Abstract
In addition to stimulating gene transcription, sex steroids trigger rapid, non-genomic responses in the extra-nuclear compartment of target cells. These events take place within seconds or minutes after hormone administration and do not require transcriptional activity of sex steroid receptors. Depending on cell systems, activation of extra-nuclear signaling pathways by sex steroids fosters cell cycle progression, prevents apoptosis, leads to epigenetic modifications and increases cell migration through cytoskeleton changes. These findings have raised the question of intracellular localization of sex steroid receptors mediating these responses. During the past years, increasing evidence has shown that classical sex steroid receptors localized in the extra-nuclear compartment or close to membranes of target cells induce these events. The emerging picture is that a process of bidirectional control between signaling activation and sex steroid receptor localization regulates the outcome of hormonal responses in target cells. This mechanism ensures cell cycle progression in estradiol-treated breast cancer cells, and its derangement might occur in progression of human proliferative diseases. These findings will be reviewed here together with unexpected examples of the relationship between sex steroid receptor localization, signaling activation and biological responses in target cells. We apologize to scientists whose reports are not mentioned or extensively discussed owing to space limitations.
Keywords: Steroid action, Signaling activation, Sex steroid receptor localization
Introduction
Sex steroids control a variety of responses in reproductive tissues, such as breast and prostate, and this activity has so far been attributed to transcriptional, genomic effects exerted by these hormones. According to this model, ligand-activated sex steroid receptors (SRs) translocate into the nucleus where they bind to hormone response element (HRE) and recruit factors required for the assembly of pre-initiation complexes (reviewed in McKenna and O’Malley 2002). After a relatively long time (from several minutes to hours or days), modifications of gene expression and protein profile occur. Finally, hormonal effects become evident.
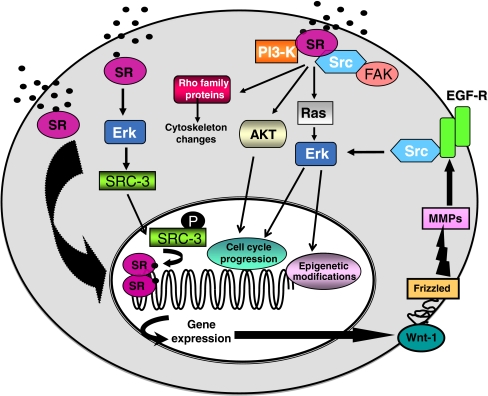
SRs also mediate rapid, non-genomic responses in the extra-nuclear compartment of target cells. These responses are insensitive to RNA or protein synthesis inhibitors and do not require transcriptional activity of sex steroid receptors. Depending on the cell milieu, activation of these pathways produces different effects, such as proliferation, survival, vasorelaxation, migration and differentiation (reviewed in Castoria et al. 2008). This dual mechanism (genomic and non-genomic) of sex steroid action does not, however, account for the complexity of steroid-elicited responses in target tissues. Much evidence shows, indeed, that genomic and non-genomic actions of sex-steroids are integrated. Thus, non-genomic action mediated by SRs regulates the downstream genomic effects of sex steroids. Conversely, transcriptional activity of sex steroid receptors controls signaling pathway activation (reviewed in Migliaccio et al. 2010; Vicent et al. 2010). Figure 1 depicts the two models of sex steroid action and their possible integration in target cells.
Fig. 1.
Models of sex steroid action in target tissue. The figure depicts a simplified model of genomic and non-genomic actions of sex steroids as well as possible integrations of the two models in target cells. According to the genomic model, sex steroid receptors (SR) translocate into the nuclear compartment, where they directly or indirectly stimulate gene transcription. Classical SR close to cell membranes or localized at caveolae recruit and activate upon ligand binding various signaling effectors, including the tyrosine kinase Src and PI-3-K, which in turn trigger cell proliferation and cytoskeleton changes or lead to epigenetic modifications (non-genomic model). Rapid activation of extra-cellular-regulated kinase (Erk) mediated by classical SR also controls phosphorylation of the co-activator SRC-3. Once phosphorylated, SRC-3 translocates into the nuclear compartment, where it positively affects the transcriptional activity of SR. These results provide evidence for an early, non-genomic action of SR on SRC-3 that regulates the downstream genomic effects of estradiol (Zheng et al. 2005). In turn, SR action in nuclei induces transcriptional up-regulation of Wnt-1, followed by activation of MMP and trans-activation of EGF-R, which then activates Src-dependent signaling. This event leads to sustained activation of MAPK
SRs are considered nucleo-cytoplasmic shuttling proteins. Target cells take advantage of this shuttling process, since it contributes to the dynamic regulation of gene transcription and activation of signaling pathways in the extra-nuclear compartment. SR trafficking thus provides a mechanism to control and integrate nuclear and extra-nuclear functions of these receptors in target cells. Therefore, there is now a strong interest in defining links between signaling pathways, SR localization and biological outcome in target cells.
Nuclear import of glucocorticoid receptor (GR) can be triggered by activation of both mitogen-activated protein kinases (MAPKs) and phosphatidylinositol 3-kinase (PI3-K) signaling; the MAPK axis has been also implicated in progesterone receptor (PR) import–export (reviewed in Pemberton and Paschal 2005). Again, in a progression model of prostate cancer, xenografts can switch from androgen-dependent to androgen-independent growth in castrated mice. Notably, during cancer progression, androgen receptor (AR) undergoes androgen-independent nuclear import through a mechanism involving MAPK pathway activation (Zhang et al. 2003). Estradiol activation of PI3-K/Akt (protein kinase B) pathway regulates estradiol receptor (ER) alpha nuclear export and cell cycle progression in MCF-7 cells (Lombardi et al. 2008). Furthermore, prostate cancer-derived as well as mesenchimal and mesenchimal-transformed cells harbor a classical androgen receptor, which is tethered by filamin A (FlnA) or its fragments to intermediate filaments of cytoskeleton (Ozanne et al. 2000; Castoria et al. 2010). In LNCaP cells, such a distribution enables AR nuclear translocation (Ozanne et al. 2000), while in fibroblasts and human fibrosarcoma cells it ensures activation of the basic machinery leading to cytoskeleton rearrangements and cell migration upon androgen stimulation (Castoria et al. 2010).
These and other findings reviewed in the following sections reveal new facets of sex steroid biology and point to the reciprocal control of rapid hormonal action and intracellular localization of SRs. Such a control mechanism might impact growth and development of target tissues, and its derangement maybe involved in proliferative breast and prostate diseases. This review aims to provide a concise up-to-date report of the complex network bridging localization of sex steroid receptors with non-genomic action of steroid hormones and biological outcome in target cells.
Estradiol receptor localization and signaling activation
Cell biology and biochemical approaches have prevalently detected ER alpha in nuclei of target cells (Stenoien et al. 2001). Because of sensitivity limits, these methods only detect the presence of a protein where its steady state concentration is above the detection threshold. Therefore, these findings do not exclude that ER alpha transiently crosses the nuclear envelope to play a role in the cytoplasm or vice versa. Initially, ER alpha shuttling from nuclei to cytoplasm was detected using interspecies heterokaryon assay (Dauvois et al. 1993). Advances in live-cell fluorescence microscopy and use of green fluorescent protein (GFP) have allowed the study of nuclear receptor behavior in live cells in real time (Pemberton and Paschal 2005). Using the GFP approach, it has been observed that ligand binding and protein-protein interactions significantly influence nucleo-cytoplasmic shuttling of ER (Maruvada et al. 2003).
