In spite of the detailed understanding reached in the last 50 years about the molecular mechanisms of muscle excitability, the localization of the Cl− conductance, GCl, in muscle has remained highly contended. In this issue, Lueck et al. address this long-standing controversy, measuring directly the Cl− current mediated by the ClC-1 channel using the patch clamp technique and by detailed localization studies in mouse muscle fibers. They convincingly conclude that the vast majority of ClC-1 channels is localized in the surface membrane (sarcolemma) and not in the T-tubules. This result contrasts with several previous reports and requires a reinterpretation of the mechanism by which the Cl− conductance limits T-tubular K+ accumulation.
Skeletal muscle activity consists of a sequence of excitatory and relaxatory events. Excitation relies on the propagation of action potentials (APs) from the neuromuscular junction along the sarcolemma via voltage-dependent Na+ channels. A specialized membrane domain, the T-tubule system, is formed by regularly spaced invaginations of the sarcolemma in correspondence to the M-lines, allowing the efficient spreading of the AP to the muscle interior. The T-tubular membrane forms tight contacts with the sarcoplasmic reticulum (SR) (“triads”). Here, voltage-gated L-type Ca2+ channels (dihydropyridine receptors [DHPRs]) act as voltage sensors that transmit the membrane depolarization to the SR-localized Ca2+ release channels (ryanodine receptors [RYRs]) to liberate Ca2+ from the SR, leading finally to muscle contraction.
Full and fast repolarization of the membrane potential is critical to allow high frequency neuronal stimulation and to prevent Na+ and Ca2+ channel inactivation. Classically, in neurons, the repolarization of the AP is mediated by delayed rectifier K+ channels. In skeletal muscle, in contrast, a large part of the repolarization appears to be mediated by the high background Cl− conductance, GCl (Bretag, 1987). GCl is almost exclusively contributed by the ClC-1 Cl− channel (Steinmeyer et al., 1991), a member of the CLC family of chloride channels and Cl−/H+ antiporters (Zifarelli and Pusch, 2007). In addition, various K+ channels are involved in AP repolarization (Kristensen and Juel, 2010). It is well established that, at rest, ∼80% of the skeletal muscle membrane conductance is carried by GCl and ∼20% by GK (Bretag, 1987). Chloride is passively distributed across the muscle fiber, such that the intracellular [Cl−] is low and ECl∼EK∼Erest∼−90 mV (Bretag, 1987; Allen et al., 2008). Extracellular (and tubular) [Cl−] is high, whereas extracellular (and tubular) [K+] is ∼5 mM. Thus, the large GCl acts like a repolarizing “buffer” system. The importance of the Cl− conductance is best illustrated by the behavior of myotonic muscles. Myotonia is a result of a failure of muscle fibers to relax, such that a voluntary contraction generates spontaneous runs of APs producing muscle stiffness. Two forms of hereditary myotonia linked to dysfunction of Cl− conductance have been characterized, with dominant and recessive mode of transmission, respectively, both caused by mutations in the gene coding for ClC-1 (Koch et al., 1992). Studies in goats and humans demonstrated that myotonic muscles are characterized by a lower GCl (Lipicky and Bryant, 1966; Lipicky et al., 1971).
Although the general role of GCl for the stability of the membrane potential is clear, it was still an open question as to whether ClC-1 is localized in the T-tubules or in the sarcolemma. This long-standing controversy has now been addressed by Lueck et al. (2010).
