Abstract
Human sweat gland epithelial cells (SGECs) have been isolated and grown in vitro, However, slow proliferation makes the culture of these cells extremely difficult. The present study was carried out to explore the modified culture medium for SGECs in vitro. Full-thickness skin samples were minced (1 mm3) and digested overnight with type II collagenase. The gland coils were removed under an inverted phase-contrast microscope. An adherent culture method was used to isolate and culture SGECs. Staining with hematoxylin and eosin was performed, followed by observation of the morphologic features of these cells. Immunofluorescence staining with antibodies to cytokeratins CK7, CK18, and CK19 and carcinoembryonic antigen (CEA) was performed to verify the presence of SGECs. Growth curves by MTT were created for cells grown in serum-free keratinocyte medium and in modified keratinocyte medium containing 2.5% fetal bovine serum (FBS). One week after culturing, the cells grew well and were polygonal or irregular in shape by inverted phase contrast microscopy. Cell fusion, with a characteristic paving-stone arrangement, reached 100% after approximately 3 weeks in culture. Immunofluorescence staining indicated expression of CK7, CK18, CK19, and CEA. Compared with SGECs grown in serum-free keratinocyte medium, the proliferation of SGECs grown in modified culture medium with low concentration of FBS at days 6, 9, and 12 was significantly accelerated (p < 0.05). This study suggests that keratinocyte medium supplemented with 2.5% FBS is effective and suitable for the culture of SGECs.
Electronic supplementary material
The online version of this article (doi:10.1007/s10616-010-9303-z) contains supplementary material, which is available to authorized users.
Keywords: Sweat gland, Epithelial cell, Cell, Culture, Modified culture medium
Introduction
The sweat gland, one of the most important appendages in the skin, is involved in secretion, excretion, and thermoregulation. Reconstruction of sweat glands has been an obstacle in skin tissue engineering. The isolation and culture of sweat gland epithelial cells (SGECs) can provide powerful tools for studying the structure and function of sweat glands at the cellular level. The isolation and culture of SGECs is very time consuming (Hongpaisan et al. 1996; Lei et al. 2008), and methods should be improved. In the present study, mechanical isolation, enzymatic digestion, and an adherent culture method were used to isolate and culture human SGECs. In addition, the traditional culture medium was modified, culture efficiency was assessed, and the presence of SGECs was verified. Results of this study provide an experimental basis for skin tissue engineering of the sweat gland.
Materials and methods
Reagents and samples
Full-thickness skin samples were obtained from the axillary region of patients undergoing plastic Surgery in the First Hospital Affiliated to General Hospital of PLA. Informed consent was obtained from all patients before skin biopsy. Type II collagenase (Gibco BRL, USA), serum-free keratinocyte media (FSKM; Gibco), epidermal growth factor (EGF; Gibco), bovine pituitary extract (Gibco), and an inverted microscope and photo acquisition system (Leica, DMI6000, German) were used in the present study. Primary antibodies to cytokeratins CK7, CK18, CK19 and carcinoembryonic antigen (CEA), and secondary antibodies conjugated to phycoerythrin (PE) or fluorescein isothiocyanate (FITC) were purchased from Abcam (Cambridge, UK).
Isolation and culture of sweat gland epithelial cells
For tissue culture, FSKM was supplemented with 5 μg/L EGF and 50 mg/L bovine pituitary extract. Full-thickness intact skin samples (0.3 cm × 2 cm) were rinsed with D-Hank buffer, and subcutaneous fat was removed. The skin was minced (1 mm3) with sharp scissors in a culture plate (60 mm in diameter). The pieces were then incubated with type II collagenase (3 mL) at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. On the following day, the gland coils were removed under an inverted phase-contrast microscope and incubated in 0.6 mL culture medium at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. When the sweat gland tissues adhered to the bottom, an additional 2 mL of culture medium was added, and the medium was changed every 2–3 days.