We recently dissected the import–export cycle of ER alpha in breast cancer MCF-7 cells. The nuclear export of ER alpha depends on chromosome region maintenance 1 (CRM1) and is regulated by estradiol activation of the PI3-K/Akt pathway (Lombardi et al. 2008). In a first attempt, the behavior of endogenous ER alpha was analyzed using two different antibodies in an immunofluorescence approach. Subsequently, the estradiol-regulated import–export cycle of ER alpha was dissected by subcloning the full-length receptor in GFP and following the fluorescence in MCF-7 cells unstimulated or stimulated with estradiol. Results from these experiments showed that after fast nuclear translocation, estradiol induces rapid LMB-sensitive ER alpha nuclear export. The entire process occurs during the initial 60 min of hormone treatment (Lombardi et al. 2008). An in vivo export assay (Henderson and Eleftheriou 2000) was then used. In this assay, putative nuclear export signals (NES) were identified by their ability to restore export activity of the NES-deficient regulator of expression of viral protein (Rev) 1.4-GFP (Rev mutant) to levels similar to those observed with the wild type pRev-GFP or the Rev 1.4-GFP NES (Rev wt) in which the NES is the canonical export sequence of the Rev protein. Different sequences of ER alpha containing leucine residues were then subcloned into Rev mutant (Lombardi et al. 2008). These constructs were transfected into MCF-7 cells and analyzed for their ability to restore nuclear export of the Rev mutant. Using this assay, we observed that the ER alpha leucine-rich sequence 444–456 induces a nucleo-cytoplasmic redistribution of Rev mutant in MCF-7 cells and that LMB treatment blocks its export activity. These data indicate that the 444–456 sequence is involved in the export of ER alpha through CRM1/exportin binding. By site-directed mutagenesis, two point mutations were introduced in the hydrophobic core of this sequence and by immunofluorescence approach it was verified that the 444–456 sequence actually contains the LMB-sensitive NES of ER alpha (Lombardi et al. 2008).
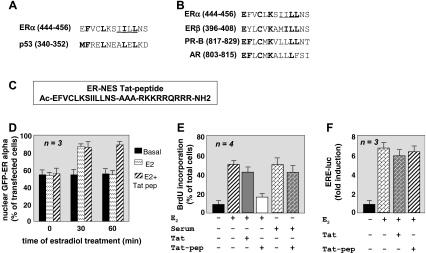
A thorough ER alpha sequence analysis showed homology between the 444–456 amino acids of ER alpha and the conserved leucine-rich and Rev-like NES of p53 (residues 340–351; Fig. 2a). A comparison of the homologous amino acids across the family of steroid receptors revealed a high level of sequence homology with ER beta, PR-B, and AR (Lombardi et al. 2008 and Fig. 2b). In contrast, we detected a less stringent sequence homology with GR and the mineralocorticoid receptor (MR; Lombardi et al. 2008). This is in agreement with the observation that GR nuclear export is independent of CRM1 (Liu and DeFranco 2000) and that the ligand binding domain of MR contains an LMB-insensitive NES (Saporita et al. 2003).
Fig. 2.
Identification of ER alpha NES and biological properties of ER alpha NES peptide in breast cancer cells. Panel A shows the ER alpha NES we mapped within the carboxyl-terminal domain of ER alpha. ER alpha NES sequence homology with the carboxyl-terminal Rev-like NES of p53 is also shown. Panel B shows that ER alpha NES sequence is highly conserved among the members of SR family In A and B, the hydrophobic core of ER alpha NES is underlined. Conserved amino acids are in bold. Panel C shows the amino acid sequence of the ER alpha NES-mimicking peptide. It was linked by a stretch of three alanins to the HIV-derived Tat peptide. Panel D shows that addition of this peptide (Tat-pep) at 1 micromolar to the cell medium traps GFP-ER in nuclei of 60 min estradiol-treated MCF-7 cells. This peptide (Tat-pep; panel E) also interferes in BrdU incorporation induced by estradiol in quiescent MCF-7 cells whereas it does not affect serum-induced BrdU incorporation in MCF-7 cells. The Tat peptide alone was used as a control at 1 micromolar (Tat; panel E). In F, neither Tat peptide (Tat) nor Tat-NES peptide (Tat-pep) interferes in ERE-luc induction mediated by ER in estradiol-treated MCF-7 cells. In D, E and F, estradiol was used at 10 nM
Based on findings presented in Fig. 2b, a full-length ER alpha NES mutant was prepared by site-directed mutagenesis and subcloned in GFP. Immunofluorescence approach showed that this mutant does not exit nuclei upon 60 min estradiol treatment, confirming that the leucine-rich sequence 444–456 of ER alpha actually contains a functional NES (Lombardi et al. 2008). Interestingly, this mutant also fails to induce estradiol-stimulated DNA synthesis, although it is still able to activate signaling or gene transcription regulated by estradiol (Lombardi et al. 2008). Lastly, a small peptide mimicking the ER alpha NES was conjugated to the Tat sequence derived from Human Immunodeficiency Virus (HIV). This small cationic peptide delivers proteins and drugs into nuclei (Joliot and Prochiantz 2004). The amino acid sequence of ER alpha NES-Tat peptide is shown in Fig. 2c. The Tat-peptide (Tat-pep) specifically sequesters ER alpha in nuclei (Fig. 2d) and interferes in hormone-triggered S-phase entry in MCF-7 cells (Fig. 2e), without affecting ERE-dependent transcriptional activity (Fig. 2f) or nuclear export of p53 and p27, which contain canonical NES (Lombardi et al. 2008). Altogether, our findings show that estradiol-induced nuclear export of ER alpha is controlled by the PI3-K pathway and is coupled with S-phase entry in breast cancer MCF-7 cells (Lombardi et al. 2008).
In search of a link between ER alpha nuclear export, PI3-K activation and cell cycle progression in breast cancer cells, the role of the forkhead (FKHR) transcription factor in these events was verified. Nuclear export of FKHR depends on its phosphorylation by Akt and nuclear FKHR regulates expression of genes involved in cell metabolic state, oxidative stress, aging and cell cycle arrest (reviewed in Accili and Arden 2004). Thus, changes in FKHR localization control the balance between cell cycle arrest and proliferation.
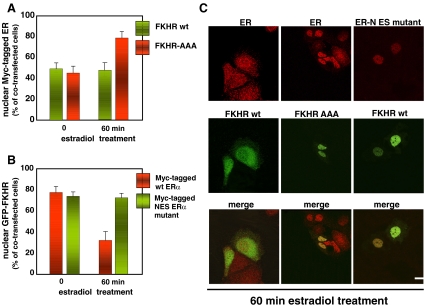
Hormone-dependent ER alpha/FKHR interaction occurs in vitro (Schuur et al. 2001; Zhao et al. 2001) and negatively regulates estrogen-dependent breast cancer growth in cultured cells and tumorigenesis in vivo (Zou et al. 2008). Estradiol-activated PI3-K/Akt pathway leads to phosphorylation of the gatekeeper Ser 256 of FKHR, analyzed by Western blot of MCF-7 cell lysates (Lombardi et al. 2008). In addition, overexpression of a triple FKHR mutant (FKHR-AAA) inhibits estradiol-induced S-phase entry in MCF-7 cells (Lombardi et al. 2008). Notably, FKHR-AAA mutant cannot be phosphorylated by Akt and permanently resides in cell nuclei, thereby inducing cell cycle arrest (Nakamura et al. 2000). Confocal microscopy approach in Fig. 3a and c shows that overexpression of FKHR-AAA mutant induces ER alpha nuclear retention in 60 min estradiol-treated MCF-7 cells. In turn, overexpression of ER alpha NES mutant traps wild type FKHR in nuclei of cells challenged for 60 min with estradiol (Fig. 3b and c). From these findings (Lombardi et al. 2008), it can be concluded that estradiol simultaneously regulates ER and FKHR nuclear export. The role of ER alpha in this process is reinforced by siRNA approach showing that ER alpha knockdown induces nuclear retention of wild type FKHR in estradiol-treated MCF-7 cells (Lombardi et al. 2008). Thus, we inferred that in ER alpha–positive breast cancer cells FKHR moves from nucleus to cytoplasm via a receptor-associated mechanism. Immunoprecipitation experiments corroborated this view, since they showed that estradiol stimulation increases association of wild type FKHR with ER alpha in MCF-7 cells. In the same experimental setting, the FKHR-AAA mutant fails to do so. Thus, FKHR phosphorylation by estradiol is required for its association with receptor, the nuclear exit of FKHR/ER complex and the consequent release of FKHR-mediated cell cycle inhibition (Lombardi et al. 2008). The reason ER alpha/FKHR association is required for nuclear export of the two proteins can at present only be the subject of speculation. It could be proposed that both ER alpha and FKHR each have a weak NES. ER alpha/FKHR complex assembly may increase the affinity of each protein for CRM1/exportin, and association of FKHR with ER alpha might favor FKHR nuclear exit by masking its nuclear localization signal (NLS). A similar mechanism occurs when FKHR exits nuclei in complex with the 14-3-3 protein (Van Der Heide et al. 2004).
