Abstract
Multiple studies demonstrate that manganese (Mn) exposure potentiates inflammatory mediator output from activated glia; this increased output is associated with enhanced mitogen activated protein kinase (MAPK: p38, ERK, and JNK) activity. We hypothesized that Mn activates MAPK by activating the kinases upstream of MAPK, i.e., MKK-3/6, MKK-1/2, and MKK-4 (responsible for activation of p38, ERK, and JNK, respectively), and/or by inhibiting a major phosphatase responsible for MAPK inactivation, MKP-1. Exposure of N9 microglia to Mn (250μM), LPS (100 ng/ml), or Mn+LPS increased MKK-3/6 and MKK-4 activity at 1 h; the effect of Mn+LPS on MKK-4 activation was greater than the rest. At 4 h, Mn, LPS, and Mn+LPS increased MKK-3/6 and MKK-1/2 phosphorylation, whereas MKK-4 was activated only by Mn and Mn+LPS. Besides activating MKK-4 via Ser257/Thr261 phosphorylation, Mn (4 h) prevented MKK-4’s phosphorylation on Ser80, which negatively regulates MKK-4 activity. Exposure to Mn or Mn+LPS (1 h) decreased both mRNA and protein expression of MKP-1, the negative MAPK regulator. In addition, we observed that at 4 h, but not at 1 h, a time point coinciding with increased MAPK activity, Mn+LPS markedly increased TNF-α , IL-6, and Cox-2 mRNA, suggesting a delayed effect. The fact that all three major groups of MKKs, MKK-1/2, MKK-3/6, and MKK-4 are activated by Mn suggests that Mn-induced activation of MAPK occurs via traditional mechanisms, which perhaps involve the MAPKs farthest upstream, MKKKs (MAP3Ks). In addition, for all MKKs, Mn-induced activation was persistent at least for 4 h, indicating a long-term effect.
Keywords: manganese, microglia, MAPK, MKK-1/2, MKK-4, MKK-3/6, MKP-1, cytokines, TNF-α, IL-6
Introduction
Manganese (Mn) is an essential metal and a common environmental contaminant. Besides mining operations, Mn is found in alloys, fertilizers, batteries, certain fungicides, and as a component of the fuel additive methylcyclopentdienyl manganese tricarbonyl (MMT; Aschner 2000; Aschner et al., 2007). Excessive exposure to Mn is of human health concern, since in certain occupational settings Mn causes specific basal ganglia parkinsonism, manganism (Aschner 2000; Aschner et al., 2007; Calne et al., 1994; Huang et al., 2007; Meco et al., 1994). Mn is thought to exert its effects, at least partially, by disrupting neuronal mitochondrial respiration, leading to increased oxidative stress and cell death (Gavin et al., 1999).
Besides direct effect on neuronal cells, Mn neurotoxicity appears to involve activation of glia. For example, astrocytes accumulate Mn and may produce reactive oxygen species (ROS) and other substances that are damaging to neurons (Aschner 2000; Hazell 2002). Importantly, it has been demonstrated that glial cells (microglia and/or astrocytes) may produce inflammatory mediators that could be involved in the mechanisms of Mn neurotoxicity (Barhoumi et al., 2004; Chang and Liu 1999; Chen et al., 2006; Crittenden and Filipov 2008; Filipov et al., 2005; Spranger et al., 1998; Zhang et al., 2010). Due to its inflammation-enhancing effects, the role of inflammation in Mn neurotoxicity appears to be particularly relevant when an additional inflammatory stimulus, such as lipopolysaccharide (LPS), is present (Chang and Liu 1999; Crittenden and Filipov 2008; Filipov et al., 2005; Spranger et al., 1998; Zhang et al., 2010).
LPS is a known environmental contaminant (Niehaus and Lange 2003) and model inflammagen due to its ability to stimulate inflammatory cells to produce cytokines, nitric oxide (NO), and ROS (Chao et al., 1992; Chen et al., 2002; Jeohn et al., 2002). Binding of LPS to CD14 and Toll-like receptor-4 (TLR4) cell surface receptors leads to the activation of intracellular kinases, including the mitogen activated protein kinases (MAPK; Bhat et al., 1998; Jeohn et al., 2002). The MAPK family of proteins is comprised of a series of kinases, beginning with the MAP kinase kinase kinases (i.e. TAK1, ASK1) that phosphorylate MAP kinase kinase (i.e. MKK-1, -2, -3, -4, -6), which subsequently phosphorylate MAPK. This MAPK cascade culminates in the activation of one or more MAPK, including the extracellular signal-regulated kinases (ERK), c-Jun N-terminal kinases (JNK), and the p38 MAPK (p38; Koistinaho and Koistinaho 2002). MAPK deactivation is dependent on the actions of dual specificity phosphatases, primarily the MAP kinase phosphatase-1 (MKP-1) in the case of p38 and ERK (Koistinaho and Koistinaho 2002; Lang et al., 2006; Wang and Liu 2007). In the case of JNK, besides action of phosphatases, its deactivation occurs via specific phosphorylation of its primary MKK, MKK-4, on Ser 80 (Park et al., 2002).
In primary microglia and microglial cell lines, LPS increases the phosphorylation of ERK, JNK, and p38, and it also increases the expression of iNOS and TNF-α in a time- and dose-dependent manner (Bhat et al., 1998; Lee et al., 1994; Lee et al., 1993). Of note, LPS-induced, p38-dependent, increases in NO and TNF-α by microglia have been shown to decrease neuronal survivability in neuronal-glial co-culture (Jeohn et al., 2002).
Within the context of Mn neurotoxicity, exposure to Mn potentiates LPS-induced production of microglial inflammatory cytokines (TNF-α & IL-6) and NO in vitro (Crittenden and Filipov 2008; Filipov et al., 2005). In addition, Mn also potentiates Cox-2 expression and ensuing prostaglandin production by inflammagen-activated mixed glial cells (Liao et al., 2007). Potentiating effect of Mn on microglial TNF-α & IL-6 production involves the activation of NF-kB and p38, as inhibitors of NF-kB or p38 were able to prevent it (Crittenden and Filipov 2008; Filipov et al., 2005). Besides p38, Mn has been reported to either activate by itself and/or to potentiate the activation of the other two major MAPKs, ERK and JNK, in glial cells stimulated with inflammagens and/or inflammatory cytokines in vitro (Bae et al., 2006; Chen et al., 2006; Moreno et al., 2008; Zhang et al., 2007).
Whether the potentiating effects of Mn on cytokine production (and other inflammatory molecules) are at the level of transcription, and the temporal relationship with MAPK activation, is unknown at present. Moreover, the mechanism(s) of prolonged p38 activation in the presence of Mn (Crittenden and Filipov 2008) and the activation of the other MAPKs by Mn is/are not understood. It may be that Mn enhances, or extends, the activation of the MKKs responsible for the activation of p38, ERK, and JNK, which primarily are MKK-3/6, MKK-1/2, and MKK-4, respectively (Enslen et al., 1998). However, cross-activation at the level of MKKs may occur, i.e., MKK-1/2 (Meja et al., 2000) and MKK-4 (Derijard et al., 1995) may also activate p38. Alternatively, Mn exposure may cause decreased expression of MKP-1, the dual-specificity phosphatase (DUSP) responsible for dephosphorylating p38 and ERK, which may lead to prolonged MAPK activation (Hammer et al., 2005; Lang et al., 2006; Wang and Liu 2007). In the case of JNK, Mn exposure may modulate the phosphorylation status of MKK-4 on Ser 80, which deactivates this MKK (Park et al., 2002).