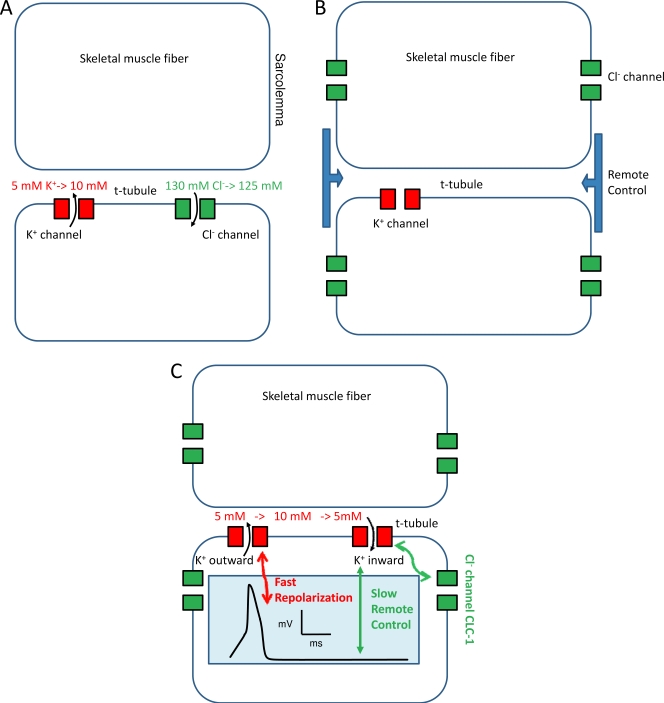
A priori it may seem advantageous to have GCl in the T-tubule for the reasons illustrated in Fig. 1 A: repolarization of each AP leads to an increase in the extracellular and tubular [K+]. Repetitive firing can lead to a rise of [K+] of up to 10 mM (Allen et al., 2008). This problem is especially severe in the restricted T-tubular space from which K+ cannot quickly diffuse away. Increased [K+] will depolarize the membrane potential, leading possibly to nerve-independent AP initiation and eventually to Na+ channel inactivation. If, instead, repolarization is mediated mostly by Cl− channels, the necessary influx of Cl− will only slightly reduce the high tubular [Cl−], thus minimizing the depolarizing effect (Fig. 1 A). The tubular localization of GCl is also in agreement with the proposed mechanism of myotonia (Adrian and Bryant, 1974): without the tubular GCl, [K+] raises much more quickly and to higher values, leading to the repetitive AP firing and muscle stiffness.
Figure 1.
Possible scenarios for localization and effect of the muscle chloride conductance. (A) A T-tubular localization of GCl could directly minimize K+ accumulation in the T-tubules. (B) In the simple remote control theory, GCl controls the T-tubular membrane potential and the tubular [K+] from its sarcolemmal localization. (C) A possible mechanism by which T-tubular outwardly rectifying K+ channels mediate fast AP repolarization and inwardly rectifying K channels mediate K+ reuptake. The tubular membrane potential is kept negative at rest after cessation of the AP. The inset shows schematically the waveform of an AP.
The principal difficulty in answering the localization question is the inaccessibility of the T-system to functional measurements and to antibody staining. An elegant way to directly assess the function of the T-system is via the optical detection of voltage-dependent fluorescent dyes. More indirect assays rely on the elimination of the T-system by “detubulating” procedures. Detubulation can be achieved by glycerol treatment or formamide treatment (Bretag, 1987). Detubulation has been used by Lueck et al. (2010); however, they avoided some of the caveats of earlier studies. Alternatively, the sarcolemma can be selectively removed in “skinned fiber” preparations in which the T-system remains as the only “plasma membrane compartment.” In skinned fibers, the membrane potential of the T-system can be polarized by high K+ (Coonan and Lamb, 1998). However, in this preparation, the T-system is even less accessible than in whole fibers.
Two types of experiments most strongly suggested a predominant localization of GCl in the T-system. First, studies measuring GCl before and after glycerol-mediated detubulation indicated predominant localization of the chloride conductance in T-tubules (Palade and Barchi, 1977; Dulhunty, 1978). However, as discussed by Bretag (1987), glycerol treatment may produce unspecific effects on muscle fibers.
Second, experiments of fiber contraction in skinned fibers strongly suggested the presence of a Cl− conductance in the T-system that recapitulated the properties of ClC-1, such as 9-AC sensitivity (Coonan and Lamb, 1998; Dutka et al., 2008). The same group estimated an absolute value of tubular Cl− conductance, concluding that a large proportion of GCl is localized in the T-system (Dutka et al., 2008). However, because the T-system is not directly accessible in skinned fibers, these estimates are based on indirect evidence.