Fig. 3.
Estradiol simultaneously induces nuclear export of ER and FKHR in MCF-7 cells. Quiescent MCF-7 were co-transfected along with the indicated constructs and then left unstimulated or stimulated for 60 min with 10 nM estradiol. Graphs in A represent the nuclear score of Myc-tagged ER in MCF-7 cells co-expressing either GFP-FKHR wt (green bars) or the GFP-FKHR-AAA mutant (red bars). Graphs in B represent the nuclear score of GFP-FKHR in MCF-7 cells co-expressing either Myc-tagged ER alpha wt (red bars) or the Myc-tagged ER alpha NES mutant (green bars). Images of confocal microscopy analysis were captured and are shown in C. They represent the staining of Myc-tagged ER alpha in MCF-7 cells expressing GFP-FKHR wt (left in green), or the GFP-FKHR-AAA mutant (middle in green) and treated for 60 min with 10 nM estradiol. Right panels represent the staining of Myc-tagged ER alpha NES mutant (red) in MCF-7 cells co-expressing GFP-FKHR wt (green) and treated for 60 min with 10 nM estradiol. Merged images are shown at the bottom. Bar, 5 μm. For experimental details see also Lombardi et al. 2008 in refs
Figure 4 depicts the model of ER alpha/FKHR nuclear export in MCF-7 cells.
Fig. 4.
Model of ER alpha nuclear export in breast cancer cells. A model of ER alpha nuclear export in breast cancer MCF-7 cells based on experimental evidence from our laboratory is shown. Estradiol-stimulated PI3K/Akt pathway leads to FKHR phosphorylation at Ser 256, thus triggering the associated export of FKHR/ER. Export of FKHR/ER removes the transcriptional repressor activity of FKHR and triggers DNA synthesis (Lombardi et al. 2008). This model is compatible with findings showing that methylation of ER alpha by PRMT1 tethers the receptor in cytoplasm of MCF-7 cells, where it recruits and activates Src, PI3-K and FAK (Le Romancer et al. 2008)
Our data reveal a novel link between non-genomic estradiol action and ER alpha export. They also identify a new and unexpected role for ER nuclear export in DNA synthesis. According to the model in Fig. 4, estradiol exerts two integrated actions: one in the extra-nuclear compartment, where it activates the PI3-K/Akt pathway (Castoria et al. 2001); the other in the nucleus, where it forms a complex with FKHR. Both actions (extra-nuclear and nuclear) converge on FKHR nuclear export and the consequent release of DNA synthesis inhibition. These findings offer an additional example of integration between non-genomic and genomic actions of SRs.
Noteworthy, Akt-2 activation by insulin-like growth factor 1 (IGF-1) also controls nuclear retention of ER alpha and FKHR-L1 in breast cancer cells (Morelli et al. 2010). These findings indicate that PI3-K/Akt/FOXO (Forkhead box O1 transcription factors) pathway activation controls ER localization in breast cancer cells, whatever the extra-cellular stimulus.
Many reports point to the role of membrane-associated ER alpha in signaling activation. Confocal microscopy studies show that estradiol rapidly induces ER alpha membrane translocation and formation of membrane ruffles as well as pseudopodia in breast cancer MCF-7 cells (Song et al. 2002). Similar effects have been observed in human endothelial and breast cancer cells. In these latter cells, estradiol-coupled ER alpha rapidly activates the small Ras-like GTPase member A (Rho A). This results in phosphorylation of the actin-binding protein moesin, with a consequent increase in cytoskeleton changes analyzed by immunofluorescence and cell motility detected by wound scratch assay (Simoncini et al. 2006; Giretti et al. 2008). It has also been reported that estradiol treatment of MCF-7 cells induces recruitment of ER alpha to IGF-1 receptor (IGF1-R). In this way, ER alpha is tethered to the plasma membrane, where it initiates signaling events (Song et al. 2004).
ER alpha has been also detected as monomer in caveolae rafts at cell surface of different cell types. It has been reported that ligand addition promotes receptor dimerization and recruitment of signaling molecules (reviewed in Hammes and Levin 2007). Recent data have clarified the molecular mechanism underlying the delivery of ER to caveolae. Accordingly, it has been proposed that the binding of ER alpha to the heat shock protein p27 (Hsp27) promotes receptor palmitoylation and increases the interaction of ER alpha with caveolin-1 at cell membranes. In this location, signaling activation is initiated. The same mechanism has been extended to AR and PR, which share a similar route in initiating signaling activation at plasma membranes in breast and prostate cancer cells (Razandi et al. 2010).
Only few studies have been conducted to analyze ER beta trafficking regulation. The finding that ER beta mediates rapid, non-genomic effects in different cell types (Migliaccio et al. 2000; Kousteni et al. 2001; Chambliss and Shaul 2002; Acconcia et al. 2005) raises the question as to the location of receptor mediating these responses. As proposed for ER alpha, a sub-population of ER beta can be detected within caveolae rafts of endothelial cells, where it mediates release of nitric oxide synthase upon estradiol stimulation (Chambliss and Shaul 2002). Subsequent studies showed that ER beta serves as a palmitoyl acyl transferase (PAT) substrate and that receptor palmitoylation is needed for ER beta localization at plasma membranes as well as for initiation of estradiol-induced rapid actions (Galluzzo et al. 2007). ER beta, however, has been prevalently detected in nuclei of target cells, where it exhibits fast mobility and highly dynamic distribution in fluorescence recovery after photobleaching (FRAP) analysis (Damdimopoulos et al. 2008; Picard et al. 2008). Phosphorylation of specific MAPK serine residues (Ser 94 and Ser 124) within the ER beta activation function 1 (AF-1) domain induces clustering of the receptor in inactive nuclear compartment (Picard et al. 2008), again reinforcing the view that signaling pathways control the outcome of target cells by modulating sub-cellular ER localization.
In conclusion, it appears that a feedback loop between signaling activation and ER trafficking ensures biological response in the different compartments of target cells.
Progesterone receptor localization and signaling activation
PR undergoes continuous nucleo-cytoplasmic shuttling (Guiochon-Mantel et al. 1989) and several putative NESs have been identified in both N-terminal and C-terminal regions of this receptor (Tyagi et al. 1998). However, because of the failure of microinjected NESs to displace PR nuclear export, it was inferred that the identified sequences cannot be considered functional NESs (Tyagi et al. 1998). Figure 2a shows that one of these putative PR-B NESs (PR 816–824 amino acid) exhibits high homology with the functional ER alpha NES. Further, LMB blocks nuclear export and proteasome-dependent degradation of PR in T47D cells (Qiu et al. 2003), thus indicating that CRM1 directly or indirectly mediates PR nuclear export.