Because of the increasing importance of MAPK activation by Mn for its toxic action, this study sought to explore the mechanism(s) of MAPK activation by Mn in glial cells as the exact mechanism of MAPK activation by Mn has not been studied before. In particular, we examined effects of Mn exposure in resting and LPS-activated microglia on the major MKKs responsible for activating MAPKs, MKK-1/2, MKK-4, and MKK-3/6. Additional objective was to determine whether Mn has an effect on the major phosphatase responsible for deactivating p38 and ERK, MKP-1. A final goal of this study was to evaluate the time-course of the expression of certain inflammatory mediators (e.g. TNF-α , IL-6, and Cox-2) in Mn-exposed activated microglia in order to determine whether the potentiating effect of Mn is at the transcription level and whether enhanced mRNA for TNF-α , IL-6, and Cox-2 by Mn coincides temporally with the activation of the MAPK pathway (e.g., p38; Crittenden and Filipov 2008).
Materials and Methods
Chemicals
Unless specified, all chemicals and reagents were purchased from Sigma-Aldrich (Sigma; St. Louis, MO) and endotoxin-free MnCl2 with purity >99% was used.
Cell Culture
The N9 mouse microglial cell line (Righi et al., 1989) was a gift kindly provided by Dr. P. Ricciardi-Castagnoli (University of Milan, Italy) and is similar to primary microglia and other cell lines, i.e. BV-2, N13, in that it produces inflammatory cytokines, such as IL-1β , IL-6, and TNF-α, as well as NO when activated by inflammagens, such as LPS (Han et al., 2007; Heyen et al., 2000; Righi et al., 1989).
The cultures were maintained (5% CO2, 95% air, at 37°C) in RPMI-1640 supplemented with 10% FBS (low endotoxin, ≤ 25 EU/ml; Hyclone, Logan UT), 0.075% sodium bicarbonate, 1 mM sodium pyruvate, 1 mM non-essential amino acids, 2 mM L-glutamine, 50 μM 2-mercaptoethanol, 25 μg/ml gentamycin, 100 U/ml penicillin G, and 100 mg/ml streptomycin (all from Invitrogen, Carlsbad, CA). For real-time quantitative PCR (qPCR) and western blot protein analysis, cells were seeded at 2.5 × 106 cells/well (5 ml volume) in 6-well plates (Costar). Cells were incubated for up to 4 h in the presence of Mn (250 μM) and/or LPS (Escherichia coli 0111: B4; 100 ng/ml) in complete (10% FBS) media.
Detailed dose justification for Mn and LPS is provided in Filipov et al. (2005) and Crittenden and Filipov (2008). Briefly, the concentrations of Mn used here were selected to be non-cytotoxic in this cell line (Filipov et al., 2005), to be in line with numerous other in vitro studies where levels of Mn (Mn2+) range from 10 μM to 4 mM with the most typical exposure range (for cultures up to 48 h) being 100 to 500 μM (Li et al., 2005; Malthankar et al., 2004), and, importantly, to be representative of Mn levels found in brains of non-human primates following exposure to Mn for 3 months (ranging from 35 to 350 μM; (Suzuki et al., 1975), as well as of basal ganglia Mn levels observed in autopsied brains of patients with cirrhosis, who have inability to eliminate Mn efficiently (Yase 1972). The concentration of LPS we have used in the present study (100 ng/ml) is relatively low in this cell line and we have already reported that when greater amounts of LPS are present in the culture medium, less Mn is required for a potentiating effect on cytokine production to be observed (Filipov et al., 2005).
Dexamethasone (Dex) was used as a positive control for MKP-1, as Dex has been shown to induce MKP-1 and to enhance the LPS-induced expression of MKP-1 (Zhou et al., 2007).
Immunoblot analysis of MKKs and MKP-1
After incubation for 1 and 4 h, cells were removed from the culture wells via scraping and the cell suspensions were centrifuged (300× g; 10 min; 4°C). Following centrifugation, the supernatants were discarded and the cell pellets were lysed in 100μl of RIPA (modified radioimmuno-precipitation) lysis buffer (1× PBS, 1% Igepal, 0.5% sodium deoxycholate, 0.1% SDS) containing PMSF (Sigma), protease and phosphatase inhibitors (Protease Inhibitor Cocktail, Sigma, and Halt Phosphatase Inhibitor Cocktail, Pierce [Rockford, IL], respectively). Protein concentration in the cell lysates was determined using the Bradford method with reagents obtained from Bio-Rad (Hercules, CA) and with bovine serum albumin (BSA) as a standard. Aliquots of each sample were diluted in reducing sample buffer and heat denatured for 5 min at 95 oC. Twenty micrograms of total protein (MKK-3/6, MKK-1/2, MKK-4, and α-tubulin), or 50 μg (MKP-1) was loaded, separated on a 10% SDS-PAGE gel, and transferred to a PVDF membrane (Immunolon-P, Millipore Corp., Billerica, MA). The membranes were blocked in 5% milk (MKK-3/6, MKP-1, and α-tubulin; 1 h at RT), or 5% BSA (MKK-1/2 and MKK-4, 1 h at 4°C) and then incubated overnight at 4°C with rabbit antibodies specific for the (i) non-phosphorylated (1:1000) and phosphorylated (Ser 189; 1:500) MKK-3/6 (Santa Cruz Biotechnology, Santa Cruz, CA), (ii) non-phosphorylated (1:1000) and phosphorylated (Ser 218/Ser 222; 1:500; Santa Cruz) MKK-1/2, (iii) non-phosphorylated (1:1000; Cell Signaling) and phosphorylated (Ser 257/Thr 261; 1:1000, R&D Systems, Minneapolis, MN, or Ser 80; 1:500, Santa Cruz) MKK-4, (iv) MKP-1 (1:500; Santa Cruz), and (v) α-tubulin (1:1000, Santa Cruz) . Following a wash (3×), the membranes were probed with goat anti-rabbit-HRP secondary antibodies (1:10,000-50,000, Bio-Rad). Next, the blots were exposed to Super Signal West Pico chemiluminescent substrate (Pierce) for 5 min and then either exposed to x-ray film, or placed in a ChemiDoc XRS HQ (Bio-Rad) imaging apparatus. Band density was analyzed using the UNSCAN-IT (Silk Scientific Inc., Orem, UH).
Real-time quantitative PCR (qPCR) analysis of MKP-1, IL-6, TNF-α , and Cox-2
Total RNA was isolated, including the recommended DNase treatment step, using the RNeasy Mini Kit (Qiagen, Valencia, CA) and quantified with a NanoDrop Spectrophotometer ND-1000 (NanoDrop Technologies). One μg of total RNA was used to synthesize the first strand cDNA with the RT2 PCR Array First Strand Kit (SA Biosciences, Frederick, MD). Using 10 ng starting RNA per sample, expression of TNF-α , IL-6, Cox-2, and MKP-1 was determined by qPCR using mouse-specific, certified primers (SA Biosciences). Reaction mixtures were assembled in optically-clear 96-well plates using the RT-PCR SYBR Green Master Mix (SA Biosciences). Amplifications were performed in an iCycler iQ (Bio-Rad) programmed for an initial step of 30 min at 50 °C and 10 min at 95 °C, followed by 40 cycles at 95 °C for 15 s and 1 min at 60 °C. To check the quality of the products, the melting curve program was run after the above cycling program. The expression of GAPDH, which was verified of not being affected by vehicle/Mn/LPS treatment, was used as a house-keeping gene for all samples. Data were analyzed using the Δ ΔCt method and are presented as relative induction (fold changes) of MKP-1, inflammatory cytokine, or Cox-2 normalized to GAPDH.
Statistical Analysis
Data were analyzed using analysis of variance (ANOVA). When statistical differences were detected (p ≤ 0.05), treatment means were separated by the Fisher’s LSD post hoc test. All data are presented as means ± S.E.M.