Purification of sweat gland epithelial cells
Under an inverted phase-contrast microscope, regions containing SGECs were distinguishable from fibroblasts and marked on each plate. In primary culture, fibroblasts were usually mixed with SGECs, which may grow either in groups or scattered. As SGECs and fibroblasts have different tolerances to trypsin, fibroblasts were detached from the flask wall first while the SGECs remained attached when digested with trypsin at appropriate time. Fibroblasts were then washed away and SGECs were left in the flask. The purification of SGEC was performed as following: the medium was removed, and the cells were rinsed with D-Hank buffer twice, followed by digestion with trypsin (2 mL) at 37 °C in a humidified atmosphere of 5% CO2 and 95% air for 2 min. When the fibroblasts contracted, and no changes were observed in SGECs, digestion was terminated by the addition of 2 mL of culture medium containing 10% FBS. Fibroblasts were detached with a transferpettor and removed by washing twice with D-Hank buffer, followed by the addition of culture medium. The above procedure was repeated as necessary.
Cell passaging and creation of growth curve
Primary SGECs were harvested conventionally by digestion and suspended in FSKM. The harvested SGECs were divided randomly into two groups. One was grown in FSKM, and the other was grown in FSKM supplemented with 2.5% fetal bovine serum (FBS, Gibco) (Low FBS). Cells were seeded onto 12-well culture plates at a density of 1 × 104 cells/cm2 and incubated at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. The medium was changed every 3 days. The number of SGECs in 3 wells was counted on days 3, 6, 9, 12, 15, and 18, and the average was used to create the growth curve.
Hematoxylin-and-eosin and immunofluorescence staining
Cells were fixed in ice-cold acetone, and hematoxylin-and-eosin (HE) and immunofluorescence staining were performed. The cells were incubated with primary antibodies (to CK7, CK18, CK19, and to CEA) at 37 °C for 30 min, followed by washing with phosphate-buffered saline. The cells were then incubated with secondary antibodies and nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI), followed by observation under an inverted fluorescence microscope. Meanwhile, the cells were stained with secondary antibody only as negative control. Human neuroblastoma cells (SY5Y) and isolated fibroblasts were stained with CK7, CK18, CK19 and CEA as negative control. Normal human skin tissue was stained using CK7, CK18, CK19 and CEA as positive control.
Statistical analysis
Statistical analysis was performed with SPSS 10.0 statistics software (SPSS Inc. Chicago, IL, USA). Data are presented as mean ± SD and Student t test was used for statistical analysis. A value of p < 0.05 was considered statistically significant.
Results
Isolation and culture of sweat gland epithelial cells
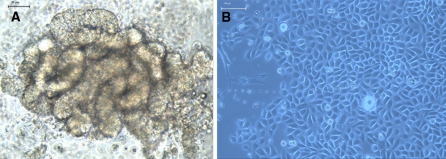
After digestion with type II collagenase, a fraction of characteristically curve-shaped gland coils was freed (Fig. 1a). The time required for SGECs to become adherent varied from 1 to 7 days, with a majority of cells adhering to the plate at 48 h. Sweat gland epithelial cells were found to detach from gland coils and were polygonal in shape. The cells continued to divide for up to 2 weeks and formed a circular monolayer surrounding the tissues. A minority of the cells continued to divide and formed multiple layers with a paving-stone arrangement (Fig. 1b). Microscopy indicated that the regions with SGECs were thickened. The cells began to senesce approximately after 3 weeks, with the development of vacuoles accompanied by the cessation of cell division.
Fig. 1.
Cultured primary sweat gland epithelial cells under an inverted phase-contrast microscope. a the culture on the first day, The eccrine sweat glands had a clear aspect, and formed an irregular ball in the single-curve tube shape (×200), bar = 50 μm. b The culture on the 12th day, The shape was polygon-like and they grew like cobblestone (×200), bar = 100 μm
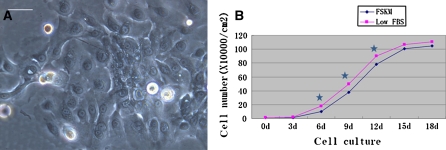
Purification and cell proliferation
No fibroblasts were observed in the passaged SGEC cultures. Over time, the cell volume increased, accompanied by the formation of irregular cell shapes and pseudopodia (Fig. 2a). The growth curve was plotted based on the number of cells at the indicated time points (Fig. 2b). Statistical analysis indicated that cell numbers in the low FBS group at days 6, 9, and 12 were significantly greater than those for cells grown in FSKM at the corresponding time points (p < 0.05).
Fig. 2.