Progestin stimulation of T47D cells rapidly activates the proto-oncogene tyrosine-protein kinase (Src)/MAPK pathway (Migliaccio et al. 1998; Boonyaratanakornkit et al. 2001; Ballaré et al. 2003; Vicent et al. 2006). Activation of this pathway controls cell cycle progression (Castoria et al. 1999) and leads to epigenetic modifications in T47D cells (Vicent et al. 2006). Again, progestins activate the PI3-K/Akt/nuclear factor-kappa B cascade to up-regulate cyclin D1 and cell proliferation in human breast cancer cells (Saitoh et al. 2005). Signaling pathway activation by progestins occurs outside of cell nuclei (Boonyaratanakornkit et al. 2007) and PR has been detected at cell membrane in some cell types (Zhu et al. 2003; Karteris et al. 2006). This localization has been attributed to the presence of palmitoylation site/membrane localization signal within the PR sequence (Pedram et al. 2007). As previously discussed, the binding of Hsp27 to PR seems to be required to direct PR to caveolae (Razandi et al. 2010). Again, a small subset of PR has been found associated to endothelial and breast cancer cell membranes, where ligand-bound PR interacts with the G-protein Gα13 to activate RhoA/Rho-associated coiled-coil containing protein kinase (ROCK) cascade and cell invasiveness mediated by moesin phosphorylation (Fu et al. 2008 a and b).
As occurs for ER alpha in MCF-7 cells, signaling activation modifies PR location. In breast cancer T47D cells, MAPKs play a dual role in PR sub-cellular trafficking. They enhance nuclear translocation of PR upon epidermal growth factor (EGF) stimulation and mediate PR nuclear export and its consequent degradation upon progestin stimulation of cells. This latter event negatively regulates PR transcriptional activity. Interestingly, both MAPK effects occur through PR Ser 294 phosphorylation. Thus, Ser 294 phosphorylation by MAPK modulates the nuclear import of unliganded PR and allows for LMB-dependent nuclear export of liganded PR (Qiu et al. 2003). Involvement of Ser 294 phosphorylation in PR nuclear export suggests that this phosphorylation either unmasks a putative PR NES or facilitates the interaction of PR with proteins associated with the nuclear export process.
The observation that LMB blocks nuclear export of PR (Qiu et al. 2003) is in apparent contrast with previous findings reporting that PR nuclear release also occurs in the presence of LMB and does not depend on Ran-GTP. These findings suggested that CRM1 pathway is not involved in PR nuclear export (Tyagi et al. 1998). It should be noted, however, that the reported differences might be due to different experimental conditions.
Conflicting data have also been reported for nuclear export of GR, which appears to be sensitive (Savory et al. 1999) or insensitive (Liu and DeFranco 2000) to LMB depending on the cell system and experimental setting. These apparent discrepancies strongly suggest that the molecular mechanism regulating PR nuclear export needs to be reviewed using more advanced approaches, such as in vivo nuclear export assay (Henderson and Eleftheriou 2000), site-directed mutagenesis of putative NESs in full-length PR, and protein-protein interaction assay. Nonetheless, it should be considered that PR, as well as other SRs, may exit nuclei through multiple and complex mechanisms that allow the cells to respond appropriately to a wide range of external cues.
Another interesting example of the link between signaling activation, PR localization and biological outcome of cells has been provided by Vallejo and co-workers, showing that progestin stimulation of rat uterine stromal cells simultaneously induces transient activation of MAPKs and PR nuclear translocation along with the activated MAPKs. Such effects lead to proliferation of these cells (Vallejo et al. 2005). Since PR expressed in rat uterine stromal cells is devoid of transcriptional activity, the role of PR nuclear translocation remains to be clarified. Nuclear PR might direct MAPK action inside nuclei to cluster MAPKs in active nuclear compartment, thus driving expression of genes involved in progestin-induced cell cycle.
The existence of a feedback loop between PR and the MAPK axis has been strengthened by findings showing that in addition to mediating rapid MAPK activation, progestin-bound PR-B induces the sustained activation of MAPKs in T47D breast cancer cells. This latter effect results from PR-mediated transcriptional up-regulation of the secreted wingless-related MMTV integration site 1 (Wnt1), which binds to the seven-transmembrane receptor Frizzled (Fz) and induces the matrix metalloprotease (MMP)-dependent cleavage of epidermal growth factor receptor (EGF-R) ligands. In this way, EGF-R is transactivated and sustained activation of the downstream Src and MAPK effectors follows (Faivre and Lange 2007 and Fig. 1).
In conclusion, it appears from these reports that location of PR acts as a sensor for MAPKs, which in turn play a regulatory role in PR import–export cycle and coupled functions.
Androgen receptor localization and signaling activation
AR regulatory functions depend on the proper sub-cellular localization of its receptor. AR is also thought to associate with a heat shock protein 90 (Hsp90)-based chaperone complex in the cytoplasm (Prescott and Coetzee 2006) until the binding of cognate ligand induces a conformational change in AR, chaperone dissociation, and subsequent AR nuclear import (Tyagi et al. 2000; Marcelli et al. 2006). Once in the nucleus, AR binds specific androgen-response elements (AREs) and enhances or represses transcription of associated androgen-responsive genes. AR also undergoes nuclear export and this process was found to be insensitive to LMB (Tyagi et al. 2000). The DNA-binding domain (DBD) of AR is sufficient to direct nuclear export of a reporter protein, and point mutations in the DBD of full-length AR reduce AR nuclear export without affecting import (Black et al. 2001). Analysis of AR sequence subsequently identified a canonical and LMB-sensitive NES in the ligand-binding domain (LBD) of AR (Saporita et al. 2003). The mechanism of AR nuclear export, however, still remains elusive.
Multiple signal transduction pathways operate upstream of AR and modulate its functions and localization. It has been previously reported that p38 alpha kinase and Jun-N terminal kinase (JNK) both phosphorylate AR at Ser 650, thereby inducing nuclear export of AR and antagonizing AR-mediated transcription in LNCaP cells (Gioeli et al. 2006). Thus, phosphorylation on Ser 650 by stress kinases may generate a signal for AR nuclear export or alternatively may relieve AR nuclear import. Since controversial data concerning the presence of a canonical NES within the AR sequence have been reported (Tyagi et al. 2000; Saporita et al. 2003), the possibility that phosphorylation on Ser 650 alters association of AR with proteins involved in nuclear export cannot be excluded. The DNA-dependent protein kinase (DNA-PK), a member of the PI3-K family, also modulates AR nuclear export in LNCaP cells (Shank et al. 2008). These and previous findings support the conclusion that multiple pathways direct AR nuclear export.
AR interacts with different scaffold proteins. It associates with caveolin 1 in low-density and caveolin-rich membrane fractions, and this association increases receptor-dependent transcriptional activity (Lu et al. 2001). Notably, immunohistochemical staining of patient specimens suggests that caveolin expression may be an independent predictor of prostate cancer progression.
