessment | Yes | Yes | NA |
| Adverse-event assessment (CTCAE version 3.0) | Yes | Yes | NA |
| Provider-assessed and patient-reported toxicity | |||
| Skin toxicity assessment | Yes | Yes | NA |
| Patient-reported toxicity | |||
| LASA, Skindex-16, and Symptom Experience Diary | Yes | Yes | Yes |
Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events; LASA, linear analogue self-assessment; NA, not applicable.
At clinic visit.
Follow-up period was the 2 weeks after radiotherapy completion.
The Skindex-16 is an analogue scale of symptoms and functional end points related to skin toxicity that may occur in the radiation treatment area (8). The Symptom Experience Diary requires the patient to rate severity of multiple skin toxicity-related signs and symptoms on a scale of 0 (do not experience) to 10 (experience all the time). The Skin Toxicity Assessment Tool is a skin-specific instrument consisting of a provider-assessed objective measure of skin changes and 5 measures of patient-reported discomfort (9). Patient-completed QOL assessment was the linear analogue self-assessment, which consists of 6 questions with responses ranging from 0 (poor QOL) to 10 (best QOL). These questions have been validated as general measures of global QOL dimensional constructs in numerous settings and have been validated at Mayo Clinic for use in cancer patients (10-13).
Statistical Analysis
The primary study end point was radiation dermatitis determined by the patient's health care provider with CTCAE version 3.0. The maximum grade of this adverse event during treatment was evaluated in each patient. The mean maximum grades were compared between the 2 treatment arms with a single 2-sample t test. We calculated that a 2-sample t test (2-sided α=0.05) with 64 patients in the MMF group and 64 patients in the placebo group had an 80% power to detect a difference of a half SD (approximately 0.4 of a severity grade based on the SD of the placebo arm in the double-blind portion of NCCTG 909252, “Phase 3 Double-Blind Evaluation of an Aloe Vera Gel as a Prophylactic Agent for Radiation-Induced Skin Toxicity”) (6). Sample size was inflated by 15% to account for missing data (eg, patient ineligibility, cancellation of trial participation). The total number planned for accrual was 148 patients, or 74 per treatment arm.
Secondary end points included incidence of severe (CTCAE grade, ≥3) radiation dermatitis, grade of adverse events at the end of radiotherapy, and maximum grade of other adverse events, the latter 2 end points as measured by the CTCAE version 3.0. These end points were compared between the treatment and the placebo arms with use of χ2 and Fisher exact methods as appropriate. Secondary end points of patient-reported skin toxicity (Skindex-16 and Skin Toxicity Assessment Tool) and QOL were analyzed by comparing mean responses between the study arms with use of the Kruskal-Wallis test.
Results
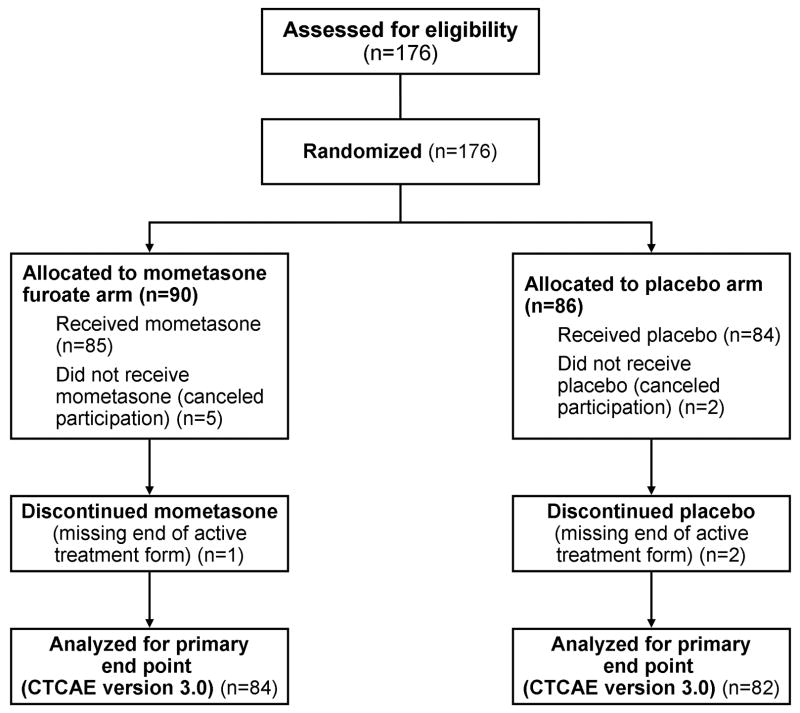
A total of 176 patients were enrolled from September 21, 2007, through December 7, 2007 (Figure 1); follow-up period was the 2 weeks after radiotherapy completion. This enrollment exceeded the original target accrual by 28 patients and resulted from an extremely rapid rate of enrollment. Ninety patients were randomly assigned to the treatment group; 86 patients were randomly assigned to the control group. After randomization, 5 patients in the MMF arm and 2 patients in the placebo arm declined participation, for a total of 169 eligible patients. Data were missing on 3 patients, leaving 166 patients eligible for evaluation of the primary end point. Baseline characteristics were equally balanced between the study agent arm and the placebo arm (Table 2).