Results
Immunoblot analysis of MKKs
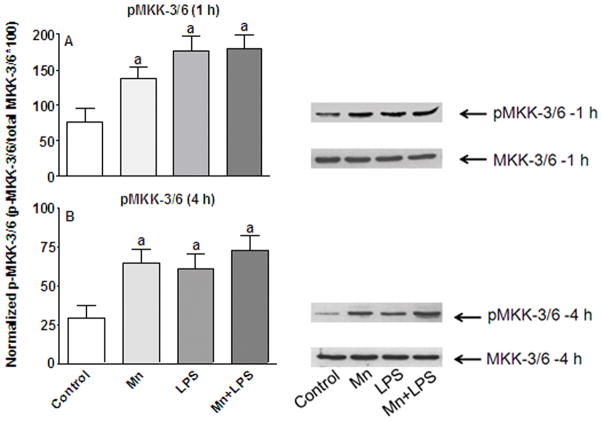
To determine the source of the enhanced/prolonged p38 activation observed in the presence of Mn (Crittenden and Filipov 2008), we first examined the induction of the major MKK upstream of p38, MKK-3/6. Following exposure to Mn (250 μM) with and without LPS (100 ng/ml) for up to 4 h, levels of non-phosphorylated MKK-3/6 did not change in response to Mn and/or LPS exposure (Figure 1). However, induction of MKK-3/6 phosphorylation was observed at 1 h post exposure to Mn, LPS, or Mn+LPS (p ≤ 0.05; Figure 1A). This effect persisted through the 4 h time point for all three treatment groups, which were significantly higher than control values (p ≤ 0.05) but not significantly different from each other (p > 0.3; Figure 1B).
Figure 1.
Effects of Mn and/or LPS on MKK-3/6 activity. Shown are quantification and representative western blots of phosphorylated MKK-3/6 (pMKK-3/6; Ser 189) and total MKK-3/6 in N9 microglia following exposure to vehicle, 250 μM Mn, 100 ng/ml LPS, or 250 μM Mn + 100 ng/ml LPS for 1 (A) and 4 (B) h. Densitometric data were normalized as a ratio of phosphorylated to total MKK-3/6 protein. All data points represent means ± SEM from at least 3 independent experiments. Data were analyzed with ANOVA and means were separated using Fisher’s LSD multiple comparison post hoc test. a Letters denote statistically significant difference from control at p ≤ 0.05.
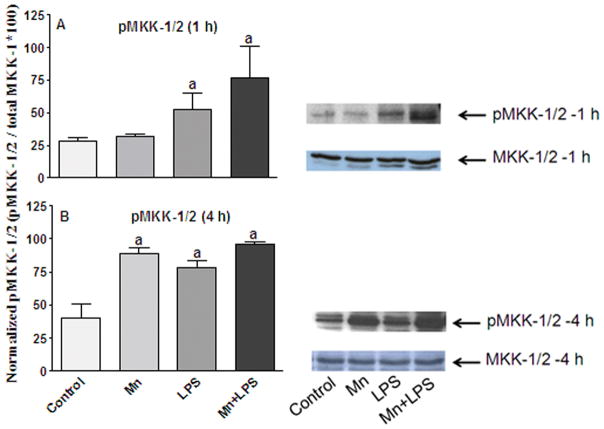
Next, we examined the induction of MKK-1/2, the major MKK upstream of ERK, which also activates p38. Similar to MKK-3/6, levels of non-phosphorylated MKK-1/2 were not affected by any of the treatments at either time point (Figure 2). At 1 and 4 h, exposure to LPS or Mn+LPS increased pMKK-1/2 levels substantially (p ≤ 0.05; Figure 2). Mn alone did not cause appreciable phosphorylation of MKK-1/2 at the 1 h time point, but it did so at 4 h (Figure 2).
Figure 2.
Effects of Mn and/or LPS on MKK-1/2 activity. Shown are quantification and representative western blots of phosphorylated MKK-1/2 (pMKK-1/2; Ser 218/Ser 222) and total MKK-1/2 in N9 microglia following exposure to vehicle, 250 μM Mn, 100 ng/ml LPS, or 250 μM Mn + 100 ng/ml LPS for 1 (A) and 4 (B) h. Densitometric data were normalized as a ratio of phosphorylated to total MKK-1/2 protein. All data points represent means ± SEM from at least 3 independent experiments. Data were analyzed with ANOVA and means were separated using Fisher’s LSD multiple comparison post hoc test. a Letters denote statistically significant difference from control at p ≤ 0.05.
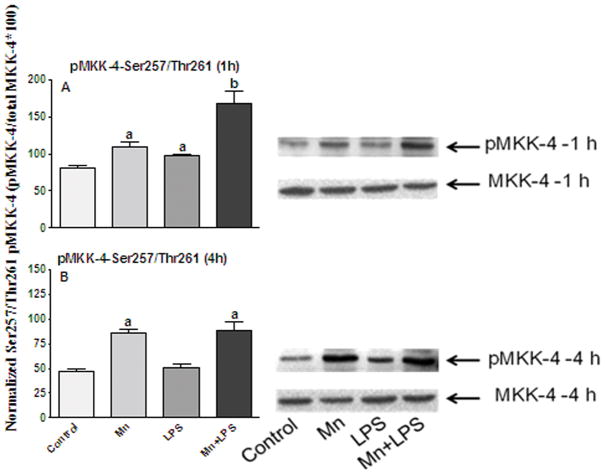
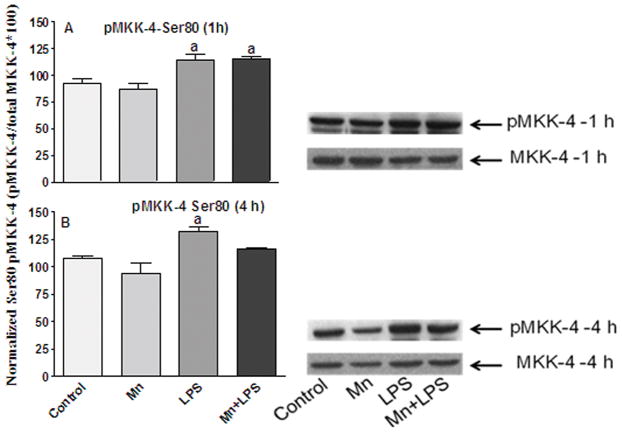
We also examined the phosphorylation status of MKK-4, the major MKK upstream of JNK, which also activates p38. In the case of MKK-4, phosphorylation on Ser 257/Thr 261 leads to activation, whereas phosphorylation on Ser 80, leads to deactivation (Park et al., 2002). Exposure to Mn and/or LPS for 1 or 4 h, nor did it change the expression levels of the non-phospho MKK-4 (Figures 3 and 4). However, at 1 h, Mn, LPS, and the combination of Mn+LPS significantly increased the phosphorylation of MKK-4 on Ser 257/Thr 261, with the effect of Mn+LPS being significantly (p ≤ 0.05) greater than the effect of Mn or LPS. At 4 h, microglia exposed to Mn or Mn+LPS had MKK-4 activity still increased (p ≤ 0.05), whereas the effect of LPS was no longer apparent (Figure 4B). After 1 h exposure, LPS and Mn+LPS induced phosphorylation of MKK-4 on Ser80 (p ≤ 0.05), whereas Mn alone was ineffective (Figure 5A). Interestingly, at 4 h Ser 80 phosphorylation of MKK-4 was only observed in cells exposed to LPS (p ≤ 0.05), whereas Mn and Mn+LPS treated cells did not differ in their Ser 80 phosphorylation status on MKK-4 from that of control cells (Figure 5B).
Figure 3.
Effects of Mn and/or LPS on MKK-4 activity modulated at Ser 257/Thr 261. Shown are quantification and representative western blots of Ser 257/Thr 261-phosphorylated MKK-4 (pMKK-4; Ser 257/Thr 261) and total MKK-4 in N9 microglia following exposure to vehicle, 250 μM Mn, 100 ng/ml LPS, or 250 μM Mn + 100 ng/ml LPS for 1 (A) and 4 (B) h. Densitometric data were normalized as a ratio of Ser 257/Thr 261-pMKK-4 to total MKK-4 protein. All data points represent means ± SEM from at least 3 independent experiments. Data were analyzed with ANOVA and means were separated using Fisher’s LSD multiple comparison post hoc test. a ,b Letters denote statistically significant difference from control, with different letters also being different from each other at p ≤ 0.05.