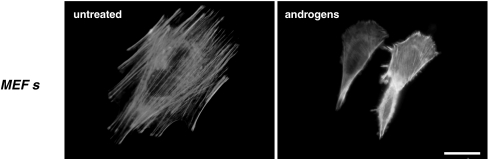
The actin-binding protein, FlnA is a master player in signaling leading to cell migration. FlnA intersects AR action and has been implicated in AR trafficking. In LNCaP cells, AR nuclear translocation observed upon androgen stimulation is mediated by interaction of AR with a proteolytic product of FlnA (Ozanne et al. 2000). Impairment of androgen-induced AR nuclear translocation has been observed in FlnA-null cells and FlnA re-expression restores the normal trafficking of AR in these cells (Ozanne et al. 2000). Thus, it appears that FlnA retains AR in the cytoplasm of resting cells and that nuclear translocation of AR occurs along the calpain product of FlnA. Relevant to these findings is the recent observation that mouse embryo fibroblasts (MEFs) as well as NIH3T3 fibroblasts and human fibrosarcoma HT1080 cells harbor low levels of classical AR, which co-localizes with FlnA at intermediate filaments of cytoskeleton (Castoria et al. 2010). Confocal microscopy analysis and immunoprecipitation approaches reveal that stimulation of these cells with physiological androgen concentration increases AR/FlnA co-localization at intermediate filaments and triggers activation of signaling pathways depending on Rac and focal adhesion kinase (FAK). Activation of these effectors finally leads to cytoskeleton remodeling and cell migration (Castoria et al. 2010). Figure 5 shows cytoskeleton changes and lamellipodia formation induced within a few minutes of androgen treatment in MEFs.
Fig. 5.
Androgens trigger cytoskeleton changes in MEFs. Quiescent MEFs on coverslips were left unstimulated or stimulated with 10 nM R1881 for 20 min. F-actin was visualized using Texas Red-phalloidin as reported (Castoria et al. 2003). Images show that androgens induce a modification in cell shape and the formation of fan-like protrusions and lamellipodia. They are representative of three independent experiments. Bar, 5 μm.
These findings provide new clues that may explain the regulatory role of extra-nuclear AR/FlnA complex in androgen-induced cell motility. In sum, it appears that the AR/FlnA complex controls cell motility when assembled in cytoplasm, while it modifies AR-mediated transcriptional machinery in nuclei. In agreement with this hypothesis, a striking correlation between FlnA cytoplasmic localization and human androgen-independent metastatic prostate cancer has been reported (Bedolla et al. 2009). A major cause of FlnA cytoplasmic retention seems to be failure to be cleaved by calpain due to its failure to be phosphorylated by protein kinase A (PKA; Bedolla et al. 2009). These results offer new opportunities for a better understanding of invasiveness and androgen independence of prostate cancers. Further, they suggest that PKA inhibitors maybe used to restore FlnA nuclear localization in patients with metastatic prostate cancer.
In different cell types extra-nuclear AR mediates rapid activation of signaling effectors, such as Src, PI3-K and MAPK (reviewed in Migliaccio et al. 2010). Thus, the extra-nuclear location of AR dictates these rapid effects, which lead to cell cycle regulation, cell growth in mouse model of prostate cancer, survival and cytoskeleton changes (Peterziel et al. 1999; Migliaccio et al. 2000, 2007; Kousteni et al. 2001; Castoria et al. 2003). These findings support the conclusion that extra-nuclear AR-mediated signaling activation plays a central role in cultured cells as well as in vivo.
In prostate cancer-derived LNCaP cells treated with androgens, extra-nuclear AR leads to Src recruitment and activation. This event occurs through cross talk between AR and ER beta expressed in these cells (Migliaccio et al. 2000). The ternary complex follows direct interaction of a proline-rich motif of AR with the Src Homology 3 (SH3)-Src domain, and a phosphorylated tyrosine of ER beta, most likely the Tyr 443 residue, with the Src Homology 2 (SH2)-Src domain (Migliaccio et al. 2000). This complex strongly activates Src and its dependent network in prostate and breast cancer cells (Migliaccio et al. 2000). AR/ER beta/Src ternary complex is also recruited to EGF-R in EGF-treated LNCaP cells, thus indicating that extra-nuclear steroid receptors transmit their signals even in the absence of steroids (Migliaccio et al. 2005). Notably, extra-nuclear AR partners, such as EGF-R and Src family kinases, are frequently deregulated in prostate cancers (reviewed in Fizazi et al. 2010) and increasing evidence from cultured cells and in vivo models points to the role of estradiol and ER in prostate cancerogenesis (reviewed in Risbridger et al. 2010). Clinical evidence has also shown that toremifene, a selective estrogen receptor modulator (SERM), exerts beneficial effects in prostate cancer treatment (Price et al. 2006).
Rapid action of androgens has also been linked to a membrane AR (mAR) that rapidly triggers Rac activation and cytoskeleton changes in LNCaP cells (Papakonstanti et al. 2003). These effects are insensitive to three different anti-androgens, suggesting that mAR is quite different from the classical AR expressed in LNCaP cells. It has also been reported that androgen stimulation of LNCaP cells activates Akt-1 and increases AR/Akt-1 interaction in lipid rafts (Cinar et al. 2007). These findings indicate that in cells which have lost the expression of phosphatase and tensin homolog (PTEN), such as LNCaP cells, androgen activation of Akt-dependent pathway requires AR location at lipid rafts, where the receptor recruits and activates kinases other than PI3-K, such as integrin-linked kinases or the raptor-mammalian target of rapamycin (mTOR) complex. Finally, these effectors activate Akt independently of PI3-K.
In summary, AR interacts with several signaling effectors or scaffolds or co-regulators that act in a variety of sub-cellular locations, bridging AR with the signaling machinery, modulating AR nuclear import–export, influencing DNA binding and gene transcription. Further investigation of these interactions may offer a way of assessing new molecules that might improve our knowledge of androgen biology in target tissues.
Conclusions
Many events regulate sex steroid receptor localization and their derangement is involved in progression of human diseases, mainly proliferative disorders. Methylation of ER alpha by protein arginine N-methyltransferase 1 (PRMT1), for instance, is required for MCF-7 cell cycle progression (Le Romancer et al. 2008). This correlates with localization of ER alpha in cytoplasm, where recruitment and activation of Src, PI3-K and FAK occur (Le Romancer et al. 2008). Notably, a subset of human breast cancer specimens displays high levels of cytoplasmic methylated ER alpha (Le Romancer et al. 2008). Expression of a shortened form of the metastatic tumor antigen 1 (MTA1s) traps ER in the cytoplasm and leads to malignant phenotypes by enhancing ER non-genomic functions in hormone-dependent breast cancer cells (Kumar et al. 2002). Breast and prostate tumors develop resistance to endocrine-based therapeutic treatments as they progress. Remarkably, the majority of resistant breast cancers retain high levels of ER alpha or PR. In addition, AR is expressed throughout prostate cancer progression. In these resistant tumors, the rapid action of extra-nuclear steroid receptors could be activated by extremely low or sub-threshold hormonal concentrations or growth factors. Thus, cytoplasm/membrane localization of sex steroid receptors might impact breast and prostate cancer progression by controlling signal transduction-dependent functions (ie cell cycle progression, anchorage-dependent growth), and SRs recruited to cell membrane may inappropriately trigger gene transcription independently of receptor nuclear localization.
Studies in cultured cells and animals have revealed important details regarding SR non-genomic regulatory complexes and their functional role. It is now appreciated that SRs interact with a plethora of signaling molecules or scaffolds or co-regulators acting in a variety of sub-cellular locations. These proteins link sex steroid receptors with basic signaling or transcriptional machinery and modulate receptor nucleo-cytoplasmic shuttling. Much evidence has shown that signaling pathway activation regulates, albeit at different levels, ER, PR, AR and GR functions by controlling receptor localization in target cells. In this review we have described the main signaling effectors controlling location of SRs and their functions in target cells. Many of these regulatory mechanisms have been discovered to date. Advances in this field may provide new insights into receptor modulation of signaling-dependent cell proliferation, invasiveness and even hormone resistance in breast and prostate cancers. Lastly, the studies so far reported raise the possibility that small molecules affecting the import–export cycle of SRs maybe efficacious in the treatment of human breast and prostate cancers.
Acknowledgements
This work was supported by grants from Associazione Italiana per la Ricerca sul Cancro.