On the other hand, other reports concluded that GCl is predominantly localized in the sarcolemma. Hodgkin and Horowicz (1960) showed that sudden changes of the Cl− concentration affected membrane potential much faster than changes of the K+ concentration, compatible with a higher “accessibility” of the Cl− conductance. Also, measurements of Cl− conductance on frog (Eisenberg and Gage, 1969) and goat (Adrian and Bryant, 1974) muscle before and after glycerol detubulation indicated predominant sarcolemma localization of GCl. Finally, immunolocalization of the ClC-1 channel in the rat and mouse detected the protein exclusively on the sarcolemma (Gurnett et al., 1995; Papponen et al., 2005).
Lueck et al. (2010) adopted a three-faceted approach to show that ClC-1 is almost exclusively expressed on the sarcolemma. The most important result of their work comes from the application of the whole cell recording configuration of the patch clamp technique to intact muscle fibers (from mouse flexor digitorum brevis). This has so far proved to be exceedingly difficult because of the large size of the fibers and the large currents, resulting in a relatively large series resistance error. However, using large pipettes and careful series resistance compensation, the authors could reliably clamp the ClC-1–mediated currents. The authors had already used this approach to characterize ClC-1 in dystrophic mouse models (Lueck et al., 2007; Wheeler et al., 2007), but only in relatively small fibers from mice up to 20 days old. Now, the authors succeeded to record from adult fibers.
In agreement with the earlier studies (Lueck et al., 2007), the properties of the Cl− currents recorded in the new work resemble in all details the recordings of ClC-1 in heterologous expression systems (Rychkov et al., 1996; Zifarelli and Pusch, 2007). To dissect the contribution of ClC-1 localized in the sarcolemma and in the T-tubule, the authors detubulated the fibers by formamide treatment. In paired experiments in which fibers were assayed before and after detubulation, they could show that a drastic decrease of membrane capacitance (up to 65%, corresponding to an 85% loss of T-tubule membrane) did not produce any reduction of ClC-1 activity or any change in channel properties, including the sensitivity to the blocker 9-AC. Confocal fluorescence microscopy confirmed the substantial detubulation after formamide treatment. Using immunofluorescence, the authors could show a separate localization of ClC-1 protein to the sarcolemma and of the DHPR in the T-system. The localization experiments of endogenous ClC-1 were complemented with experiments in which tagged ClC-1 protein was reintroduced in a transgenic mouse model for myotonia (HSALR mice), which are characterized by a reduced intrinsic expression of ClC-1. In these mice, the reintroduction of ClC-1 “cures” the myotonia (Wheeler et al., 2007), showing that the localization of the exogenous ClC-1 is similar to that of the endogenous protein. Moreover, although immunolabeled RYR1 showed a typical T-tubule distribution, the fluorescence signal from tagged ClC-1 was confined to the sarcolemma.
In conclusion, this work provides compelling and exhaustive evidence of ClC-1 localization to the sarcolemma, without any significant contribution of the T-system. A particularly strong point is that the authors did not see any reduction of ClC-1 currents after detubulation. If a significant fraction of ClC-1 were expressed in the T-tubules, currents should be reduced after detubulation.
A further strong point is that the immunolabeling experiments were well controlled by DHPR and RYR detection in (or close to) the T-system. That said, the contrast with those previous studies that concluded a significant localization of GCl in the T-system requires an explanation. Although potential technical shortcomings of some studies have been highlighted, there is also the possibility of a residual localization of ClC-1 in T-tubule “necks.” Lueck et al. (2010) found these necks to be resistant to formamide treatment. It might be that the necks are included in skinned fibers, possibly explaining the presence of a certain chloride conductance in the T-system in that preparation (Dutka et al., 2008).