Morphological characteristics and growth state of first passage sweat gland epithelial cells. a the P1 cell culture on the third day, the cell volume increased, and formed irregular cell shapes and pseudopodia (×200), bar = 50 μm. b The growth curve of the human eccrine sweat gland epithelial cells with two different media (star indicating p < 0.05)
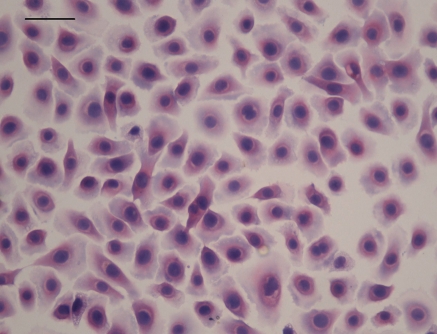
Staining with hematoxylin and eosin
A state of 100% cell confluence was observed in the FBS group after 15 days in culture. Staining with HE indicated that the confluent cell monolayer developed into multiple cell layers with a characteristic paving-stone arrangement. Nuclei were round, and the cell shape was polygonal or oval. A minority of cells showed an irregular shape (Fig. 3).
Fig. 3.
Morphological characteristic of cultured primary sweat gland epithelial cells on 15th day (×200), bar = 50 μm. HE staining
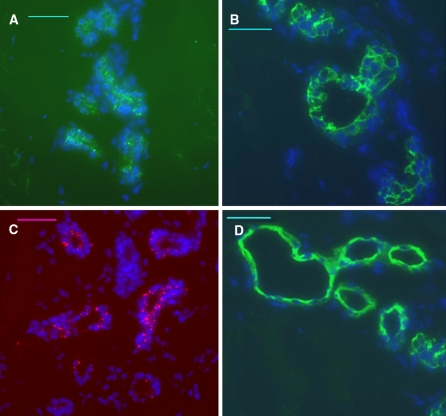
Immunocytochemical identification of sweat gland epithelial cells
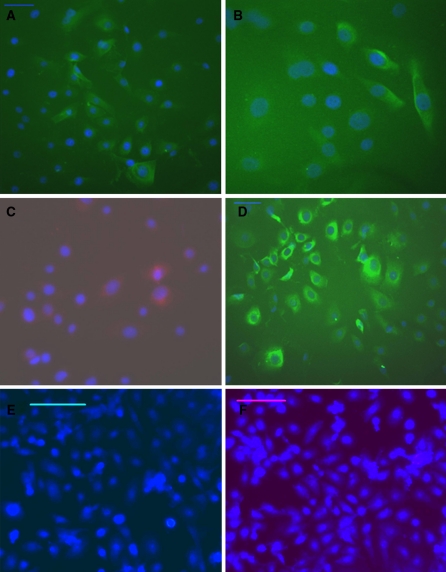
Staining for the sweat gland in human skin tissue with CK7, CK18, CK19 and CEA antibodies was positive (Fig. 4). Immunofluorescence staining indicated that these isolated cells were positive for CK7 (Fig. 5a), CK18 (Fig. 5b), CK19 (Fig. 5c), and CEA (Fig. 5d). Staining of these cells with secondary antibodies only (Fig. 5e, f), and staining for human neuroblastoma cells (Supplemental Fig. 1) and fibroblasts (Supplemental Fig. 2, a: cultured fibroblast at third passage, b: CK7 staining for fibroblast, c: CK18 staining for fibroblast, d: CEA staining for fibroblast, e: CK19 staining for fibroblast. Scale bar, 100 μm) were used as negative controls, and the staining results were all negative. These results indicated that the isolated cells were indeed SGECs.
Fig. 4.
Immunofluorescence staining of normal human skin tissue (×200), bar = 50 μm. The sweat glands in human skin tissue were positive for CK7, CK18, CK19 and CEA. The nucleus was stained blue with DAPI. (a CK7, b CK18, c CK19, d CEA)
Fig. 5.