Figure 1.
Flow of Patients in the Phase 3 Trial. CTCAE indicates Common Terminology Criteria for Adverse Events.
Table 2.
Baseline Characteristics of Study Participants
| Characteristicsa | Group | Total (N=169) | P Value | |
|---|---|---|---|---|
| Mometasone Furoate (n=85) | Placebo (n=84) | |||
| Age, y | .38 | |||
| Median | 60 | 57 | 58 | |
| Range | (35-89) | (27-85) | (27-89) | |
| Radiotherapy fields | .81 | |||
| Breast (postlumpectomy) | 71 (84) | 69 (82) | 140 (83) | |
| Chest wall (postmastectomy) | 14 (16) | 15 (18) | 29 (17) | |
| Regional lymph nodes | .44 | |||
| Treated | 18 (21) | 22 (26) | 40 (24) | |
| Not treated | 67 (79) | 62 (74) | 129 (76) | |
| Planned total radiation dose | .33 | |||
| 50.0-55.0 Gy | 17 (20) | 12 (14) | 29 (17) | |
| >55.0 Gy | 68 (80) | 72 (86) | 140 (83) | |
Categorical data are expressed as number and percentage of patients.
There was no significant difference in the mean maximum grade of provider-assessed radiation dermatitis (1.2 in MMF arm vs 1.3 in placebo arm; P=.18) (Table 3). Similarly, there was no significant difference in the incidence of provider-assessed severe (CTCAE grade, ≥3) radiation dermatitis or the provider-assessed maximum radiation dermatitis grade.
Table 3.
Provider-Assessed Primary and Secondary End Points With Use of CTCAE Version 3.0
| Group | ||||
|---|---|---|---|---|
| End Pointa | Mometasone Furoate (n=84) | Placebo (n=82) | Total (N=166) | P Value |
| Provider-assessed primary end point Maximum radiation dermatitis grade | ||||
| Mean (SD) | 1.2 (0.85) | 1.3 (0.80) | 1.3 (0.83) | .18 |
| Range | (0.0-3.0) | (0.0-3.0) | (0.0-3.0) | |
| Provider-assessed secondary end points Incident of severe (grade, ≥3) radiation dermatitis | ||||
| No | 80 (95) | 78 (95) | 158 (95) | .97 |
| Yes | 4 (5) | 4 (5) | 8 (5) | |
| Maximum radiation dermatitis grade | ||||
| 0 | 20 (24) | 13 (16) | 33 (20) | .51 |
| 1 | 34 (40) | 32 (39) | 66 (40) | |
| 2 | 26 (31) | 33 (40) | 59 (36) | |
| 3 | 4 (5) | 4 (5) | 8 (5) | |
Categorical data are expressed as number and percentage of patients.