Figure 4.
Effects of Mn and/or LPS on MKK-4 activity modulated at Ser 80. Shown are quantification and representative western blots of Ser 80-phosphorylated MKK-4 (pMKK-4; Ser 80) and total MKK-4 in N9 microglia following exposure to vehicle, 250 μM Mn, 100 ng/ml LPS, or 250 μM Mn + 100 ng/ml LPS for 1 (A) and 4 (B) h. Densitometric data were normalized as a ratio of Ser 80-pMKK-4 to total MKK-4 protein. All data points represent means ± SEM from at least 3 independent experiments. Data were analyzed with ANOVA and means were separated using Fisher’s LSD multiple comparison post hoc test. a Letters denote statistically significant difference from control at p ≤ 0.05.
Figure 5.
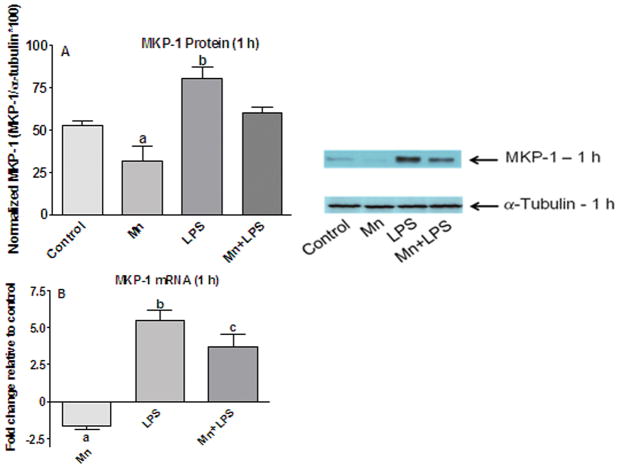
Effects of Mn and/or LPS on MKP-1. Shown are representative western blots and quantification of MKP-1 protein (A) and mRNA (B) levels following exposure to vehicle, 250 μM Mn, 100 ng/ml LPS, or 250 μM Mn + 100 ng/ml LPS for 1 h. Densitometric data were normalized as a ratio of MPK-1 to α-tubulin protein. All data points represent means ± SEM from at least 3 (protein) or 4 (mRNA) independent experiments. Data were analyzed with ANOVA and means were separated using Fisher’s LSD multiple comparison post hoc test. a ,b,c Letters denote statistically significant difference from control, with different letters also being different from each other at p ≤ 0.05.
Immunoblot and qPCR analyses of MKP-1
Based on published observations in primary microglia (Zhou et al., 2007), Dex was used as a positive MKP-1 control; similar to primary microglia, Dex+LPS, increased the MKP-1 protein and mRNA levels at1 h in N9 microglia more than any of the other treatments (data not shown). At 1 h, MKP-1 protein levels were increased only in LPS-treated cells (p ≤ 0.05; Figure 5A). Interestingly, MKP-1 protein levels in cells exposed to Mn were decreased (p ≤ 0.05) relative to controls and a similar decrease was observed in Mn+LPS exposed cells relative to cells exposed to LPS alone (Figure 5A).
Similar to the MKP-1 protein levels, decreased expression of MKP-1 mRNA was observed for Mn exposed cells at 1 h (p ≤ 0.05; Figure 5B). Although Mn+LPS increased the overall expression of MKP-1, the effect was less than the effect of LPS alone (p ≤ 0.05; Figure 5B). Again, the decrease in MKP-1 mRNA expression (almost 2-fold) was similar for Mn and Mn+LPS, in comparison to their respective controls (vehicle and LPS; Figure 5B).
qPCR analysis of TNF-α , IL-6, and Cox-2
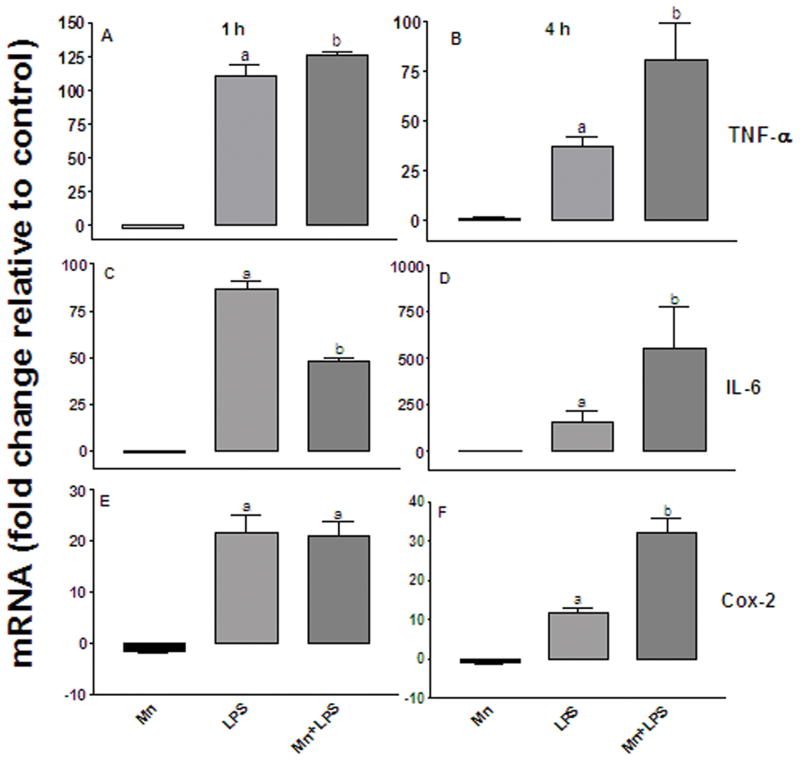
To examine the temporal expression of the inflammatory cytokines TNF-α and IL-6, as well as Cox-2, we quantified TNF-α , IL-6, and Cox-2 mRNA at 1 and 4 h post exposure to Mn and/or LPS. Two hundred and fifty μM Mn did not affect TNF-α mRNA at either time point (Figures 6A and 6B). Exposure to 100 ng/ml LPS on the other hand, significantly (p ≤ 0.05) increased TNF-α mRNA about 100-fold at 1 h; this effect diminished by 4 h (Figures 6A and 6B). Similar to the effect observed with LPS alone, Mn+LPS significantly (p ≤ 0.05) increased TNF-α mRNA at 1 h, with the effect of Mn+LPS on TNF-α expression being moderately greater than the effect of LPS alone (Figure 6A). While the LPS effect diminished substantially by 4 h, the Mn+LPS persisted, thus allowing for much greater TNF-α mRNA fold increase at 4 h in the cells exposed to Mn+LPS (p ≤ 0.05; Figure 6B).
Figure 6.
Fold change (up/down from vehicle control) in TNF-α (A and B), IL-6 (C and D), and Cox-2 (E and F) mRNA expression following exposure to 250 μM Mn, 100 ng/ml LPS, or 250μM Mn + 100 ng/ml LPS for 1 (left-hand graphs) and 4 (right-hand graphs) h. All data points represent means ± SEM from 4 independent experiments. Data were analyzed with ANOVA and means were separated using Fisher’s LSD multiple comparison post hoc test. a ,b Letters denote statistically significant difference from control, with different letters also being different from each other at p ≤ 0.05.
In accord with the TNF-α mRNA data, LPS significantly increased expression of IL-6 mRNA at 1 h and 4 h ((p ≤ 0.05; Figures 6C and 6D). Similar to TNF-α , no effect on IL-6 mRNA expression was observed following exposure to Mn alone (Figures 6C and 6D). Interestingly, although Mn+LPS increased IL-6 expression at 1 h, this effect was not greater than the effect of LPS alone (Figure 6C). However, similar to TNF-α , the expression of IL-6 mRNA in cells exposed to Mn+LPS increased over time from 1 to 4 h, almost 10-fold (Figure 6D). This effect was specific for Mn+LPS, as IL-6 mRNA in LPS-exposed cells, although significantly greater than vehicle, did not increase between the 1 and 4 h time points (Figures 6C and 6D).