If all of GCl is in the sarcolemma, how can we explain the role of GCl in AP repolarization in the T-system and its role in preventing K+ accumulation there? It is well accepted that the AP actively invades the T-system using voltage-gated Na+ channels (Allen et al., 2008). The precise localization and distribution of K+ channels and other K+ transport proteins in the T-system are less clear (Kristensen and Juel, 2010). Inwardly rectifying K+ channels are localized to the T-system, but these are not involved in AP repolarization, but rather in K+ reuptake and stabilization of the resting potential (Kristensen and Juel, 2010). However, even if little is known about the distribution of Kv K+ channels that classically serve AP repolarization (Kristensen and Juel, 2010), it is likely that the T-tubular membrane contains at least some K+ conductance that helps in the repolarization of the tubular AP. To explain the function of sarcolemmal GCl for the control of T-tubular membrane potential and T-tubular [K+], Lueck et al. (2010) invoked a “remote control” mechanism (Fig. 1 B). According to this view, the membrane potential of the T-system is controlled by the relatively distantly localized sarcolemmal Cl− conductance. It might seem difficult to imagine that the T-system is almost isopotential to the sarcolemma. However, at least in mammalian skeletal muscle, sarcolemma and T-tubule membranes change their potential practically simultaneously. This was true both for AP-mediated potential changes as well as passive (“electrotonic”) potential changes (DiFranco et al., 2005). Nevertheless, small drifts of the T-tubular potential caused by K+ accumulation might not be captured by these measurements, and may lead to small differences in the T-tubule and sarcolemmal potential. To rationalize the T-tubular localization of GCl, Lueck et al. propose that the localization of ClC-1 within the T-tubules could compromise the buffering action of GCl because ECl passively follows EK. If ClC-1 is instead localized on the sarcolemma, distant from the K+ accumulation, sarcolemmal ECl would remain negative, ensuring high buffering power (Fig. 1 C).
An additional advantage of a sarcolemmal localization of CLC-1 is that the resting conductance of the T-tubular membrane is small. This allows faster and less energy-consuming AP conduction because fewer voltage-gated Na+ channels are necessary for AP propagation. In fact, assuming that within the T-tubules fast AP repolarization occurs predominantly by outwardly rectifying K+ channels (Fig. 1 C), the large sarcolemmal GCl could keep the resting potential very negative after cessation of the AP via a “slow remote control,” such that accumulated K+ can be reabsorbed efficiently and quickly via inwardly rectifying K+ channels (Fig. 1 C).
Acknowledgments
The financial support of our work by Telethon Italy (no. GGP08064), the Compagnia San Paolo, and the Italian Institute of Technology (“Progetto Seed”) is gratefully acknowledged.
References
- Adrian R.H., Bryant S.H. 1974. On the repetitive discharge in myotonic muscle fibres. J. Physiol. 240:505–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D.G., Lamb G.D., Westerblad H. 2008. Skeletal muscle fatigue: cellular mechanisms. Physiol. Rev. 88:287–332 10.1152/physrev.00015.2007 [DOI] [PubMed] [Google Scholar]
- Bretag A.H. 1987. Muscle chloride channels. Physiol. Rev. 67:618–724 [DOI] [PubMed] [Google Scholar]
- Coonan J.R., Lamb G.D. 1998. Effect of transverse-tubular chloride conductance on excitability in skinned skeletal muscle fibres of rat and toad. J. Physiol. 509:551–564 10.1111/j.1469-7793.1998.551bn.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranco M., Capote J., Vergara J.L. 2005. Optical imaging and functional characterization of the transverse tubular system of mammalian muscle fibers using the potentiometric indicator di-8-ANEPPS. J. Membr. Biol. 208:141–153 10.1007/s00232-005-0825-9 [DOI] [PubMed] [Google Scholar]