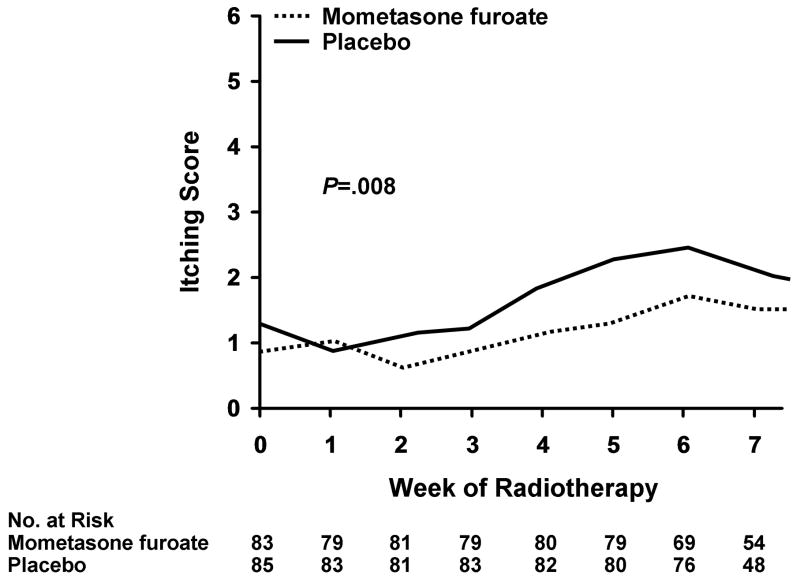
A number of secondary end points were positive for a reduction in skin toxicity in the MMF group. Itching, irritation, persistence of symptoms, recurrence of toxicity symptoms, and annoyance with the dermatitis were all reduced in a statistically significant fraction in the treatment group compared with the placebo group in the Skindex-16 (Table 4). The total Skindex-16 score was 1.4 in the MMF arm and 1.7 in the placebo arm (P=.07), suggesting a trend toward a more favorable outcome in patients treated with MMF. Patients in the MMF arm also reported less discomfort and burning (P=.02), less itching (P=.002), and less redness (P=.003) (Table 5) via the Skin Toxicity Assessment Tool and Symptom Experience Diary. Significantly less itching was observed in the patients of the MMF arm after approximately week 2 (Figure 2). There was no difference in overall QOL with the linear analogue self-assessment instrument: the MMF group had a mean score of 8.3 and median score of 9.0 and the placebo group had a mean score of 8.4 and median score of 9.0. Similarly, there was no difference in the 6 linear analogue self-assessment subdomains.
Table 4.
Patient-Reported Maximum Skindex-16 Toxicity Scorea
| Maximum Skindex-16 Score by Group | ||||
|---|---|---|---|---|
| Toxicity Characteristic | Mometasone Furoate (n=83) | Placebo (n=84) | Change in Score | P Value |
| Itching | 2.3 | 3.1 | −0.8 | .008 |
| Burning or stinging | 2.6 | 3.1 | −0.5 | .06 |
| Pain | 2.5 | 2.9 | −0.4 | .15 |
| Irritation | 2.6 | 3.4 | −0.8 | .01 |
| Persistance or recurrence | 2.3 | 3.0 | −0.7 | .02 |
| Worry about skin condition | 1.5 | 1.8 | −0.3 | .45 |
| Appearance | 1.6 | 2.0 | −0.4 | .10 |
| Frustration | 1.5 | 1.8 | −0.3 | .23 |
| Embarassment | 0.7 | 1.2 | −0.5 | .07 |
| Annoyance | 1.2 | 1.8 | −0.6 | .04 |
| Depression | 0.8 | 1.1 | −0.3 | .24 |
| Interaction with others | 0.8 | 1.0 | −0.2 | .30 |
| Desire to be with people | 0.7 | 0.8 | −0.1 | .43 |
| Shows affection | 0.8 | 1.0 | −0.2 | .79 |
| Effect on daily activities | 1.2 | 1.4 | −0.2 | .24 |
| Work or do what enjoy | 1.2 | 1.4 | −0.2 | .43 |
| Total Skindex-16 score | 1.4 | 1.7 | −0.2 | .07 |
Lower score indicates less toxicity.
Table 5.