As recent data have suggested that non-cytokine inflammatory molecules, such as prostaglandin E-2 (PGE2), may be important in Mn-induced inflammation (Liao et al., 2007), we quantified Cox-2 mRNA in the presence of Mn and/or LPS at 1 and 4 h. Although exposure to LPS or Mn+LPS increased (p ≤ 0.05) the expression of COX-2 mRNA by 20-fold at 1 h, the effect of Mn in combination with LPS was not greater than LPS alone (Figure 6E). However, similar to TNF-α and IL-6, by the 4 h time point, Mn+LPS exposure had increased Cox-2 mRNA even further to 30-fold. In contrast, the LPS-induced increase in Cox-2 mRNA, although still present, was only 11-fold, which was significantly (p ≤ 0.05) less that the increase caused by Mn+LPS (Figure 6F).
Discussion
Previous research has demonstrated that Mn exposure, in combination with an inflammagen, can increase the production of iNOS/NO (Barhoumi et al., 2004; Chang and Liu 1999; Chen et al., 2006; Filipov et al., 2005), PGE2 (Liao et al., 2007), as well of the inflammatory cytokines TNF-α (Chen et al., 2006; Filipov et al., 2005; Zhang et al., 2010), IL-1β (Zhang et al., 2010), and IL-6 (Filipov et al., 2005) by glial cells. Although Mn alone increases ROS, i.e., hydrogen peroxide, production in microglia (Zhang et al., 2007), its ability to induce cytokine production is very limited, suggesting that prior/ongoing microglial activation is necessary for Mn-potentiation of, at least, TNF-α , IL-1β and IL-6, to be observed (Filipov et al., 2005; Zhang et al., 2010).
The cause of the Mn-induced potentiation is not understood. However, several potential mechanisms have been explored. In earlier studies, the effects of Mn on the production of inflammatory molecules by activated glia was shown to involve the activation of the transcription factor NF-κB (Barhoumi et al., 2004; Chen et al., 2006; Filipov et al., 2005). Additionally, Mn activates either by itself or in conjunction with an inflammagen, the three major MAPKs, ERK, p38, and JNK, in glial cells (Bae et al., 2006; Chen et al., 2006; Crittenden and Filipov 2008; Moreno et al., 2008; Zhang et al., 2007). Of these, the activation of p38 appears to be long-lasting (Crittenden and Filipov 2008).
In our current study, we examined key signaling molecules that may be responsible for the Mn-caused MAPK activation. The upstream MKKs, MKK-3 and -6, can activate p38, leading to the production of inflammatory mediators (Fujishiro et al., 2001; Igarashi et al., 2000). However, based on our results, the enhanced and prolonged p38 activation observed in Mn-exposed, LPS-stimulated N9 microglia (Crittenden and Filipov 2008) does not appear to be solely driven by enhanced MKK-3/6 activation. Indeed, at both time points examined in our study, the combination of Mn+LPS was no more effective than LPS alone in inducing MKK-3/6 phosphorylation. On the other hand, Mn by itself did increase MKK-3/6 activity indicating that Mn is capable of activating p38 through MKK-3/6. In addition, in contrast to the still ongoing activation of p38 in cells exposed to Mn or Mn+LPS, p38 activation by LPS alone at 4 h had already subsided (Crittenden and Filipov 2008), suggesting that factors other than MKK-3/6 are involved.
Another possible explanation for increased and/or prolonged p38 phosphorylation caused by Mn exposure which we investigated in this study is decreased activity of the phosphatase MKP-1 which is responsible for deactivating p38 and ERK. The DUSPs, particularly MKP-1, are important regulators of the innate immune responses. Overexpression of MKP-1 in macrophages has been shown to inhibit phosphorylation of MAPK and production of inflammatory cytokines (Chen et al., 2002; Shepherd et al., 2004; Zhao et al., 2005). Conversely, reduced expression of MKP-1 results in increased production of the inflammatory cytokines TNF-α and IL-6 (Chi et al., 2006; Hammer et al., 2006). This observation is in line with our current findings which demonstrate that exposure to Mn for 1 h leads to reduced expression of MKP-1 protein. As TLR signaling not only promotes the expression of MKP-1 mRNA, but also prolongs the half-life of the transcript, it was necessary to examine both protein and mRNA expression in the presence of Mn (Hammer et al., 2005). We observed that MKP-1 mRNA was inhibited by Mn. Likewise, compared to the LPS alone treatment, the decreased MKP-1 following exposure to Mn+LPS was of the same magnitude as the decrease caused by the Mn-only treatment (as compared to vehicle control), suggesting that the reduction in MKP-1 mRNA and protein expression could contribute to the prolonged p38 activation, as we have reported (Crittenden and Filipov 2008). However, Mn alone does not increase IL-6 and TNF-α (Filipov et al., 2005). Thus, these data corroborate our previous findings where Mn alone increased p38 activity, but did not increase IL-6 and TNF-α cytokine production and further highlight the fact that persistent increase of p38 by Mn is necessary, but not sufficient, for the increased cytokine production previously observed (Crittenden and Filipov 2008). It is also interesting to note that while kinases control primarily the degree of MAPK pathways activation, the phosphatases, such as MKP-1, control both degree and duration of activation (Hornberg et al., 2005; Junttila et al., 2008).
MKK-4, the MKK upstream of JNK, was activated by Mn, LPS and Mn+LPS, with the effect of the combined exposure being greater than the rest at 1 h. Interestingly, LPS effect on MKK-4 activation had subsided by 4 h, whereas the effect of Mn and Mn+LPS was still present, suggesting that activation of JNK in glial cells exposed to Mn (Bae et al., 2006; Chen et al., 2006) occurs via activation of its upstream MKK, MKK-4 via phosphorylation of Ser 257/Thr 261. Our data also hints that of the three major MAPKs, in microglia, JNK activation by inflammagens, such as LPS, is the most likely to be potentiated by Mn especially at early time points. Thus, microglial JNK activation may at least partly responsible for the increased inflammatory mediator output and corresponding enhanced neurotoxicity as reported for dopaminergic neurons (Zhang et al., 2010). Mechanistically, MKK-4 is an interesting MKK in that its activity is inhibited by a specific phosphorylation at Ser 80 (Park et al., 2002). Ser 80 phosphorylation on MKK-4 is done by the protein serine-threonine kinase Akt (Park et al., 2002), which is induced by inflammagens, such as LPS, as a normal way of feedback regulation of the pathway. Our data suggest that, as expected, LPS increased MKK-4 phosphorylation on Ser 80; this phosphorylation persisted through 4 h. Presence of Mn, however, eliminated LPS effects at 4 h in cultures treated with Mn+LPS and it also numerically decreased basal Ser 80 phosphorylation of MKK-4. The net result of the effect of Mn on Ser 80 phosphorylation is allowing MKK-4 to stay activated longer.
We also examined the effects of Mn with and without the presence of LPS on MKK-1/2, the MKKs primarily responsible for activation of ERK. At 1 h, only LPS and Mn in combination with LPS increased phosphorylation of MKK-1/2, with the activation caused by Mn+LPS not being greater than the one caused by LPS; Mn caused a delayed (4 h) activation of MKK-1/2 on its own. Thus, it appears that the glial activation of ERK by Mn reported previously (Bae et al., 2006; Chen et al., 2006; Moreno et al., 2008; Zhang et al., 2007) occurs via activation of its upstream MKKs, MKK-1/2. Moreover, at least in microglia, our current data suggest that in the absence of an additional inflammatory stimulus, ERK activation by Mn is delayed.