Immunofluorescence staining of passaged sweat gland epithelial cells on the second day (×200), bar = 50 μm (a, b, c, d), 100 μm (e, f). Cultured cells were positive for CK7, CK18, CK19, CEA, and strongly marked the cytoplasm with a green or red color (a–d), staining with the only secondary antibody (e, f) as negative control without primary antibody. The nucleus was stained blue with DAPI. (a CK7, b CK18, c CK19, d CEA, e IgG-FITC, f IgG-PE)
Discussion
Loss and dysfunction of sweat glands are frequently observed clinically. In patients with massive burns, the sweat glands are destroyed or the secretory duct is obstructed by scar tissue, which leads to abnormal sweat secretion (Schon et al. 1999), resulting in a poor quality of life and causing distress to patients. The only way to restore sweat secretion is to replace the damaged sweat glands. Therefore, it is important to isolate and culture SGECs and to explore the biological characteristics of these cells.
With the traditional method, few SGECs are harvested (Mork et al. 1995). In the present study, the traditional method was modified and improved. Collagenase was used for digestion, and a transferpettor was used to detach SGECs from tissues. Digestion with collagenase for a limited duration digests collagen in the extracellular matrix, allowing for dissociation of sweat glands from skin tissues, and no adverse effects on the proliferation of sweat glands were observed. Detaching the cells with a transferpettor minimized cell damage while removing other cells. The appropriate amount of medium was indispensible for the adherence and growth of SGECs. Humidity and nutrition were not adequate in too low a volume of medium, and the cells remained in suspension in the presence of too much medium. In the present study, only 0.6 mL of medium was added before adherence, and the sweat gland tissue was supplemented with 2 mL of medium after adherence, which resulted in survival of approximately 70% of the sweat gland tissue.
With the traditional method, harvested SGECs are frequently contaminated by fibroblasts, which show high levels of differentiation and division. The presence of fibroblasts affects the growth of SGECs if they are not removed. It has been hypothesized that fibroblasts cannot survive in serum-free culture medium. Therefore, serum-free medium has been used to remove fibroblasts, which is time consuming. In addition, serum-free medium exerts adverse effects on the growth of SGECs, leading to decreased proliferation. In the present study, based on the hypothesis that fibroblasts are more sensitive to trypsin than are SGECs, we removed fibroblasts with trypsin after a 2-min digestion, with the result that a majority of SGECs survived. In addition, no harmful effects on SGEC proliferation were noted.
Our present results indicated that by 6 days of culture, the proliferation of primary SGECs was markedly increased, becoming maximal at 15 days. No evident proliferation was noted at 18 days. These findings are consistent with a previous report (Lei et al. 2008). Currently, no specific culture medium has been developed for sweat gland cells. Dulbecco modified Eagle medium has been used to culture SGECs (Reddy et al. 1992), but this medium is not optimal. In the present study, FSKM supplemented with 2.5% FBS was used as the culture medium, and the results indicated that this medium accelerated the proliferation of SGECs. Morphologic and immunohistochemical analyses and observation of growth characteristics were performed to qualitatively investigate cultured SGECs. Epithelial cells are characteristically oval, irregular, or polygonal in shape, and the nucleus is round. When the cells fuse, a paving-stone arrangement occurs. Cytokeratin is the main structural protein in epithelial cells and is also a marker of differentiation; it is also specific for epithelial cells. The CK18 is specifically expressed in secretory cells (Schon et al. 1999), and CK19 is expressed in sweat gland cells and sebaceous gland cells. In addition, CEA is expressed within the ducts and secretory coils of eccrine sweat glands (Hammarstrom 1999). Our present results are consistent with previous studies with respect to sweat gland markers (Fu et al. 2005). The strong staining for CK7 in the present study is also consistent with previous reports (Beer et al. 2006). In this study, both negative and positive control experiments were carried out and results are shown in Fig. 4 and 5 and Supplemental Fig. 1 and 2. Our finding that CK7, CK18, and CK19, and particularly CEA, were expressed in the cultured cells indicate that these isolated cells are indeed SGECs and not other types of epithelial cells.
The culture of SGECs is potentially important in biological and clinical fields, and provides a basis for further studies to explore the pathogenesis and pathophysiology of sweat gland-related diseases (Saga 2002). This method is also important with respect to tissue engineering of skin. Our present results confirm the effectiveness of culture medium supplemented with a low concentration of FBS in the culture of SGECs. Interestingly, mesenchymal stem cells (MSCs) derived from umbilical cord have been found to differentiate into several cell types (Secco et al. 2008). Theoretically, with a cell coculture method (Corti et al. 2005), MSCs may be made to differentiate into SGECs. We hope to investigate this potential in future studies.