Maximum Patient-Reported Skin Toxicity Assessment Tool (STAT) and Symptom Experience Diary (SED) Toxicity Scoresa
| Maximum Score by Group | |||
|---|---|---|---|
| Characteristics | Mometasone Furoate (n=83) | Placebo (n=84) | P Value |
| STAT | |||
| Discomfort or burning | 1.5 | 2.1 | .02 |
| Itching | 1.5 | 2.2 | .002 |
| Pulling | 1.0 | 1.4 | .07 |
| Discomfort or tenderness | 2.1 | 2.5 | .11 |
| SED | |||
| Redness | 5.1 | 6.8 | .003 |
| Dry peeling | 2.7 | 3.4 | .52 |
| Wet peeling | 1.3 | 1.6 | .97 |
| Weeping | 1.2 | 1.4 | .65 |
| Rash | 2.6 | 4.0 | .01 |
| Swelling | 2.3 | 2.4 | .70 |
| Fatigue | 4.5 | 5.0 | .24 |
| Decrease in color | 2.3 | 2.1 | .72 |
| Band, stripes, or lines | 1.7 | 1.5 | .72 |
Lower score indicates less toxicity.
Figure 2.
Mean Itching Score by Week of Radiotherapy. The score was measured with the Skindex-16 instrument. Maximum mean Skindex-16 score was 2.3 for the mometasone furoate group and 3.1 for the placebo group.
Adverse event monitoring by providers using CTCAE version 3.0 showed a higher mean maximum grade of any type of toxicity in the placebo group than the treatment group (P=.04) (Table 6). This difference was largely secondary to the maximum grade of pruritus being higher in the placebo group (P=.005), with 6% of patients reporting grade 3 pruritus versus 1% in the MMF group and 61% of patients reporting mild pruritus in the placebo group versus 36% in the MMF group.
Table 6.
Provider-Assessed Maximum CTCAE Version 3.0 Toxicity Grade
| Group, No. (%) | |||
|---|---|---|---|
| Toxicity Type and Gradea | Mometasone Furoate (n=84) | Placebo (n=82) | P Value (Wilcoxon rank sum test) |
| Pruritus | .005 | ||
| 0 | 33 (39) | 13 (16) | |
| 1 | 36 (43) | 50 (61) | |
| 2 | 14 (17) | 14 (17) | |
| 3 | 1 (1) | 5 (6) | |
| 4 | 0 | 0 | |
| Worst grade of any type of toxicity | .04 | ||
| 0 | 23 (27) | 5 (6) | |
| 1 | 34 (40) | 50 (61) | |
| 2 | 25 (30) | 20 (24) | |
| 3 | 2 (2) | 7 (9) | |
| 4 | 0 | 0 | |
Abbreviation: CTCAE, Common Terminology Criteria for Adverse Events.
Grades are 0, none; 1, mild; 2, moderate; 3, severe; and 4, life-threatening.
Discussion
Radiation dermatitis is a common adverse effect of radiotherapy in patients receiving irradiation to the breast and the chest wall. Clinically, the severity of this symptom is related to its dose and fractionation delivered to the skin, as well as such patient-related factors as obesity, smoking, and use of radiosensitizing chemotherapy (4,14).
Acute radiation dermatitis is associated with an inflammatory cascade thought to be cytokine mediated, although its exact mechanisms are unclear. In vitro studies of irradiated human skin have shown alterations to the normal histologic characteristics that include a marked decrease in basal cell proliferation, endothelial cell damage and resultant vasodilation with altered membrane permeability, and inflammatory cytokine release (1,15,16). Corticosteroids act as anti-inflammatory agents by regulating leukocyte adhesion to endothelial cells, inducing vasoconstriction, decreasing capillary permeability, and inhibiting leukocyte proliferation and migration. They have been shown to decrease the expression of interleukin 6 in vitro (17).
No clear consensus about the superiority of any single topical agent in the prevention and treatment of radiation dermatitis has emerged, despite decades of investigations (3,6,18-22). Corticosteroid preparations have been investigated as agents for the treatment of radiation dermatitis since shortly after synthetic corticosteroids were first used clinically (23).