Although we reported increased production of inflammatory cytokines by microglia exposed to Mn+LPS for 24 and 48 h in vitro (Crittenden and Filipov 2008; Filipov et al., 2005), we did not examine cytokine mRNA expression. Previous studies have suggested that Mn-inflammagen exposure results in increased TNF-α (Chen et al., 2006) and Cox-2 (Liao et al., 2007) mRNA expression by glial cells at 6 h post exposure. In accord with these data, compared to the effects of LPS alone, we observed that TNF-α , IL-6, and Cox-2 mRNA levels in cells exposed to Mn+LPS were markedly greater at 4 h post exposure. In contrast, at 1 h post exposure, except for a moderate potentiating effect on TNF-α , presence of Mn in the culture medium did not enhance inflammatory mediator mRNA above the effects of LPS alone. Interestingly, at this early time point, IL-6 mRNA levels in cells exposed to Mn+LPS were significantly less than the levels induced by LPS alone. In this regard, mechanistically, it is well known that IL-6 signaling differs from that of TNF-α , with the JAK-STAT3 pathway being very important for IL-6, but not for TNF-α . Thus, it may be that Mn exposure leads to early inhibition or that pathway. How exactly Mn achieves this effect is unknown at present. However, one possibility is via hydrogen peroxide (H2O2). Microglia exposed to Mn produce H2O2 (Zhang et al., 2007) and while enhanced microglial output of H2O2 is detrimental to dopaminergic neurons (Zhang et al., 2009), as of late, H2O2 has emerged not as an inducer of NFκ B, but as a modulator, i.e., an agent that modulates the activation of the NFκ B pathway by other stimuli, such as LPS (Oliveira-Marques et al., 2009). This inhibition is overcome later and the potentiating effects of Mn on IL-6 are seen. A possible mechanism for it is through the earlier increased TNF-α , as it has already been shown that TNF-α will increase IL-6 via NFκB-dependent and JAK-STAT3-dependent pathways (Tanabe et al., 2010). Thus, if H2O2 synergizes with LPS in activating the NFκB-dependent pathways and it also moderately inhibits JAK-STAT3-dependent pathways, perhaps by targeting JAK1, as it has been suggested for ROS (Kurdi and Booz 2007), the increased TNF-α would overcome the JAK-STAT3 inhibition and, ultimately, lead to increased IL-6.
Intuitively, it would be reasonable to assume a correlation between an increased inflammatory mediator message expression and prolonged MAPK activity. Indeed, as we reported for p38, Mn+LPS effects on p38 were not different from the effects of LPS at 1 h post exposure. However, by 4 h, LPS-induced activation of p38 was no longer present, while the presence of Mn in the exposure solution maintained p38 in its activated state (Crittenden and Filipov 2008). Besides p38, it may be that ERK and JNK also play a role in the inflammation-potentiating properties of Mn and/or that there is a MAPK cross-talk that is being affected by Mn at the MKK level. In this regard, involvement of role of JNK and ERK for the Mn-induced potentiation of TNF-α and COX-2 in mixed glial cells has been suggested (Chen et al., 2006) and our current data indicate that MKK-4, the MKK upstream of JNK that also regulates p38, is targeted by Mn.Besides its role in glial activation, the role of MAPK activation by Mn has also been studied in neuronal cells. For example, activation of JNK, specifically through its upstream kinase, MKK-4, appears to play a role in Mn-induced apoptosis in PC 12cells (Hirata et al., 1998). Considering the potentiating effects of Mn on MKK-4 activation by LPS in our study with microglia, it appears that the JNK pathway is a primary and cell-type independent target for Mn and that this pathway warrants further investigation within the context of Mn neurotoxicity. Other than JNK, p38 is activated by Mn in PC12 cells (Roth et al., 2000). Interestingly, it appears that, at least in SN4741 dopaminergic neuronal cell lines, ERK and p38 activation by Mn is neuroprotective, whereas JNK activation is pro-apoptotic (Kim et al., 2008), suggesting that MAPK activation by Mn is a wide-spread phenomenon and that Mn may, to a certain extent, use similar MAPK activation pathways in diverse cell types. The fact that all three major groups of MAP2Ks, MKK-1/2, MKK-3/6, and MKK-4 are activated by Mn suggests that Mn-induced activation of MAPK occurs via traditional mechanisms, which perhaps involve the MAPKs farthest upstream, MAP3Ks. In addition, for all MKKs, Mn-induced activation was persistent at least for 4 h, indicating a long-term effect. Taken together, as depicted on Figure 7, Mn causes over-activation of MAPK signaling, in part by dysregulation of feedback control, especially in the case of JNK (MKK-4) and p38 (MKP-1). In the presence of an inflammagen, this MAPK over-activation is associated with enhanced inflammatory mediator expression and may lead to prolonged neuroinflammation, resulting in excessive neuronal damage and death.
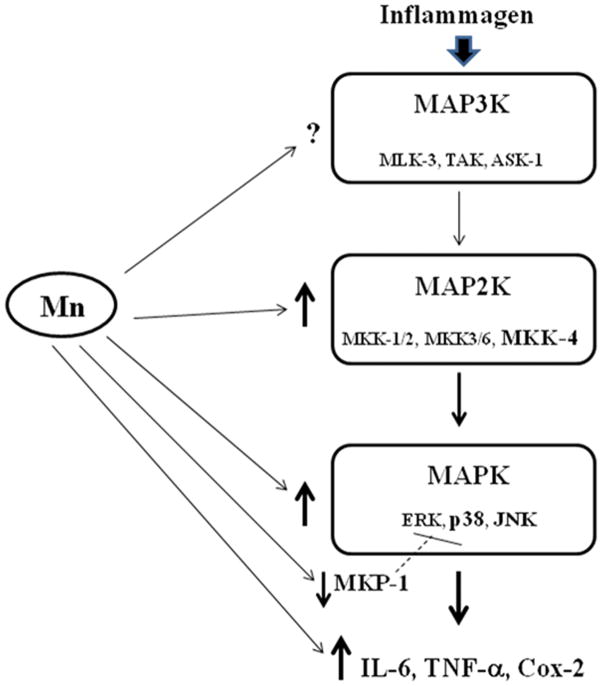
Figure 7.
Simplified MAPK signaling pathway diagram depicting places where Mn has (demonstrated previously and in this study) an effect. In microglial cells, this pathway can be activated by a variety of stimuli, including inflammagens, such as LPS. Arrows beside components of the pathway denote increases or decreases caused by Mn (↑, ↓, respectively). Potential effects of Mn on MAP3K have not been studied yet and are hence marked with a question mark (?). Abbreviations: Mitogen activated protein kinase kinase kinase (MAP3K, i.e., MLK3, TAK, ASK1); mitogen activated protein kinase kinase (MAP2K, i.e. MKK-1/2, MKK-4, MKK-3/6); mitogen activated protein kinase (MAPK, i.e., extracellular regulated protein kinase [ERK], p38, c-Jun N-terminal kinase [JNK]); mitogen activated kinase phosphatase-1 (MKP-1); interleukin-6 (IL-6); tumor necrosis factor-alpha (TNF-α ); cyclooxygenase-2 (Cox-2).
Acknowledgments
We thank Dr. Sang-Ryul Lee, Dr. Celia Dodd, and Irina Georgieva for their technical assistance. This project was supported by research grants from the National Institute of Environmental Health Sciences (NIH), ES011654 and ES016965 to NMF.