M. Di Donato is an AIRC fellowship recipient.
We declare that we do not have competing financial interests.
References
- Acconcia F, Ascenzi P, Bocedi A, Spisni E, Tomasi V, Trentalance A, Visca P, Marino M. Palmitoylation-dependent estrogen receptor alpha membrane localization: regulation by 17beta-estradiol. Mol Biol Cell. 2005;16:231–237. doi: 10.1091/mbc.E04-07-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. doi: 10.1016/S0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- Ballaré C, Uhrig M, Bechtold T, Sancho E, Domenico M, Migliaccio A, Auricchio F, Beato M. Two domains of the progesterone receptor interact with the estrogen receptor and are required for progesterone activation of the c-Src/Erk pathway in mammalian cells. Mol Cell Biol. 2003;23:1994–2008. doi: 10.1128/MCB.23.6.1994-2008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedolla RG, Wang Y, Asuncion A, Chamie K, Siddiqui S, Mudryj MM, Prihoda TJ, Siddiqui J, Chinnaiyan AM, Mehra R, Vere White RW, Ghosh PM. Nuclear versus cytoplasmic localization of filamin A in prostate cancer: immunohistochemical correlation with metastases. Clin Cancer Res. 2009;15:788–796. doi: 10.1158/1078-0432.CCR-08-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black BE, Holaska JM, Rastinejad F, Paschal BM. DNA binding domains in diverse nuclear receptors function as nuclear export signals. Curr Biol. 2001;11:1749–1758. doi: 10.1016/S0960-9822(01)00537-1. [DOI] [PubMed] [Google Scholar]
- Boonyaratanakornkit V, Scott MP, Ribon V, Sherman L, Anderson SM, Maller JL, Miller WT, Edwards DP. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol Cell. 2001;8:269–280. doi: 10.1016/S1097-2765(01)00304-5. [DOI] [PubMed] [Google Scholar]
- Boonyaratanakornkit V, McGowan E, Sherman L, Mancini MA, Cheskis BJ, Edwards DP. The role of extranuclear signaling actions of progesterone receptor in mediating progesterone regulation of gene expression and the cell cycle. Mol Endocrinol. 2007;21:359–375. doi: 10.1210/me.2006-0337. [DOI] [PubMed] [Google Scholar]
- Castoria G, Barone MV, Domenico M, Bilancio A, Ametrano D, Migliaccio A, Auricchio F. Non-transcriptional action of estrogen and progestin triggers DNA synthesis. EMBO J. 1999;18:2500–2510. doi: 10.1093/emboj/18.9.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castoria G, Migliaccio A, Bilancio A, Domenico M, Falco A, Lombardi M, Fiorentino R, Varricchio L, Barone MV, Auricchio F. PI3-kinase in concert with Src promotes the S-phase entry of estradiol-stimulated MCF-7 cells. EMBO J. 2001;20:6050–6059. doi: 10.1093/emboj/20.21.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castoria G, Lombardi M, Barone MV, Bilancio A, Domenico M, Bottero D, Vitale F, Migliaccio A, Auricchio F. Androgen-stimulated DNA synthesis and cytoskeletal changes in fibroblasts by a nontranscriptional receptor action. J Cell Biol. 2003;161:547–556. doi: 10.1083/jcb.200211099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castoria G, Migliaccio A, D’Amato L, Stasio R, Ciociola A, Lombardi M, Bilancio A, Domenico M, Falco A, Auricchio F. Integrating signals between cAMP and MAPK pathways in breast cancer. Front Biosci. 2008;13:1318–1327. doi: 10.2741/2764. [DOI] [PubMed] [Google Scholar]
- Castoria G, D’Amato L, Ciociola A, Giovannelli P, Giraldi T, Sepe L, Paolella G, Maria Vittoria Barone MV, Migliaccio A, Auricchio F (2010) Androgen-induced cell migration: role of AR/filamin A association. PloSOne, (in press) [DOI] [PMC free article] [PubMed]
- Chambliss KL, Shaul PW. Estrogen modulation of endothelial nitric oxide synthase. Endocr Rev. 2002;23:665–686. doi: 10.1210/er.2001-0045. [DOI] [PubMed] [Google Scholar]
- Cinar B, Mukhopadhyay NK, Meng G, Freeman MR. Phosphoinositide 3-kinase-independent non-genomic signals transit from the androgen receptor to Akt1 in membrane raft microdomains. J Biol Chem. 2007;282:29584–29593. doi: 10.1074/jbc.M703310200. [DOI] [PubMed] [Google Scholar]
- Damdimopoulos AE, Spyrou G, Gustafsson JA. Ligands differentially modify the nuclear mobility of estrogen receptors alpha and beta. Endocrinology. 2008;149:339–345. doi: 10.1210/en.2007-0198. [DOI] [PubMed] [Google Scholar]
- Dauvois S, White R, Parker MG. The antiestrogen ICI 182780 disrupts estrogen receptor nucleocytoplasmic shuttling. J Cell Sci. 1993;106:1377–1388. doi: 10.1242/jcs.106.4.1377. [DOI] [PubMed] [Google Scholar]
- Faivre EJ, Lange CA. Progesterone receptors upregulate Wnt-1 to induce epidermal growth factor receptor transactivation and c-Src-dependent sustained activation of Erk1/2 mitogen-activated protein kinase in breast cancer cells. Mol Cell Biol. 2007;2:466–480. doi: 10.1128/MCB.01539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fizazi K, Sternberg CN, Fitzpatrick JM, Watson RW, Tabesh M. Role of targeted therapy in the treatment of advanced prostate cancer. BJU Int. 2010;105:748–767. doi: 10.1111/j.1464-410X.2010.09236.x. [DOI] [PubMed] [Google Scholar]
- Fu XD, Giretti MS, Baldacci C, Garibaldi S, Flamini M, Sanchez AM, Gadducci A, Genazzani AR, Simoncini T. Extra-nuclear signaling of progesterone receptor to breast cancer cell movement and invasion through the actin cytoskeleton. PLoS ONE. 2008;3:e2790. doi: 10.1371/journal.pone.0002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzo P, Caiazza F, Moreno S, Marino M. Role of ERbeta palmitoylation in the inhibition of human colon cancer cell proliferation. Endocr Relat Cancer. 2007;14:153–167. doi: 10.1677/ERC-06-0020. [DOI] [PubMed] [Google Scholar]
- Gioeli D, Black BE, Gordon V, Spencer A, Kesler CT, Eblen ST, Paschal BM, Weber MJ. Stress kinase signaling regulates androgen receptor phosphorylation, transcription, and localization. Mol Endocrinol. 2006;20:503–515. doi: 10.1210/me.2005-0351. [DOI] [PubMed] [Google Scholar]
- Giretti MS, Fu XD, Rosa G, Sarotto I, Baldacci C, Garibaldi S, Mannella P, Biglia N, Sismondi P, Genazzani AR, Simoncini T. Extra-nuclear signalling of estrogen receptor to breast cancer cytoskeletal remodelling, migration and invasion. PLoS ONE. 2008;3:e2238. doi: 10.1371/journal.pone.0002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiochon-Mantel A, Loosfelt H, Lescop P, Sar S, Atger M, Perrot-Applanat M, Milgrom E. Mechanisms of nuclear localization of the progesterone receptor: evidence for interaction between monomers. Cell. 1989;57:1147–1154. doi: 10.1016/0092-8674(89)90052-4. [DOI] [PubMed] [Google Scholar]
- Hammes SR, Levin ER. Extranuclear steroid receptors: nature and actions. Endocr Rev. 2007;28:726–741. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
- Henderson BR, Eleftheriou A. A comparison of the activity, sequence specificity, and CRM1-dependence of different nuclear export signals. Exp Cell Res. 2000;256:213–224. doi: 10.1006/excr.2000.4825. [DOI] [PubMed] [Google Scholar]