Scott and Kalz (24) found that a single application of various corticosteroid preparations was capable of either ameliorating or delaying the onset of radiation dermatitis in a small cohort of patients receiving radiotherapy with grenz rays. Bjornberg and colleagues (25) performed a double-blind comparison of fluocinolone acetonide versus placebo in 26 patients and demonstrated a statistically significant decrease in the degree of overall dermatitis at 3 weeks after initiation of therapy, but that difference was no longer significant at 6 weeks (25). Bjornberg and colleagues (26) also conducted a double-blind comparison of bethamethasone-17-valerate versus Vaseline (Unilever United States, Inc, Englewood Cliffs, New Jersey) and versus Eucerine (Beiersdorf AG, Hamburg, Germany) in 26 patients. A statistically significant difference was shown in favor of bethamethasone over Vaseline or Eucerine. This difference was no longer significant at 6 weeks, however. Rechecks of the skin at 2 to 4 months showed no statistical difference among the 3 groups in regard to atrophic skin lesions in the treatment area (26). Later trials exploring the use of corticosteroids did not find a statistically significant difference between patients treated with corticosteroid preparations and those treated with other agents or placebo (27-29).
More recently, 2 double-blind, randomized trials have evaluated the use of corticosteroids in preventing radiation dermatitis. The first of these trials evaluated MMF cream plus emollient cream compared with a placebo emollient cream (2). Those patients receiving MMF and emollient cream experienced less radiation dermatitis than the placebo group (P=.003). There was also an indication of less burning sensation (P=.069) and less pain (P=.087) in MMF-treated patients. The second trial evaluated the topical preparations 0.1% methylprednisolone versus 0.5% dexpanthenol in a cohort of patients undergoing radiotherapy for breast cancer (30). The authors reported that, while neither agent reduced the incidence of radiation dermatitis, both agents delayed onset of maximal clinical symptoms by 1 week when compared with the control cohort.
Analysis of the primary study end point in the present trial, the maximum grade of provider-assessed radiation dermatitis with use of CTCAE version 3.0, showed no significant difference in the grades of dermatitis. Most patients had only grade 1 or 2 toxicity, and the narrow range of toxicity limited our ability to assess MMF's impact on radiation dermatitis. However, secondary patient-reported measures of toxicity, including the Skindex-16, Skin Toxicity Assessment Tool, and Symptom Experience Diary, did suggest a modest benefit in patients treated with MMF. Moreover, overall toxicity, evaluated with CTCAE version 3.0, was decreased in MMF-treated patients, primarily because of decreased pruritus in the MMF group. Overall patient QOL did not appear to be affected by use of the study agent and the reduction in toxicity reported for skin symptoms.
These findings are consistent with those of Bostrom et al (2), whose results suggested a modest reduction in radiation dermatitis in MMF-treated patients during breast radiotherapy. Our results also are consistent with those of Schmuth et al (30), who found that 0.1% methylprednisolone decreased patient symptoms, despite the lack of a statistically significant reduction in the incidence of radiation dermatitis in our study. The current study was designed to identify which patients might benefit most from mometasone on the basis of known risk factors, such as obesity, smoking history, radiotherapy daily fraction size, and history of chemotherapy (4,14). Patients were not stratified for fraction size, although there was not a statistically significant difference between treatment groups based on total radiotherapy. Another unknown factor at this time is whether mometasone is effective when its use is delayed until the onset of patient-reported symptoms, typically around the third week of therapy. This factor will be addressed in a future North Central Cancer Treatment Group trial.
Patient-reported outcomes have been defined by the US Food and Drug Administration as a measure of the patient's health status that is obtained from the patient without interpretation by the physician. In recent years, interest has grown in using reports obtained directly from patients without an intervening interpretation by their care providers. The patient-reported outcome measures in the present study delineated a wider spectrum of toxicity compared with provider-assessed measures using CTCAE version 3.0. Although the primary study end point did not show a positive impact of MMF on reducing radiation dermatitis, the secondary measures suggest a value to the prophylactic use of MMF in reducing skin toxicity during breast radiotherapy (31,32).
Conclusions
No reduction in radiation dermatitis during radiotherapy for breast cancer was observed by medical providers with the use of MMF, assessed with the primary study end point, and with the maximum grade of radiation dermatitis, assessed with CTCAE version 3.0. However, MMF use reduced skin toxicity symptoms compared with placebo in terms of pruritus, burning, redness, and other measures assessed by secondary measures in this study.
Acknowledgments
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic and was supported by US National Institutes of Health Grant CA 124477 (PI, Charles Loprinzi, MD).