Conclusion
Our experimental results showed that human sweat gland epithelial cells (SGECs) have been successfully isolated from normal skin sample and abundantly cultured in the modified culture medium. The present study demonstrated that the keratinocyte medium (KM) supplemented with 2.5% FBS was effective and suitable for the culture of SGECs, and it will provide an experimental basis for skin tissue engineering of the sweat gland.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The technical assistance of Dr. Yin Zhou is gratefully acknowledged. The study was supported by grants from the National health public welfare special scientific research foundation of China (200802066) and the People’s Liberation Army health science research foundation of China (06Z054).
Footnotes
Ran Tao and Yanfu Han contributed equally to this work.
References
- Beer GM, Baumuller S, Zech N, Wyss P, Strasser D, Varga Z, Seifert B, Hafner J, Mihic-Probst D. Immunohistochemical differentiation and localization analysis of sweat glands in the adult human axilla. Plast Reconstr Surg. 2006;117:2043–2049. doi: 10.1097/01.prs.0000210681.90799.b1. [DOI] [PubMed] [Google Scholar]
- Corti S, Locatelli F, Papadimitriou D, Strazzer S, Bonato S, Comi GP. Nuclear repmgrmnming and adult stem cell potential. Histol Histopathol. 2005;20:977–986. doi: 10.14670/HH-20.977. [DOI] [PubMed] [Google Scholar]
- Fu X, Li J, Sun X, Sun T, Sheng Z. Epidermal stem cells are the source of sweat glands in human fetal skin: evidence of synergetic development of stem cells, sweat glands, growth factors, and matrix metalloproteinases. Wound Repair Regen. 2005;13:102–108. doi: 10.1111/j.1067-1927.2005.130113.x. [DOI] [PubMed] [Google Scholar]
- Hammarstrom S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999;9:67–81. doi: 10.1006/scbi.1998.0119. [DOI] [PubMed] [Google Scholar]
- Hongpaisan J, Zhang AL, Mork AC, Roomans GM. Use of primary cell cultures and intact isolated glandular epithelia for X-ray microanalysis. J Microsc. 1996;184:22–34. doi: 10.1046/j.1365-2818.1996.1110668.x. [DOI] [PubMed] [Google Scholar]
- Lei X, Wu J, Lu Y, Zhu T. Effects of acetylcholine chloride on intracellular calcium concentration of cultured sweat gland epithelial cells. Arch Dermatol Res. 2008;300:335–341. doi: 10.1007/s00403-008-0847-0. [DOI] [PubMed] [Google Scholar]
- Mork AC, Hongpaisan J, Roomans GM. Ion transport in primary cultures from human sweat gland coils studied with X-ray microanalysis. Cell Biol Int. 1995;19:151–159. doi: 10.1006/cbir.1995.1056. [DOI] [PubMed] [Google Scholar]
- Reddy MM, Bell CL, Quinton PM. Evidence of two discinct epithelial cell types in primary cultures from human sweat gland secretory coil. Am J Physiol. 1992;262:C891–C898. doi: 10.1152/ajpcell.1992.262.4.C891. [DOI] [PubMed] [Google Scholar]
- Saga K. Structure and function of human sweat glands studied with histochemistry and cytochemistry. Prog Histochem Cytochem. 2002;37:323–386. doi: 10.1016/S0079-6336(02)80005-5. [DOI] [PubMed] [Google Scholar]
- Schon M, Benwood J, O’Connell-Willstaedt T, Rheinwald JG. Human Sweat gland myoepithelial cells express a unique set of eytokeratins and reveal the potential for alternative epithdial and mesenehymal diferentiation states in culture. J Cell Sci. 1999;112:1925–1936. doi: 10.1242/jcs.112.12.1925. [DOI] [PubMed] [Google Scholar]
- Secco M, Zucconi E, Vieira NM, Fogaça LL, Cerqueira A, Carvalho MD, Jazedje T, Okamoto OK, Muotri AR, Zatz M. Multipotent stem cells from umbilical cord: cord is richer than blood! Stem Cells. 2008;26:146–150. doi: 10.1634/stemcells.2007-0381. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.