- Dulhunty A.F. 1978. The dependence of membrane potential on extracellular chloride concentration in mammalian skeletal muscle fibres. J. Physiol. 276:67–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutka T.L., Murphy R.M., Stephenson D.G., Lamb G.D. 2008. Chloride conductance in the transverse tubular system of rat skeletal muscle fibres: importance in excitation-contraction coupling and fatigue. J. Physiol. 586:875–887 10.1113/jphysiol.2007.144667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R.S., Gage P.W. 1969. Ionic conductances of the surface and transverse tubular membranes of frog sartorius fibers. J. Gen. Physiol. 53:279–297 10.1085/jgp.53.3.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurnett C.A., Kahl S.D., Anderson R.D., Campbell K.P. 1995. Absence of the skeletal muscle sarcolemma chloride channel ClC-1 in myotonic mice. J. Biol. Chem. 270:9035–9038 10.1074/jbc.270.16.9035 [DOI] [PubMed] [Google Scholar]
- Hodgkin A.L., Horowicz P. 1960. The effect of sudden changes in ionic concentrations on the membrane potential of single muscle fibres. J. Physiol. 153:370–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M.C., Steinmeyer K., Lorenz C., Ricker K., Wolf F., Otto M., Zoll B., Lehmann-Horn F., Grzeschik K.H., Jentsch T.J. 1992. The skeletal muscle chloride channel in dominant and recessive human myotonia. Science. 257:797–800 10.1126/science.1379744 [DOI] [PubMed] [Google Scholar]
- Kristensen M., Juel C. 2010. Potassium-transporting proteins in skeletal muscle: cellular location and fibre-type differences. Acta Physiol. (Oxf.). 198:105–123 10.1111/j.1748-1716.2009.02043.x [DOI] [PubMed] [Google Scholar]
- Lipicky R.J., Bryant S.H. 1966. Sodium, potassium, and chloride fluxes in intercostal muscle from normal goats and goats with hereditary myotonia. J. Gen. Physiol. 50:89–111 10.1085/jgp.50.1.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipicky R.J., Bryant S.H., Salmon J.H. 1971. Cable parameters, sodium, potassium, chloride, and water content, and potassium efflux in isolated external intercostal muscle of normal volunteers and patients with myotonia congenita. J. Clin. Invest. 50:2091–2103 10.1172/JCI106703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueck J.D., Mankodi A., Swanson M.S., Thornton C.A., Dirksen R.T. 2007. Muscle chloride channel dysfunction in two mouse models of myotonic dystrophy. J. Gen. Physiol. 129:79–94 10.1085/jgp.200609635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueck J.D., Rossi A.E., Thornton C.A., Campbell K.P., Dirksen R.T. 2010. Sarcolemmal-restricted localization of functional ClC-1 channels in mouse skeletal muscle. J. Gen. Physiol. 136:597–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade P.T., Barchi R.L. 1977. Characteristics of the chloride conductance in muscle fibers of the rat diaphragm. J. Gen. Physiol. 69:325–342 10.1085/jgp.69.3.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papponen H., Kaisto T., Myllylä V.V., Myllylä R., Metsikkö K. 2005. Regulated sarcolemmal localization of the muscle-specific ClC-1 chloride channel. Exp. Neurol. 191:163–173 10.1016/j.expneurol.2004.07.018 [DOI] [PubMed] [Google Scholar]
- Rychkov G.Y., Pusch M., Astill D.S., Roberts M.L., Jentsch T.J., Bretag A.H. 1996. Concentration and pH dependence of skeletal muscle chloride channel ClC-1. J. Physiol. 497:423–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmeyer K., Ortland C., Jentsch T.J. 1991. Primary structure and functional expression of a developmentally regulated skeletal muscle chloride channel. Nature. 354:301–304 10.1038/354301a0 [DOI] [PubMed] [Google Scholar]
- Wheeler T.M., Lueck J.D., Swanson M.S., Dirksen R.T., Thornton C.A. 2007. Correction of ClC-1 splicing eliminates chloride channelopathy and myotonia in mouse models of myotonic dystrophy. J. Clin. Invest. 117:3952–3957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zifarelli G., Pusch M. 2007. CLC chloride channels and transporters: a biophysical and physiological perspective. Rev. Physiol. Biochem. Pharmacol. 158:23–76 10.1007/112_2006_0605 [DOI] [PubMed] [Google Scholar]