Abbreviations
- CTCAE
Common Terminology Criteria for Adverse Events
- IL-6
interleukin-6
- MMF
mometasone furoate
- QOL
quality of life
Footnotes
Presented as a late-breaking abstract at the 50th Annual Meeting of the American Society for Therapeutic Radiology and Oncology, Boston, Massachusetts, September 22, 2008.
Conflict of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Registration numbers: NCCTG-N06C4 and NCT00438659.
Contributor Information
Robert C. Miller, Department of Radiation Oncology, Mayo Clinic, Rochester, Minnesota.
David J. Schwartz, Minneapolis Radiation Oncology, P.A., Minneapolis, Minnesota.
Jeff A. Sloan, Division of Biomedical Informatics and Biostatistics, Mayo Clinic, Rochester, Minnesota.
Patricia C. Griffin, Upstate Carolina CCOP, Spartanburg, South Carolina.
Richard L. Deming, Iowa Oncology Research Association CCOP, Des Moines, Iowa.
Jon C. Anders, Wichita Community Clinical Oncology Program, Wichita, Kansas.
Thomas J. Stoffel, Cedar Rapids Oncology Project CCOP, Cedar Rapids, Iowa.
Robert E. Haselow, Metro-Minnesota Community Clinical Oncology Program, St. Louis Park, Minnesota.
Paul L. Schaefer, Toledo Community Hospital Oncology Program CCOP, Toledo, Ohio.
James D. Bearden, III, Upstate Carolina CCOP, Spartanburg, South Carolina.
Pamela J. Atherton, Division of Biomedical Informatics and Biostatistics, Mayo Clinic, Rochester, Minnesota.
Charles L. Loprinzi, Department of Radiation Oncology, Mayo Clinic, Rochester, Minnesota.
James A. Martenson, Department of Radiation Oncology, Mayo Clinic, Rochester, Minnesota.
References
- 1.Hopewell JW. The skin: its structure and response to ionizing radiation. Int J Radiat Biol. 1990 Apr;57(4):751–73. doi: 10.1080/09553009014550911. [DOI] [PubMed] [Google Scholar]
- 2.Bostrom A, Lindman H, Swartling C, Berne B, Bergh J. Potent corticosteroid cream (mometasone furoate) significantly reduces acute radiation dermatitis: results from a double-blind, randomized study. Radiother Oncol. 2001 Jun;59(3):257–65. doi: 10.1016/s0167-8140(01)00327-9. [DOI] [PubMed] [Google Scholar]
- 3.Fisher J, Scott C, Stevens R, Marconi B, Champion L, Freedman GM, et al. Randomized phase III study comparing Best Supportive Care to Biafine as a prophylactic agent for radiation-induced skin toxicity for women undergoing breast irradiation: Radiation Therapy Oncology Group (RTOG) 97-13. Int J Radiat Oncol Biol Phys. 2000 Dec 1;48(5):1307–10. doi: 10.1016/s0360-3016(00)00782-3. [DOI] [PubMed] [Google Scholar]
- 4.Wells M, Macmillan M, Raab G, MacBride S, Bell N, MacKinnon K, et al. Does aqueous or sucralfate cream affect the severity of erythematous radiation skin reactions? a randomised controlled trial. Radiother Oncol. 2004 Nov;73(2):153–62. doi: 10.1016/j.radonc.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 5.Wickline MM. Prevention and treatment of acute radiation dermatitis: a literature review. Oncol Nurs Forum. 2004 Mar-Apr;31(2):237–47. doi: 10.1188/04.ONF.237-247. [DOI] [PubMed] [Google Scholar]
- 6.Williams MS, Burk M, Loprinzi CL, Hill M, Schomberg PJ, Nearhood K, et al. Phase III double-blind evaluation of an aloe vera gel as a prophylactic agent for radiation-induced skin toxicity. Int J Radiat Oncol Biol Phys. 1996 Sep 1;36(2):345–9. doi: 10.1016/s0360-3016(96)00320-3. [DOI] [PubMed] [Google Scholar]
- 7.Common terminology criteria for adverse events v3.0 (CTCAE) Rockville (MD): National Cancer Institute (US); 2006. Aug 9, Cancer therapy evaluation program; p. 72. Internet. cited 2009 Feb 4. cited 2009 Feb 4. Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. [Google Scholar]