- Karteris E, Zervou S, Pang Y, Dong J, Hillhouse EW, Randeva HS, Thomas P. Progesterone signaling in human myometrium through two novel membrane G protein-coupled receptors: potential role in functional progesterone withdrawal at term. Mol Endocrinol. 2006;20:1519–1534. doi: 10.1210/me.2005-0243. [DOI] [PubMed] [Google Scholar]
- Kousteni S, Bellido T, Plotkin LI, O’Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104:719–730. [PubMed] [Google Scholar]
- Kumar R, Wang RA, Mazumdar A, Talukder AH, Mandal M, Yang Z, Bagheri-Yarmand R, Sahin A, Hortobagyi G, Adam L, Barnes CJ, Vadlamudi RK. A naturally occurring MTA1 variant sequesters oestrogen receptor-alpha in the cytoplasm. Nature. 2002;418:654–657. doi: 10.1038/nature00889. [DOI] [PubMed] [Google Scholar]
- Joliot A, Prochiantz A. Transduction peptides: from technology to physiology. Nat Cell Biol. 2004;6:189–196. doi: 10.1038/ncb0304-189. [DOI] [PubMed] [Google Scholar]
- Romancer M, Treilleux I, Leconte N, Robin-Lespinasse Y, Sentis S, Bouchekioua-Bouzaghou K, Goddard S, Gobert-Gosse S, Corbo L. Regulation of estrogen rapid signaling through arginine methylation by PRMT1. Mol Cell. 2008;31:212–221. doi: 10.1016/j.molcel.2008.05.025. [DOI] [PubMed] [Google Scholar]
- Liu J, DeFranco DB. Protracted nuclear export of glucocorticoid receptor limits its turnover and does not require the exportin 1/CRM1-directed nuclear export pathway. Mol Endocrinol. 2000;14:40–51. doi: 10.1210/me.14.1.40. [DOI] [PubMed] [Google Scholar]
- Lombardi M, Castoria G, Migliaccio A, Barone MV, Stasio R, Ciociola A, Bottero D, Yamaguchi H, Appella E, Auricchio F. Hormone-dependent nuclear export of estradiol receptor and DNA synthesis in breast cancer cells. J Cell Biol. 2008;182:327–340. doi: 10.1083/jcb.200712125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu ML, Schneider MC, Zheng Y, Zhang X, Richie JP. Caveolin-1 interacts with androgen receptor. A positive modulator of androgen receptor mediated transactivation. J Cell Biol. 2001;276:13442–13451. doi: 10.1074/jbc.M006598200. [DOI] [PubMed] [Google Scholar]
- Marcelli M, Stenoien DL, Szafran AT, Simeoni S, Agoulnik IU, Weigel NL, Moran T, Mikic I, Price JH, Mancini MA. Quantifying effects of ligands on androgen receptor nuclear translocation, intranuclear dynamics, and solubility. J Cell Biochem. 2006;98:770–788. doi: 10.1002/jcb.20593. [DOI] [PubMed] [Google Scholar]
- Maruvada P, Baumann CT, Hager GL, Yen PM. Dynamic shuttling and intranuclear mobility of nuclear hormone receptors. J Biol Chem. 2003;278:12425–12432. doi: 10.1074/jbc.M202752200. [DOI] [PubMed] [Google Scholar]
- McKenna NJ, O’Malley BW. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell. 2002;108:465–474. doi: 10.1016/S0092-8674(02)00641-4. [DOI] [PubMed] [Google Scholar]
- Migliaccio A, Piccolo D, Castoria G, Domenico M, Bilancio A, Lombardi M, Gong W, Beato M, Auricchio F. Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. EMBO J. 1998;17:2008–2018. doi: 10.1093/emboj/17.7.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio A, Castoria G, Domenico M, Falco A, Bilancio A, Lombardi M, Barone MV, Ametrano D, Zannini MS, Abbondanza C, Auricchio F. Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. EMBO J. 2000;19:5406–5417. doi: 10.1093/emboj/19.20.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio A, Domenico M, Castoria G, Nanayakkara M, Lombardi M, Falco A, Bilancio A, Varricchio L, Ciociola A, Auricchio F. Steroid receptor regulation of EGF signaling through Src in breast and prostate cancer cells: steroid antagonist action. Cancer Res. 2005;65:10585–10593. doi: 10.1158/0008-5472.CAN-05-0912. [DOI] [PubMed] [Google Scholar]
- Migliaccio A, Varricchio L, Falco A, Castoria G, Arra C, Yamaguchi H, Ciociola A, Lombardi M, Stasio R, Barbieri A, Baldi A, Barone MV, Appella E, Auricchio F. Inhibition of the SH3 domain-mediated binding of Src to the androgen receptor and its effect on tumor growth. Oncogene. 2007;26:6619–6629. doi: 10.1038/sj.onc.1210487. [DOI] [PubMed] [Google Scholar]
- Migliaccio A, Castoria G, Auricchio F (2010) Non genomic action of sex steroid hormones. In: Bunce CM, Campbell MJ (eds), Nuclear Receptors, Proteins and Cell Regulation vol 8, pp 365–379. doi:10.1007/978-90-481-3303-1_15. Springer Science and Business Media BV
- Morelli C, Lanzino M, Garofalo C, Maris P, Brunelli E, Casaburi I, Catalano S, Bruno R, Sisci D, Andò S. Akt2 inhibition enables the forkhead transcription factor FoxO3a to have a repressive role in estrogen receptor alpha transcriptional activity in breast cancer cells. Mol Cell Biol. 2010;30:857–870. doi: 10.1128/MCB.00824-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N, Ramaswamy S, Vazquez F, Signoretti S, Loda M, Sellers WR. Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Mol Cell Biol. 2000;20:8969–8982. doi: 10.1128/MCB.20.23.8969-8982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozanne DM, Brady ME, Cook S, Gaughan L, Neal DE, Robson CN. Androgen receptor nuclear translocation is facilitated by the F-actin cross-linking protein filamin. Mol Endocrinol. 2000;14:1618–1626. doi: 10.1210/me.14.10.1618. [DOI] [PubMed] [Google Scholar]
- Papakonstanti EA, Kampa M, Castanas E, Stournaras C. A rapid, nongenomic, signaling pathway regulates the actin reorganization induced by activation of membrane testosterone receptors. Mol Endocrinol. 2003;17:870–881. doi: 10.1210/me.2002-0253. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Sainson RC, Kim JK, Hughes CC, Levin ER. A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem. 2007;282:22278–22288. doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- Pemberton LF, Paschal BM. Mechanisms of receptor-mediated nuclear import and nuclear export. Traffic. 2005;6:187–198. doi: 10.1111/j.1600-0854.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- Peterziel H, Mink S, Schonert A, Becker M, Klocker H, Cato AC. Rapid signalling by androgen receptor in prostate cancer cells. Oncogene. 1999;18:6322–6329. doi: 10.1038/sj.onc.1203032. [DOI] [PubMed] [Google Scholar]