References
- Aschner M. Manganese: brain transport and emerging research needs. Environ Health Perspect. 2000;108(Suppl 3):429–32. doi: 10.1289/ehp.00108s3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Guilarte TR, Schneider JS, Zheng W. Manganese: recent advances in understanding its transport and neurotoxicity. Toxicol Appl Pharmacol. 2007;221(2):131–47. doi: 10.1016/j.taap.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae JH, Jang BC, Suh SI, Ha E, Baik HH, Kim SS, Lee MY, Shin DH. Manganese induces inducible nitric oxide synthase (iNOS) expression via activation of both MAP kinase and PI3K/Akt pathways in BV2 microglial cells. Neurosci Lett. 2006;398(1–2):151–4. doi: 10.1016/j.neulet.2005.12.067. [DOI] [PubMed] [Google Scholar]
- Barhoumi R, Faske J, Liu X, Tjalkens RB. Manganese potentiates lipopolysaccharide-induced expression of NOS2 in C6 glioma cells through mitochondrial–dependent activation of nuclear factor kappaB. Brain Res Mol Brain Res. 2004;122(2):167–79. doi: 10.1016/j.molbrainres.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Bhat NR, Zhang P, Lee JC, Hogan EL. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J Neurosci. 1998;18(5):1633–41. doi: 10.1523/JNEUROSCI.18-05-01633.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calne DB, Chu NS, Huang CC, Lu CS, Olanow W. Manganism and idiopathic parkinsonism: similarities and differences. Neurology. 1994;44(9):1583–6. doi: 10.1212/wnl.44.9.1583. [DOI] [PubMed] [Google Scholar]
- Chang JY, Liu LZ. Manganese potentiates nitric oxide production by microglia. Brain Res Mol Brain Res. 1999;68(1–2):22–8. doi: 10.1016/s0169-328x(99)00082-0. [DOI] [PubMed] [Google Scholar]
- Chao CC, Hu S, Close K, Choi CS, Molitor TW, Novick WJ, Peterson PK. Cytokine release from microglia: differential inhibition by pentoxifylline and dexamethasone. J Infect Dis. 1992;166(4):847–53. doi: 10.1093/infdis/166.4.847. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Ou YC, Lin SY, Liao SL, Chen SY, Chen JH. Manganese modulates pro-inflammatory gene expression in activated glia. Neurochem Int. 2006;49(1):62–71. doi: 10.1016/j.neuint.2005.12.020. [DOI] [PubMed] [Google Scholar]
- Chen P, Li J, Barnes J, Kokkonen GC, Lee JC, Liu Y. Restraint of proinflammatory cytokine biosynthesis by mitogen-activated protein kinase phosphatase-1 in lipopolysaccharide-stimulated macrophages. J Immunol. 2002;169(11):6408–16. doi: 10.4049/jimmunol.169.11.6408. [DOI] [PubMed] [Google Scholar]
- Chi H, Barry SP, Roth RJ, Wu JJ, Jones EA, Bennett AM, Flavell RA. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc Natl Acad Sci U S A. 2006;103(7):2274–9. doi: 10.1073/pnas.0510965103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden PL, Filipov NM. Manganese-induced potentiation of in vitro proinflammatory cytokine production by activated microglial cells is associated with persistent activation of p38 MAPK. Toxicol In Vitro. 2008;22(1):18–27. doi: 10.1016/j.tiv.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derijard B, Raingeaud J, Barrett T, Wu IH, Han J, Ulevitch RJ, Davis RJ. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267(5198):682–5. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- Enslen H, Raingeaud J, Davis RJ. Selective activation of p38 mitogen-activated protein (MAP) kinase isoforms by the MAP kinase kinases MKK3 and MKK6. J Biol Chem. 1998;273(3):1741–8. doi: 10.1074/jbc.273.3.1741. [DOI] [PubMed] [Google Scholar]
- Filipov NM, Seegal RF, Lawrence DA. Manganese potentiates in vitro production of proinflammatory cytokines and nitric oxide by microglia through a nuclear factor kappa B-dependent mechanism. Toxicol Sci. 2005;84(1):139–48. doi: 10.1093/toxsci/kfi055. [DOI] [PubMed] [Google Scholar]
- Fujishiro M, Gotoh Y, Katagiri H, Sakoda H, Ogihara T, Anai M, Onishi Y, Ono H, Funaki M, Inukai K, et al. MKK6/3 and p38 MAPK pathway activation is not necessary for insulin-induced glucose uptake but regulates glucose transporter expression. J Biol Chem. 2001;276(23):19800–6. doi: 10.1074/jbc.M101087200. [DOI] [PubMed] [Google Scholar]
- Gavin CE, Gunter KK, Gunter TE. Manganese and calcium transport in mitochondria: implications for manganese toxicity. Neurotoxicology. 1999;20(2–3):445–53. [PubMed] [Google Scholar]
- Hammer M, Mages J, Dietrich H, Servatius A, Howells N, Cato AC, Lang R. Dual specificity phosphatase 1 (DUSP1) regulates a subset of LPS-induced genes and protects mice from lethal endotoxin shock. J Exp Med. 2006;203(1):15–20. doi: 10.1084/jem.20051753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer M, Mages J, Dietrich H, Schmitz F, Striebel F, Murray PJ, Wagner H, Lang R. Control of dual-specificity phosphatase-1 expression in activated macrophages by IL-10. Eur J Immunol. 2005;35(10):2991–3001. doi: 10.1002/eji.200526192. [DOI] [PubMed] [Google Scholar]
- Han S, Lee K, Yeo J, Kweon H, Woo S, Lee M, Baek H, Kim S, Park K. Effect of honey bee venom on microglial cells nitric oxide and tumor necrosis factor-alpha production stimulated by LPS. J Ethnopharmacol. 2007;111(1):176–81. doi: 10.1016/j.jep.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Hazell AS. Astrocytes and manganese neurotoxicity. Neurochem Int. 2002;41(4):271–7. doi: 10.1016/s0197-0186(02)00013-x. [DOI] [PubMed] [Google Scholar]
- Heyen JR, Ye S, Finck BN, Johnson RW. Interleukin (IL)-10 inhibits IL-6 production in microglia by preventing activation of NF-kappaB. Brain Res Mol Brain Res. 2000;77(1):138–47. doi: 10.1016/s0169-328x(00)00042-5. [DOI] [PubMed] [Google Scholar]
- Hirata Y, Adachi K, Kiuchi K. Activation of JNK pathway and induction of apoptosis by manganese in PC12 cells. J Neurochem. 1998;71(4):1607–15. doi: 10.1046/j.1471-4159.1998.71041607.x. [DOI] [PubMed] [Google Scholar]
- Hornberg JJ, Binder B, Bruggeman FJ, Schoeberl B, Heinrich R, Westerhoff HV. Control of MAPK signalling: from complexity to what really matters. Oncogene. 2005;24(36):5533–42. doi: 10.1038/sj.onc.1208817. [DOI] [PubMed] [Google Scholar]
- Huang CC, Chu NS, Lu CS, Chen RS, Schulzer M, Calne DB. The natural history of neurological manganism over 18 years. Parkinsonism Relat Disord. 2007;13(3):143–5. doi: 10.1016/j.parkreldis.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Yamaguchi H, Hirata A, Daimon M, Tominaga M, Kato T. Insulin activates p38 mitogen-activated protein (MAP) kinase via a MAP kinase kinase (MKK) 3/MKK 6 pathway in vascular smooth muscle cells. Eur J Clin Invest. 2000;30(8):668–77. doi: 10.1046/j.1365-2362.2000.00671.x. [DOI] [PubMed] [Google Scholar]
- Jeohn GH, Cooper CL, Wilson B, Chang RC, Jang KJ, Kim HC, Liu B, Hong JS. p38 MAP kinase is involved in lipopolysaccharide-induced dopaminergic neuronal cell death in rat mesencephalic neuronglia cultures. Ann N Y Acad Sci. 2002;962:332–46. doi: 10.1111/j.1749-6632.2002.tb04078.x. [DOI] [PubMed] [Google Scholar]
- Junttila MR, Li SP, Westermarck J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 2008;22(4):954–65. doi: 10.1096/fj.06-7859rev. [DOI] [PubMed] [Google Scholar]