- 8.Chren MM, Lasek RJ, Sahay AP, Sands LP. Measurement properties of Skindex-16: a brief quality-of-life measure for patients with skin diseases. J Cutan Med Surg. 2001 Mar-Apr;5(2):105–10. doi: 10.1007/BF02737863. Epub 2001 Mar 21. [DOI] [PubMed] [Google Scholar]
- 9.Berthelet E, Truong PT, Musso K, Grant V, Kwan W, Moravan V, et al. Preliminary reliability and validity testing of a new Skin Toxicity Assessment Tool (STAT) in breast cancer patients undergoing radiotherapy. Am J Clin Oncol. 2004 Dec;27(6):626–31. doi: 10.1097/01.coc.0000138965.97476.0f. [DOI] [PubMed] [Google Scholar]
- 10.Bretscher M, Rummans T, Sloan J, Kaur J, Bartlett A, Borkenhagen L, et al. Quality of life in hospice patients: a pilot study. Psychosomatics. 1999 Jul-Aug;40(4):309–13. doi: 10.1016/S0033-3182(99)71224-7. [DOI] [PubMed] [Google Scholar]
- 11.Grunberg SM, Groshen S, Steingass S, Zaretsky S, Meyerowitz B. Comparison of conditional quality of life terminology and visual analogue scale measurements. Qual Life Res. 1996 Feb;5(1):65–72. doi: 10.1007/BF00435970. [DOI] [PubMed] [Google Scholar]
- 12.Gudex C, Dolan P, Kind P, Williams A. Health state valuations from the general public using the visual analogue scale. Qual Life Res. 1996 Dec;5(6):521–31. doi: 10.1007/BF00439226. [DOI] [PubMed] [Google Scholar]
- 13.Hyland ME, Sodergren SC. Development of a new type of global quality of life scale, and comparison of performance and preference for 12 global scales. Qual Life Res. 1996 Oct;5(5):469–80. doi: 10.1007/BF00540019. [DOI] [PubMed] [Google Scholar]
- 14.Freedman GM, Anderson PR, Li J, Eisenberg DF, Hanlon AL, Wang L, et al. Intensity modulated radiation therapy (IMRT) decreases acute skin toxicity for women receiving radiation for breast cancer. Am J Clin Oncol. 2006 Feb;29(1):66–70. doi: 10.1097/01.coc.0000197661.09628.03. [DOI] [PubMed] [Google Scholar]
- 15.Archambeau JO, Pezner R, Wasserman T. Pathophysiology of irradiated skin and breast. Int J Radiat Oncol Biol Phys. 1995 Mar 30;31(5):1171–85. doi: 10.1016/0360-3016(94)00423-I. [DOI] [PubMed] [Google Scholar]
- 16.Boisnic S, Branchet-Gumila MC, Nizri D, Ben Slama L. Histochemical and biochemical modifications induced by experimental irradiation of human skin maintained in survival conditions and modulation by application of an emulsion containing trolamine. Int J Tissue React. 2003;25(1):9–18. [PubMed] [Google Scholar]
- 17.Cronstein BN, Kimmel SC, Levin RI, Martiniuk F, Weissmann G. A mechanism for the antiinflammatory effects of corticosteroids: the glucocorticoid receptor regulates leukocyte adhesion to endothelial cells and expression of endothelial-leukocyte adhesion molecule 1 and intercellular adhesion molecule 1. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):9991–5. doi: 10.1073/pnas.89.21.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halperin EC, Gaspar L, George S, Darr D, Pinnell S. A double-blind, randomized, prospective trial to evaluate topical vitamin C solution for the prevention of radiation dermatitis. CNS Cancer Consortium. Int J Radiat Oncol Biol Phys. 1993 Jun 15;26(3):413–6. doi: 10.1016/0360-3016(93)90958-x. [DOI] [PubMed] [Google Scholar]