- Picard N, Charbonneau C, Sanchez M, Licznar A, Busson M, Lazennec G, Tremblay A. Phosphorylation of activation function-1 regulates proteasome-dependent nuclear mobility and E6-associated protein ubiquitin ligase recruitment to the estrogen receptor beta. Mol Endocrinol. 2008;22:317–330. doi: 10.1210/me.2007-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott J, Coetzee GA. Molecular chaperones throughout the life cycle of the androgen receptor. Cancer Lett. 2006;231:12–19. doi: 10.1016/j.canlet.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Price D, Stein B, Sieber P, Tutrone R, Bailen J, Goluboff E, Burzon D, Bostwick D, Steiner M. Toremifene for the prevention of prostate cancer in men with high grade prostatic intraepithelial neoplasia: results of a double-blind, placebo controlled, phase IIB clinical trial. J Urol. 2006;176:965–970. doi: 10.1016/j.juro.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Qiu M, Olsen A, Faivre E, Horwitz KB, Lange CA. Mitogen-activated protein kinase regulates nuclear association of human progesterone receptors. Mol Endocrinol. 2003;17:628–642. doi: 10.1210/me.2002-0378. [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Levin ER. Heat shock protein 27 is required for sex steroid receptor trafficking to and functioning at the plasma membrane. Mol Cell Biol. 2010;30:3249–3261. doi: 10.1128/MCB.01354-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbridger GP, Davis ID, Birrell SN, Tilley WD. Breast and prostate cancer: more similar than different. Nat Rev Cancer. 2010;10:205–212. doi: 10.1038/nrc2795. [DOI] [PubMed] [Google Scholar]
- Saitoh M, Ohmichi M, Takahashi K, Kawagoe J, Ohta T, Doshida M, Takahashi T, Igarashi H, Mori-Abe A, Du B, Tsutsumi S, Kurachi H. Medroxyprogesterone acetate induces cell proliferation through up-regulation of cyclin D1 expression via phosphatidylinositol 3-kinase/Akt/nuclear factor-kappaB cascade in human breast cancer cells. Endocrinology. 2005;146:4917–4925. doi: 10.1210/en.2004-1535. [DOI] [PubMed] [Google Scholar]
- Saporita AJ, Zhang Q, Navai N, Dincer Z, Hahn J, Cai X, Wang Z. Identification and characterization of a ligand-regulated nuclear export signal in androgen receptor. J Biol Chem. 2003;278:41998–42005. doi: 10.1074/jbc.M302460200. [DOI] [PubMed] [Google Scholar]
- Savory JG, Hsu B, Laquian IR, Giffin W, Reich T, Haché RJ, Lefebvre YA. Discrimination between NL1- and NL2-mediated nuclear localization of the glucocorticoid receptor. Mol Cell Biol. 1999;19:1025–1037. doi: 10.1128/mcb.19.2.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuur ER, Loktev AV, Sharma M, Sun Z, Roth RA, Weigel RJ. Ligand-dependent interaction of estrogen receptor-alpha with members of the forkhead transcription factor family. J Biol Chem. 2001;276:33554–33560. doi: 10.1074/jbc.M105555200. [DOI] [PubMed] [Google Scholar]
- Shank LC, Kelley JB, Gioeli D, Yang CS, Spencer A, Allison LA, Paschal BM. Activation of the DNA-dependent protein kinase stimulates nuclear export of the androgen receptor in vitro. J Biol Chem. 2008;283:10568–80. doi: 10.1074/jbc.M800810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoncini T, Scorticati C, Mannella P, Fadiel A, Giretti MS, Fu XD, Baldacci C, Garibaldi S, Caruso A, Fornari L, Naftolin F, Genazzani AR. Estrogen receptor alpha interacts with Galpha13 to drive actin remodeling and endothelial cell migration via the RhoA/Rho kinase/moesin pathway. Mol Endocrinol. 2006;20:1756–1771. doi: 10.1210/me.2005-0259. [DOI] [PubMed] [Google Scholar]
- Song RX, McPherson RA, Adam L, Bao Y, Shupnik M, Kumar R, Santen RJ. Linkage of rapid estrogen action to MAPK activation by ERalpha-Shc association and Shc pathway activation. Mol Endocrinol. 2002;16:116–127. doi: 10.1210/me.16.1.116. [DOI] [PubMed] [Google Scholar]
- Song RX, Barnes CJ, Zhang Z, Bao Y, Kumar R, Santen RJ. The role of Shc and insulin-like growth factor 1 receptor in mediating the translocation of estrogen receptor alpha to the plasma membrane. Proc Natl Acad Sci USA. 2004;101:2076–2081. doi: 10.1073/pnas.0308334100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenoien DL, Nye AC, Mancini MG, Patel K, Dutertre M, O’Malley BW, Smith CL, Belmont AS, Mancini MA. Ligand-mediated assembly and real-time cellular dynamics of estrogen receptor alpha-coactivator complexes in living cells. Mol Cell Biol. 2001;21:4404–4412. doi: 10.1128/MCB.21.13.4404-4412.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi RK, Amazit L, Lescop P, Milgrom E, Guiochon-Mantel A. Mechanisms of progesterone receptor export from nuclei: role of nuclear localization signal, nuclear export signal, and ran guanosine triphosphate. Mol Endocrinol. 1998;12:1684–1695. doi: 10.1210/me.12.11.1684. [DOI] [PubMed] [Google Scholar]
- Tyagi RK, Lavrovsky Y, Ahn SC, Song CS, Chatterjee B, Roy AK. Dynamics of intracellular movement and nucleocytoplasmic recycling of the ligand-activated androgen receptor in living cells. Mol Endocrinol. 2000;14:1162–1174. doi: 10.1210/me.14.8.1162. [DOI] [PubMed] [Google Scholar]
- Vallejo G, Ballaré C, Barañao JL, Beato M, Saragüeta P. Progestin activation of nongenomic pathways via cross talk of progesterone receptor with estrogen receptor beta induces proliferation of endometrial stromal cells. Mol Endocrinol. 2005;19:3023–3037. doi: 10.1210/me.2005-0016. [DOI] [PubMed] [Google Scholar]
- Heide LP, Hoekman MF, Smidt MP. The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem J. 2004;380:297–309. doi: 10.1042/BJ20040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicent GP, Ballaré C, Nacht AS, Clausell J, Subtil-Rodríguez A, Quiles I, Jordan A, Beato M. Induction of progesterone target genes requires activation of Erk and Msk kinases and phosphorylation of histone H3. Mol Cell. 2006;24:367–381. doi: 10.1016/j.molcel.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Vicent GP, Nacht AS, Zaurín R, Ballaré C, Clausell J, Beato M. Role of kinases and chromatin remodeling in progesterone signaling to chromatin. Mol Endocrinol. 2010 doi: 10.1210/me.2010-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Johnson M, Le KH, Sato M, Ilagan R, Iyer M, Gambhir SS, Wu L, Carey M. Interrogating androgen receptor function in recurrent prostate cancer. Cancer Res. 2003;63:4552–4560. [PubMed] [Google Scholar]
- Zhao HH, Herrera RE, Coronado-Heinsohn E, Yang MC, Ludes-Meyers JH, Seybold-Tilson KJ, Nawaz Z, Yee D, Barr FG, Diab SG, Brown PH, Fuqua SA, Osborne CK. Forkhead homologue in rhabdomyosarcoma functions as a bifunctional nuclear receptor-interacting protein with both coactivator and corepressor functions. J Biol Chem. 2001;276:27907–27912. doi: 10.1074/jbc.M104278200. [DOI] [PubMed] [Google Scholar]
- Zheng FF, Wu RC, Smith CL, O’Malley BW. Rapid estrogen-induced phosphorylation of the SRC-3 coactivator occurs in an extranuclear complex containing estrogen receptor. Mol Cell Biol. 2005;25:8273–8284. doi: 10.1128/MCB.25.18.8273-8284.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci USA. 2003;100:2231–2236. doi: 10.1073/pnas.0336132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Tsai WB, Cheng CJ, Hsu C, Chung YM, Li PC, Lin SH, Hu MC. Forkhead box transcription factor FOXO3a suppresses estrogen-dependent breast cancer cell proliferation and tumorigenesis. Breast Cancer Res. 2008;10:R21. doi: 10.1186/bcr1872. [DOI] [PMC free article] [PubMed] [Google Scholar]