- Kim S, Park E, Kim SJ, Chun HS. Differential role of mitogen-activated protein kinases in response to manganese treatment in substantia nigra dopaminergic neurons. J Health Sci. 2008;54 (2):244–249. [Google Scholar]
- Koistinaho M, Koistinaho J. Role of p38 and p44/42 mitogen-activated protein kinases in microglia. Glia. 2002;40(2):175–83. doi: 10.1002/glia.10151. [DOI] [PubMed] [Google Scholar]
- Kurdi M, Booz GW. Evidence that IL-6-type cytokine signaling in cardiomyocytes is inhibited by oxidative stress: parthenolide targets JAK1 activation by generating ROS. J Cell Physiol. 2007;212(2):424–31. doi: 10.1002/jcp.21033. [DOI] [PubMed] [Google Scholar]
- Lang R, Hammer M, Mages J. DUSP meet immunology: dual specificity MAPK phosphatases in control of the inflammatory response. J Immunol. 2006;177(11):7497–504. doi: 10.4049/jimmunol.177.11.7497. [DOI] [PubMed] [Google Scholar]
- Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter SW, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372(6508):739–46. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- Lee SC, Liu W, Dickson DW, Brosnan CF, Berman JW. Cytokine production by human fetal microglia and astrocytes. Differential induction by lipopolysaccharide and IL-1 beta. J Immunol. 1993;150(7):2659–67. [PubMed] [Google Scholar]
- Li GJ, Zhao Q, Zheng W. Alteration at translational but not transcriptional level of transferrin receptor expression following manganese exposure at the blood-CSF barrier in vitro. Toxicol Appl Pharmacol. 2005;205(2):188–200. doi: 10.1016/j.taap.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao SL, Ou YC, Chen SY, Chiang AN, Chen CJ. Induction of cyclooxygenase-2 expression by manganese in cultured astrocytes. Neurochem Int. 2007;50(7–8):905–15. doi: 10.1016/j.neuint.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Malthankar GV, White BK, Bhushan A, Daniels CK, Rodnick KJ, Lai JC. Differential lowering by manganese treatment of activities of glycolytic and tricarboxylic acid (TCA) cycle enzymes investigated in neuroblastoma and astrocytoma cells is associated with manganese-induced cell death. Neurochem Res. 2004;29(4):709–17. doi: 10.1023/b:nere.0000018841.98399.ce. [DOI] [PubMed] [Google Scholar]
- Meco G, Bonifati V, Vanacore N, Fabrizio E. Parkinsonism after chronic exposure to the fungicide maneb (manganese ethylene-bis-dithiocarbamate) Scand J Work Environ Health. 1994;20(4):301–5. doi: 10.5271/sjweh.1394. [DOI] [PubMed] [Google Scholar]
- Meja KK, Seldon PM, Nasuhara Y, Ito K, Barnes PJ, Lindsay MA, Giembycz MA. p38 MAP kinase and MKK-1 co-operate in the generation of GM-CSF from LPS-stimulated human monocytes by an NF-kappa B-independent mechanism. Br J Pharmacol. 2000;131(6):1143–53. doi: 10.1038/sj.bjp.0703684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JA, Sullivan KA, Carbone DL, Hanneman WH, Tjalkens RB. Manganese potentiates nuclear factor-kappaB-dependent expression of nitric oxide synthase 2 in astrocytes by activating soluble guanylate cyclase and extracellular responsive kinase signaling pathways. Journal of neuroscience research. 2008;86(9):2028–38. doi: 10.1002/jnr.21640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehaus I, Lange JH. Endotoxin: is it an environmental factor in the cause of Parkinson's disease? Occup Environ Med. 2003;60(5):378. doi: 10.1136/oem.60.5.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira-Marques V, Marinho HS, Cyrne L, Antunes F. Role of hydrogen peroxide in NF-kappaB activation: from inducer to modulator. Antioxid Redox Signal. 2009;11(9):2223–43. doi: 10.1089/ars.2009.2601. [DOI] [PubMed] [Google Scholar]
- Park HS, Kim MS, Huh SH, Park J, Chung J, Kang SS, Choi EJ. Akt (protein kinase B) negatively regulates SEK1 by means of protein phosphorylation. J Biol Chem. 2002;277(4):2573–8. doi: 10.1074/jbc.M110299200. [DOI] [PubMed] [Google Scholar]
- Righi M, Mori L, De Libero G, Sironi M, Biondi A, Mantovani A, Donini SD, Ricciardi-Castagnoli P. Monokine production by microglial cell clones. Eur J Immunol. 1989;19 (8):1443–8. doi: 10.1002/eji.1830190815. [DOI] [PubMed] [Google Scholar]
- Roth JA, Feng L, Walowitz J, Browne RW. Manganese-induced rat pheochromocytoma (PC12) cell death is independent of caspase activation. Journal of neuroscience research. 2000;61 (2):162–71. doi: 10.1002/1097-4547(20000715)61:2<162::AID-JNR7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Shepherd EG, Zhao Q, Welty SE, Hansen TN, Smith CV, Liu Y. The function of mitogen-activated protein kinase phosphatase-1 in peptidoglycan-stimulated macrophages. J Biol Chem. 2004;279(52):54023–31. doi: 10.1074/jbc.M408444200. [DOI] [PubMed] [Google Scholar]
- Spranger M, Schwab S, Desiderato S, Bonmann E, Krieger D, Fandrey J. Manganese augments nitric oxide synthesis in murine astrocytes: a new pathogenetic mechanism in manganism? Exp Neurol. 1998;149(1):277–83. doi: 10.1006/exnr.1997.6666. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Mouri T, Nishiyama K, Fujii N. Study of subacute toxicity of manganese dioxide in monkeys. Tokushima J Exp Med. 1975;22:5–10. [PubMed] [Google Scholar]
- Tanabe K, Matsushima-Nishiwaki R, Yamaguchi S, Iida H, Dohi S, Kozawa O. Mechanisms of tumor necrosis factor-alpha-induced interleukin-6 synthesis in glioma cells. J Neuroinflammation. 2010;7:16. doi: 10.1186/1742-2094-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Liu Y. Regulation of innate immune response by MAP kinase phosphatase-1. Cell Signal. 2007;19(7):1372–82. doi: 10.1016/j.cellsig.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yase Y. The pathogenesis of amyotrophic lateral sclerosis. Lancet. 1972;2(7772):292–6. doi: 10.1016/s0140-6736(72)92903-0. [DOI] [PubMed] [Google Scholar]
- Zhang P, Hatter A, Liu B. Manganese chloride stimulates rat microglia to release hydrogen peroxide. Toxicol Lett. 2007;173(2):88–100. doi: 10.1016/j.toxlet.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Lokuta KM, Turner DE, Liu B. Synergistic dopaminergic neurotoxicity of manganese and lipopolysaccharide: differential involvement of microglia and astroglia. J Neurochem. 2010;112(2):434–43. doi: 10.1111/j.1471-4159.2009.06477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Wong TA, Lokuta KM, Turner DE, Vujisic K, Liu B. Microglia enhance manganese chloride-induced dopaminergic neurodegeneration: role of free radical generation. Exp Neurol. 2009;217(1):219–30. doi: 10.1016/j.expneurol.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Shepherd EG, Manson ME, Nelin LD, Sorokin A, Liu Y. The role of mitogen-activated protein kinase phosphatase-1 in the response of alveolar macrophages to lipopolysaccharide: attenuation of proinflammatory cytokine biosynthesis via feedback control of p38. J Biol Chem. 2005;280(9):8101–8. doi: 10.1074/jbc.M411760200. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Ling EA, Dheen ST. Dexamethasone suppresses monocyte chemoattractant protein-1 production via mitogen activated protein kinase phosphatase-1 dependent inhibition of Jun N-terminal kinase and p38 mitogen-activated protein kinase in activated rat microglia. J Neurochem. 2007;102(3):667–78. doi: 10.1111/j.1471-4159.2007.04535.x. [DOI] [PubMed] [Google Scholar]