- 19.Heggie S, Bryant GP, Tripcony L, Keller J, Rose P, Glendenning M, et al. A Phase III study on the efficacy of topical aloe vera gel on irradiated breast tissue. Cancer Nurs. 2002 Dec;25(6):442–51. doi: 10.1097/00002820-200212000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Liguori V, Guillemin C, Pesce GF, Mirimanoff RO, Bernier J. Double-blind, randomized clinical study comparing hyaluronic acid cream to placebo in patients treated with radiotherapy. Radiother Oncol. 1997 Feb;42(2):155–61. doi: 10.1016/s0167-8140(96)01882-8. [DOI] [PubMed] [Google Scholar]
- 21.Lokkevik E, Skovlund E, Reitan JB, Hannisdal E, Tanum G. Skin treatment with bepanthen cream versus no cream during radiotherapy: a randomized controlled trial. Acta Oncol. 1996;35(8):1021–6. doi: 10.3109/02841869609100721. [DOI] [PubMed] [Google Scholar]
- 22.Vuong T, Franco E, Lehnert S, Lambert C, Portelance L, Nasr E, et al. Silver leaf nylon dressing to prevent radiation dermatitis in patients undergoing chemotherapy and external beam radiotherapy to the perineum. Int J Radiat Oncol Biol Phys. 2004 Jul 1;59(3):809–14. doi: 10.1016/j.ijrobp.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 23.Marshall AH. The action cortisone on experimental acute roentgen dermatitis. Acta Radiol. 1953 Jan;39(1):73–7. doi: 10.3109/00016925309136687. [DOI] [PubMed] [Google Scholar]
- 24.Scott A, Kalz F. The effect of the topical application of corticotrophin, hydrocortisone, and fluorocortisone on the process of cutaneous inflammation. J Invest Dermatol. 1956 May;26(5):361–78. doi: 10.1038/jid.1956.47. [DOI] [PubMed] [Google Scholar]
- 25.Bjornberg A, Hellgren L, Olsson S. Treatment of radiation dermatitis with fluocinolone acetonide. Acta Radiol Ther Phys Biol. 1965 Apr;3:129–34. doi: 10.3109/02841866509133086. [DOI] [PubMed] [Google Scholar]
- 26.Bjornberg A, Hellgren L, Magnusson B, Mattsson I, Rosengren B. Topical treatment of radiation dermatitis with bethamethasone-17-valerate, vaseline and eucerine: a double-blind comparison. Clin Radiol. 1967 Oct;18(4):463–4. doi: 10.1016/s0009-9260(67)80064-3. [DOI] [PubMed] [Google Scholar]
- 27.Glees JP, Mameghan-Zadeh H, Sparkes CG. Effectiveness of topical steroids in the control of radiation dermatitis: a randomised trial using 1% hydrocortisone cream and 0.05% clobetasone butyrate (Eumovate) Clin Radiol. 1979 Jul;30(4):397–403. doi: 10.1016/s0009-9260(79)80217-2. [DOI] [PubMed] [Google Scholar]
- 28.Huter KA, Muller HG. Radiation reaction of the skin in the use of glucocorticoids. Strahlentherapie. 1959 Mar;108(3):475–7. German. [PubMed] [Google Scholar]
- 29.Potera ME, Lookingbill DP, Stryker JA. Prophylaxis of radiation dermatitis with a topical cortisone cream. Radiology. 1982 Jun;143(3):775–7. doi: 10.1148/radiology.143.3.7079509. [DOI] [PubMed] [Google Scholar]
- 30.Schmuth M, Wimmer MA, Hofer S, Sztankay A, Weinlich G, Linder DM, et al. Topical corticosteroid therapy for acute radiation dermatitis: a prospective, randomized, double-blind study. Br J Dermatol. 2002 Jun;146(6):983–91. doi: 10.1046/j.1365-2133.2002.04751.x. [DOI] [PubMed] [Google Scholar]
- 31.Sloan JA, Berk L, Roscoe J, Fisch MJ, Shaw EG, Wyatt G, et al. National Cancer Institute Integrating patient-reported outcomes into cancer symptom management clinical trials supported by the National Cancer Institute-sponsored clinical trials networks. J Clin Oncol. 2007 Nov 10;25(32):5070–7. doi: 10.1200/JCO.2007.12.7670. [DOI] [PubMed] [Google Scholar]
- 32.Trotti A, Colevas AD, Setser A, Basch E. Patient-reported outcomes and the evolution of adverse event reporting in oncology. J Clin Oncol. 2007 Nov 10;25(32):5121–7. doi: 10.1200/JCO.2007.12.4784. [DOI] [PubMed] [Google Scholar]